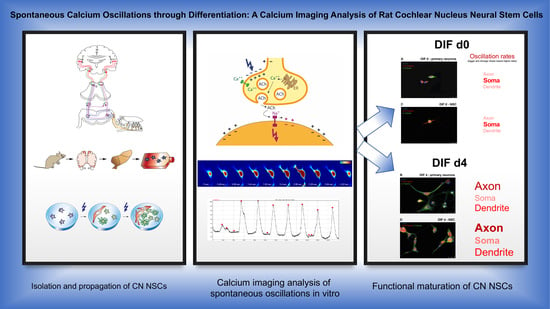
Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Preparation, Cell Culture and Neurosphere Assay
2.2. Calcium Imaging, Loading Protocol, and Immunocytochemistry
2.3. Data Analysis and Image Processing
3. Results
3.1. Differentiating CN NSCs Show Spontaneous Calcium Oscillations
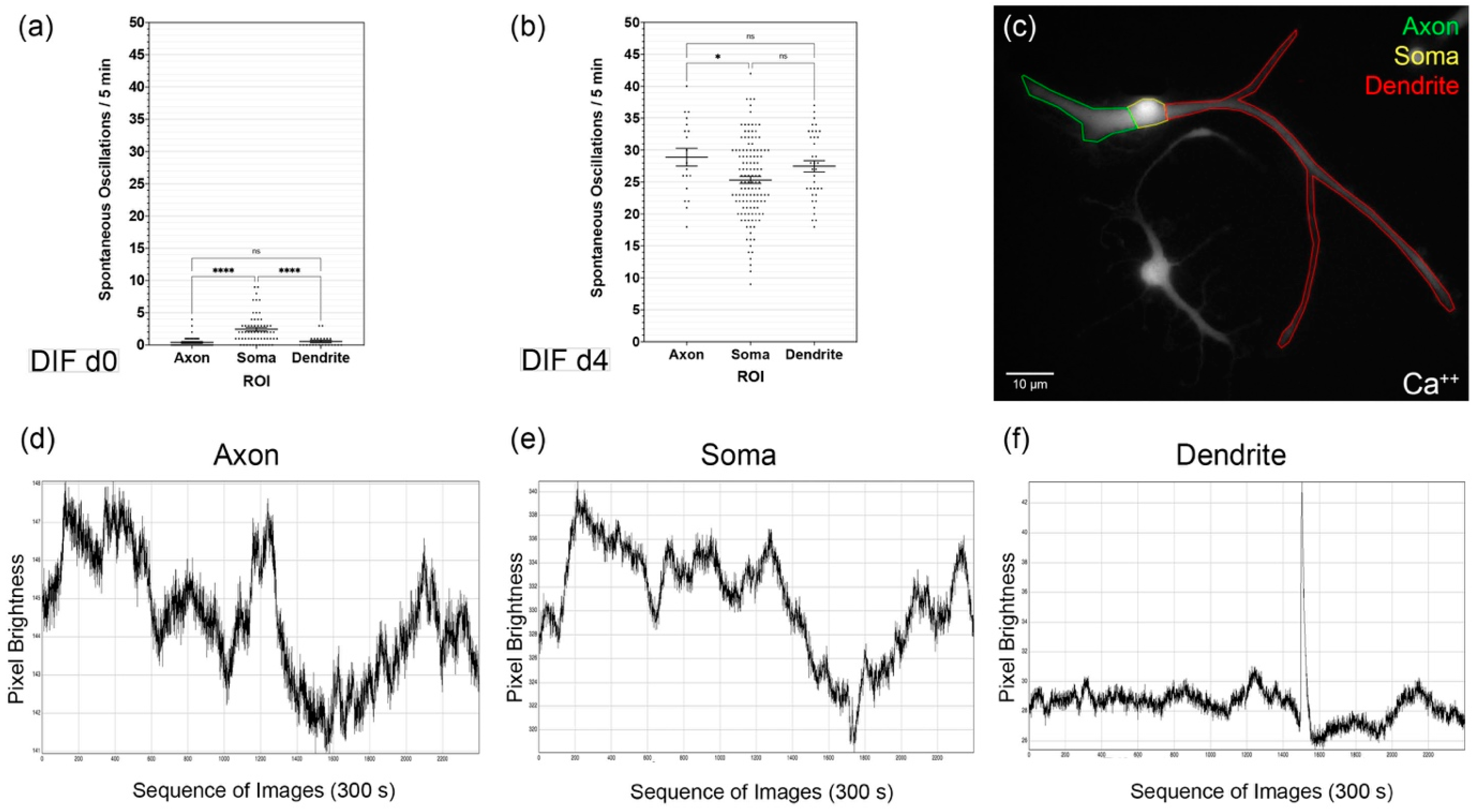
3.2. Spontaneous Calcium Activities in the Maturing CN NSC Subregions
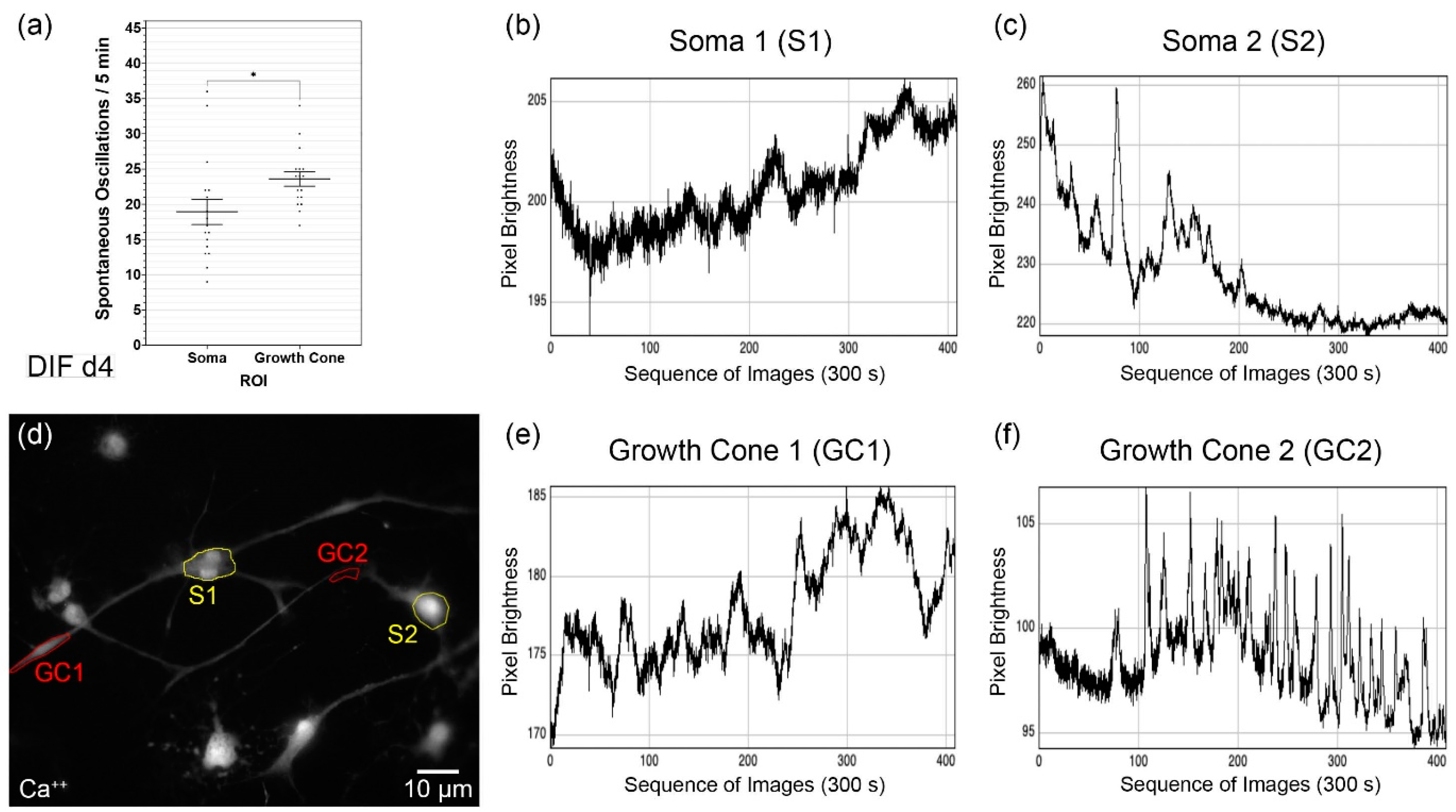
3.3. Analysis of the Growth Cone Activities
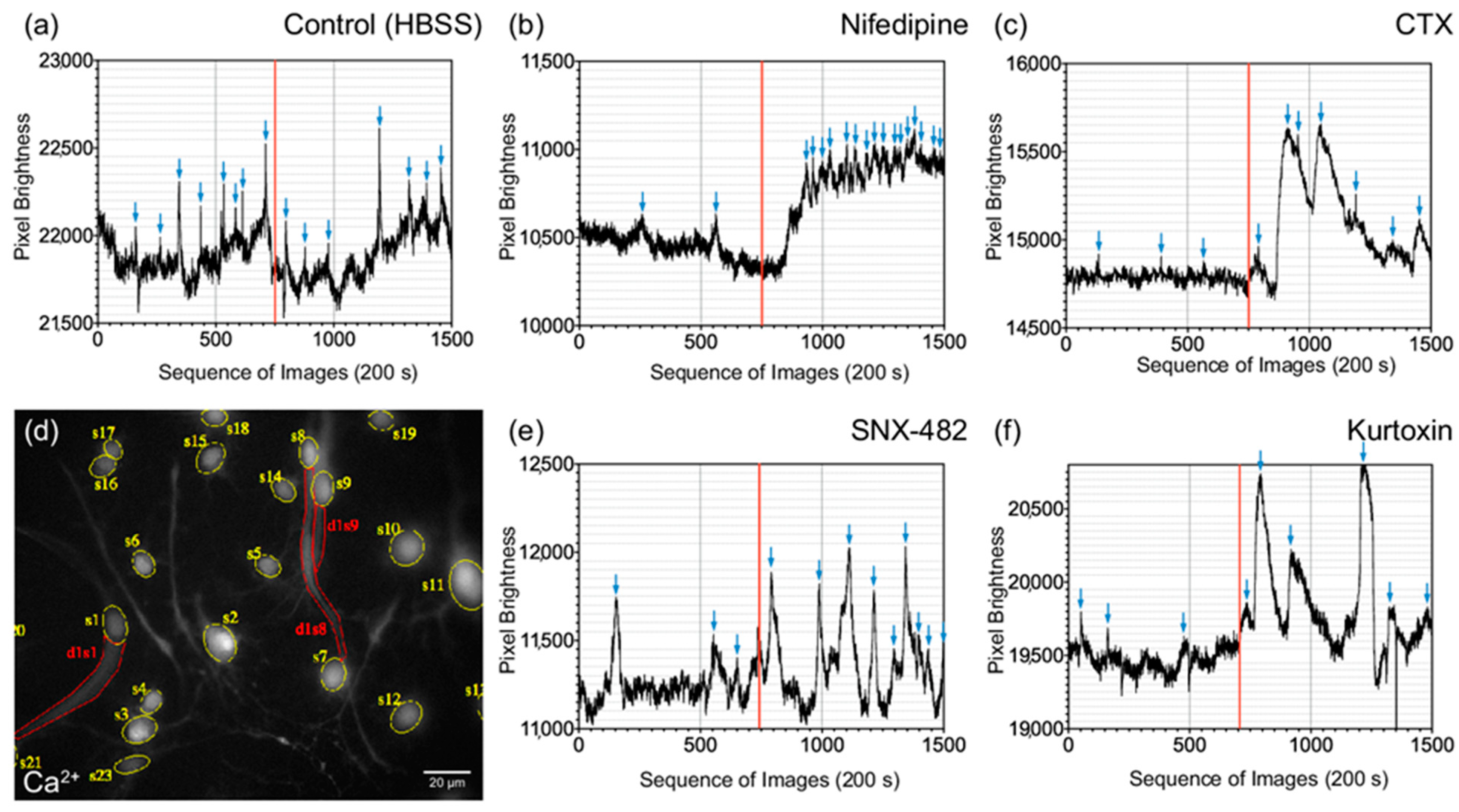
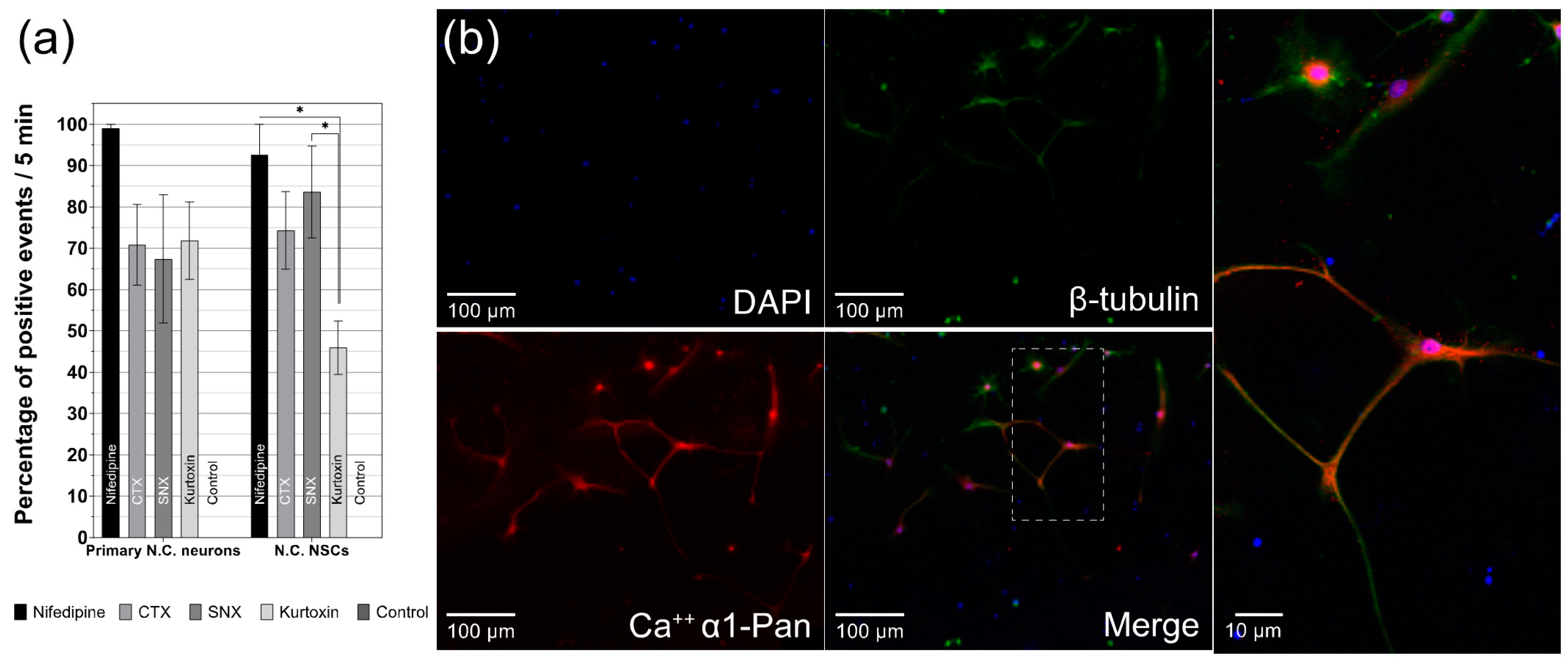
3.4. Influence of Specific Voltage-Gated Calcium Channel Inhibitors on Primary Neurons and NSCs of the Cochlear Nucleus
3.5. Differentiated CN NSCs Have a Specific Sensitivity to Calcium Channel Inhibitors
4. Discussion
4.1. CN NSC Differentiation Assay and Calcium Imaging Allow In Vitro Analyses in the Longitudinal Course
4.2. CN NSC Differentiation Is Associated with Increased Spontaneous Activity and Specific Intracellular Patterns
4.3. Voltage-Dependent Calcium Channels Cause Spontaneous Oscillations in the Early Phase of CN NSC Maturation and Allow Exogenous Influence
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homans, N.C.; Metselaar, R.M.; Dingemanse, J.G.; van der Schroeff, M.P.; Brocaar, M.P.; Wieringa, M.H.; Baatenburg de Jong, R.J.; Hofman, A.; Goedegebure, A. Prevalence of Age-Related Hearing Loss, Including Sex Differences, in Older Adults in a Large Cohort Study. Laryngoscope 2017, 127, 725–730. [Google Scholar] [CrossRef]
- Roccio, M.; Senn, P.; Heller, S. Novel Insights into Inner Ear Development and Regeneration for Targeted Hearing Loss Therapies. Hear. Res. 2019, 107859. [Google Scholar] [CrossRef]
- Thomas, D.; Tovey, S.C.; Collins, T.J.; Bootman, M.D.; Berridge, M.J.; Lipp, P. A Comparison of Fluorescent Ca2+indicator Properties and Their Use in Measuring Elementary and Global Ca2+ signals. Cell Calcium 2000, 28, 213–223. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical Calcium Indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Temple, S. A Self-Renewing Multipotential Stem Cell in Embryonic Rat Cerebral Cortex. Nature 1994, 372, 263–266. [Google Scholar] [CrossRef] [PubMed]
- McKay, R. Stem Cells in the Central Nervous System. Science 1997, 276, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, J. Are New Neurons Formed in the Brains of Adult Mammals? Science 1962, 135, 1127–1128. [Google Scholar] [CrossRef] [Green Version]
- Alessio, N.; Siniscalco, D.; Peluso, G.; Galderisi, U. New Frontiers in Stem Cell Research and Translational Approaches. Biology 2020, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Pickles, J.O. Chapter 1—Auditory pathways: Anatomy and physiology. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; The Human Auditory System; Elsevier: Amsterdam, The Netherlands, 2015; Volume 129, pp. 3–25. [Google Scholar]
- Kandel, E.; Schwartz, J.; Jessell, T.; Siegelbaum, S.; Hudspeth, A.J. Principles of Neural Science, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2012; ISBN 978-0-07-139011-8. [Google Scholar]
- Campagnola, L.; Manis, P.B. A Map of Functional Synaptic Connectivity in the Mouse Anteroventral Cochlear Nucleus. J. Neurosci. 2014, 34, 2214–2230. [Google Scholar] [CrossRef] [Green Version]
- Liberman, M.C. Central Projections of Auditory Nerve Fibers of Differing Spontaneous Rate, II: Posteroventral and Dorsal Cochlear Nuclei. J. Comp. Neurol. 1993, 327, 17–36. [Google Scholar] [CrossRef]
- Mitochondrial Oxidative Damage and Apoptosis in Age-Related Hearing Loss. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4086639/ (accessed on 4 August 2021).
- Fischel-Ghodsian, N. Mitochondrial Deafness. Ear Hear. 2003, 24, 303–313. [Google Scholar] [CrossRef]
- Drusin, M.A.; Lubor, B.; Losenegger, T.; Selesnick, S. Trends in Hearing Rehabilitation Use among Vestibular Schwannoma Patients. Laryngoscope 2020, 130, 1558–1564. [Google Scholar] [CrossRef]
- Baldi, A.; Tenaglia, S.; D’Anna, S. Auditory Dysfunction. Manif. Stroke 2012, 30, 26–29. [Google Scholar] [CrossRef]
- Colletti, V.; Shannon, R.; Carner, M.; Veronese, S.; Colletti, L. Outcomes in Nontumor Adults Fitted with the Auditory Brainstem Implant: 10 Years’ Experience. Otol. Neurotol. 2009, 30, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Tierney, T.S.; Russell, F.A.; Moore, D.R. Susceptibility of Developing Cochlear Nucleus Neurons to Deafferentation-Induced Death Abruptly Ends Just before the Onset of Hearing. J. Comp. Neurol. 1997, 378, 295–306. [Google Scholar] [CrossRef]
- Born, D.E.; Rubel, E.W. Afferent Influences on Brain Stem Auditory Nuclei of the Chicken: Neuron Number and Size Following Cochlea Removal. J. Comp. Neurol. 1985, 231, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ebisu, M.; Morita, T.; Takeuchi, T.; Umemura, T. Age-Related Changes in the Cochlea and Cochlear Nuclei of Dogs. J. Vet. Med. Sci. 1998, 60, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.S.; Otto, S.R.; Shannon, R.V.; Hitselberger, W.E.; Brackmann, D.E. Auditory Brainstem Implants. Neurotherapeutics 2008, 5, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Matthies, C.; Brill, S.; Varallyay, C.; Solymosi, L.; Gelbrich, G.; Roosen, K.; Ernestus, R.-I.; Helms, J.; Hagen, R.; Mlynski, R.; et al. Auditory Brainstem Implants in Neurofibromatosis Type 2: Is Open Speech Perception Feasible? J. Neurosurg. 2014, 120, 546–558. [Google Scholar] [CrossRef]
- Rak, K.; Wasielewski, N.V.; Radeloff, A.; Völker, J.; Scherzed, A.; Jablonka, S.; Hagen, R.; Mlynski, R. Isolation and Characterization of Neural Stem Cells from the Neonatal Rat Cochlear Nucleus. Cell Tissue Res. 2011, 343, 499–508. [Google Scholar] [CrossRef]
- Volkenstein, S.; Oshima, K.; Sinkkonen, S.T.; Corrales, C.E.; Most, S.P.; Chai, R.; Jan, T.A.; van Amerongen, R.; Cheng, A.G.; Heller, S. Transient, Afferent Input-Dependent, Postnatal Niche for Neural Progenitor Cells in the Cochlear Nucleus. Proc. Natl. Acad. Sci. USA 2013, 110, 14456–14461. [Google Scholar] [CrossRef] [Green Version]
- Rak, K.; Völker, J.; Frenz, S.; Scherzed, A.; Radeloff, A.; Hagen, R.; Mlynski, R. Dynamic Changes of the Neurogenic Potential in the Rat Cochlear Nucleus during Post-Natal Development. Exp. Brain Res. 2013, 226, 393–406. [Google Scholar] [CrossRef]
- Rak, K.; Völker, J.; Jürgens, L.; Völker, C.; Frenz, S.; Scherzad, A.; Schendzielorz, P.; Jablonka, S.; Mlynski, R.; Radeloff, A.; et al. Cochlear Nucleus Whole Mount Explants Promote the Differentiation of Neuronal Stem Cells from the Cochlear Nucleus in Co-Culture Experiments. Brain Res. 2015, 1616, 58–70. [Google Scholar] [CrossRef]
- Thivierge, J.-P. How Does Non-Random Spontaneous Activity Contribute to Brain Development? Neural Netw. 2009, 22, 901–912. [Google Scholar] [CrossRef]
- Demerens, C.; Stankoff, B.; Logak, M.; Anglade, P.; Allinquant, B.; Couraud, F.; Zalc, B.; Lubetzki, C. Induction of Myelination in the Central Nervous System by Electrical Activity. Proc. Natl. Acad. Sci. USA 1996, 93, 9887–9892. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidelberger, R.; Heinemann, C.; Neher, E.; Matthews, G. Calcium Dependence of the Rate of Exocytosis in a Synaptic Terminal. Nature 1994, 371, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, J.H.; Sakmann, B. Control of Synaptic Strength and Timing by the Release-Site Ca2+ Signal. Nat. Neurosci. 2005, 8, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.M.; Jacobs, R.A.; Hudspeth, A.J. Colocalization of Ion Channels Involved in Frequency Selectivity and Synaptic Transmission at Presynaptic Active Zones of Hair Cells. J. Neurosci. 1990, 10, 3664–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, G.J.; Santamaria, F.; Tanaka, K. Local Calcium Signaling in Neurons. Neuron 2003, 40, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Di Capite, J.; Ng, S.W.; Parekh, A.B. Decoding of Cytoplasmic Ca2+ Oscillations through the Spatial Signature Drives Gene Expression. Curr. Biol. 2009, 19, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, A.B.; Putney, J.W. Store-Operated Calcium Channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 Is a Ca2+ Sensor That Activates CRAC Channels and Migrates from the Ca2+ Store to the Plasma Membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef]
- Hartmann, J.; Karl, R.M.; Alexander, R.P.D.; Adelsberger, H.; Brill, M.S.; Rühlmann, C.; Ansel, A.; Sakimura, K.; Baba, Y.; Kurosaki, T.; et al. STIM1 Controls Neuronal Ca2+ Signaling, MGluR1-Dependent Synaptic Transmission, and Cerebellar Motor Behavior. Neuron 2014, 82, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Baba, A.; Yasui, T.; Fujisawa, S.; Yamada, R.X.; Yamada, M.K.; Nishiyama, N.; Matsuki, N.; Ikegaya, Y. Activity-Evoked Capacitative Ca2+ Entry: Implications in Synaptic Plasticity. J. Neurosci. 2003, 23, 7737–7741. [Google Scholar] [CrossRef] [Green Version]
- Emptage, N.J.; Reid, C.A.; Fine, A. Calcium Stores in Hippocampal Synaptic Boutons Mediate Short-Term Plasticity, Store-Operated Ca2+ Entry, and Spontaneous Transmitter Release. Neuron 2001, 29, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Lalonde, J.; Saia, G.; Gill, G. Store-Operated Calcium Entry Promotes the Degradation of the Transcription Factor Sp4 in Resting Neurons. Sci. Signal. 2014, 7, ra51. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.; Leclerc, C. The Choice between Epidermal and Neural Fate: A Matter of Calcium. Int. J. Dev. Biol. 2003, 48, 75–84. [Google Scholar] [CrossRef]
- Spitzer, N.C. Electrical Activity in Early Neuronal Development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef]
- Somasundaram, A.; Shum, A.K.; McBride, H.J.; Kessler, J.A.; Feske, S.; Miller, R.J.; Prakriya, M. Store-Operated CRAC Channels Regulate Gene Expression and Proliferation in Neural Progenitor Cells. J. Neurosci. 2014, 34, 9107–9123. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.S.; Spitzer, N.C. Calcium Signaling in Neuronal Development. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.Q.; Poo, M. Calcium Signaling in Neuronal Motility. Annu. Rev. Cell Dev. Biol. 2007, 23, 375–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmetsch, R.E.; Lewis, R.S. Signaling between Intracellular Ca2+ Stores and Depletion-Activated Ca2+ Channels Generates [Ca2+]i Oscillations in T Lymphocytes. J. Gen. Physiol. 1994, 103, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Manent, J.-B.; Demarque, M.; Jorquera, I.; Pellegrino, C.; Ben-Ari, Y.; Aniksztejn, L.; Represa, A. A Noncanonical Release of GABA and Glutamate Modulates Neuronal Migration. J. Neurosci. 2005, 25, 4755–4765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.; Davis, M.B.; Kriegstein, A.R. GABA and Glutamate Depolarize Cortical Progenitor Cells and Inhibit DNA Synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium Oscillations Increase the Efficiency and Specificity of Gene Expression. Nature 1998, 392, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B. Decoding Cytosolic Ca2+ Oscillations. Trends Biochem. Sci. 2011, 36, 78–87. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Lewis, R.S.; Goodnow, C.C.; Healy, J.I. Differential Activation of Transcription Factors Induced by Ca2+ Response Amplitude and Duration. Nature 1997, 386, 855–858. [Google Scholar] [CrossRef]
- Weinstein, D.C.; Hemmati-Brivanlou, A. Neural Induction. Annu. Rev. Cell Dev. Biol. 1999, 15, 411–433. [Google Scholar] [CrossRef]
- Leclerc, C.; Néant, I.; Moreau, M. The Calcium: An Early Signal That Initiates the Formation of the Nervous System during Embryogenesis. Front. Mol. Neurosci. 2012, 5. [Google Scholar] [CrossRef] [Green Version]
- Papanayotou, C.; De Almeida, I.; Liao, P.; Oliveira, N.M.M.; Lu, S.-Q.; Kougioumtzidou, E.; Zhu, L.; Shaw, A.; Sheng, G.; Streit, A.; et al. Calfacilitin Is a Calcium Channel Modulator Essential for Initiation of Neural Plate Development. Nat. Commun. 2013, 4, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deisseroth, K.; Singla, S.; Toda, H.; Monje, M.; Palmer, T.D.; Malenka, R.C. Excitation-Neurogenesis Coupling in Adult Neural Stem/Progenitor Cells. Neuron 2004, 42, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Gaudillière, B.; Konishi, Y.; de la Iglesia, N.; lan Yao, G.; Bonni, A. A CaMKII-NeuroD Signaling Pathway Specifies Dendritic Morphogenesis. Neuron 2004, 41, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wen, Z.; Shi, D.; Xie, Z. Muscarinic Acetylcholine Receptors Involved in the Regulation of Neural Stem Cell Proliferation and Differentiation in Vitro. Cell Biol. Int. 2004, 28, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Louhivuori, L.M.; Louhivuori, V.; Wigren, H.K.; Hakala, E.; Jansson, L.C.; Nordström, T.; Castrén, M.L.; Åkerman, K.E. Role of Low Voltage Activated Calcium Channels in Neuritogenesis and Active Migration of Embryonic Neural Progenitor Cells. Stem Cells Dev. 2013, 22, 1206–1219. [Google Scholar] [CrossRef]
- Yasuda, T.; Bartlett, P.F.; Adams, D.J. K(Ir) and K(v) Channels Regulate Electrical Properties and Proliferation of Adult Neural Precursor Cells. Mol. Cell. Neurosci. 2008, 37, 284–297. [Google Scholar] [CrossRef]
- Spitzer, N.C.; Gu, X.; Olson, E. Action Potentials, Calcium Transients and the Control of Differentiation of Excitable Cells. Curr. Opin. Neurobiol. 1994, 4, 70–77. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Spitzer, N.C. Activity-Dependent Neurotransmitter-Receptor Matching at the Neuromuscular Junction. Proc. Natl. Acad. Sci. USA 2007, 104, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Gomez, T.M.; Spitzer, N.C. In Vivo Regulation of Axon Extension and Pathfinding by Growth-Cone Calcium Transients. Nature 1999, 397, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.G.; Landmesser, L.T. Normal Patterns of Spontaneous Activity Are Required for Correct Motor Axon Guidance and the Expression of Specific Guidance Molecules. Neuron 2004, 43, 687–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozuka, Y.; Fukuda, S.; Namba, T.; Seki, T.; Hisatsune, T. GABAergic Excitation Promotes Neuronal Differentiation in Adult Hippocampal Progenitor Cells. Neuron 2005, 47, 803–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosenitsch, T.A.; Katz, D.M. Physiological Patterns of Electrical Stimulation Can Induce Neuronal Gene Expression by Activating N-Type Calcium Channels. J. Neurosci. 2001, 21, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Völker, J.; Engert, J.; Völker, C.; Bieniussa, L.; Schendzielorz, P.; Hagen, R.; Rak, K. Isolation and Characterization of Neural Stem Cells from the Rat Inferior Colliculus. Stem Cells Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Voelker, J.; Engert, J.; Voelker, C.; Bieniussa, L.; Schendzielorz, P.; Hagen, R.; Rak, K. Different Neurogenic Potential in the Subnuclei of the Postnatal Rat Cochlear Nucleus. Stem Cells Int. 2021, 2021, 8871308. [Google Scholar] [CrossRef]
- Altman, J. Autoradiographic Investigation of Cell Proliferation in the Brains of Rats and Cats. Anat. Rec. 1963, 145, 573–591. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Draguhn, A.; Reichinnek, S.; Both, M. Optimized Temporally Deconvolved Ca2+ Imaging Allows Identification of Spatiotemporal Activity Patterns of CA1 Hippocampal Ensembles. NeuroImage 2014, 94, 239–249. [Google Scholar] [CrossRef]
- Matsubara, S.; Matsuda, T.; Nakashima, K. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells 2021, 10, 1145. [Google Scholar] [CrossRef]
- Zhao, X.; Rouhiainen, A.; Li, Z.; Guo, S.; Rauvala, H. Regulation of Neurogenesis in Mouse Brain by HMGB1. Cells 2020, 9, 1714. [Google Scholar] [CrossRef]
- Farrag, M.; Leipzig, N.D. Subcutaneous Maturation of Neural Stem Cell-Loaded Hydrogels Forms Region-Specific Neuroepithelium. Cells 2018, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Luhmann, H.J.; Sinning, A.; Yang, J.-W.; Reyes-Puerta, V.; Stüttgen, M.C.; Kirischuk, S.; Kilb, W. Spontaneous Neuronal Activity in Developing Neocortical Networks: From Single Cells to Large-Scale Interactions. Front. Neural Circuits 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espósito, M.S.; Piatti, V.C.; Laplagne, D.A.; Morgenstern, N.A.; Ferrari, C.C.; Pitossi, F.J.; Schinder, A.F. Neuronal Differentiation in the Adult Hippocampus Recapitulates Embryonic Development. J. Neurosci. 2005, 25, 10074–10086. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced Synaptic Plasticity in Newly Generated Granule Cells of the Adult Hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Babola, T.A.; Li, S.; Gribizis, A.; Lee, B.J.; Issa, J.B.; Wang, H.C.; Crair, M.C.; Bergles, D.E. Homeostatic Control of Spontaneous Activity in the Developing Auditory System. Neuron 2018, 99, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Coate, T.M.; Scott, M.K.; Gurjar, M. Current Concepts in Cochlear Ribbon Synapse Formation. Synapse 2019, 73, e22087. [Google Scholar] [CrossRef]
- Gage, F.H.; Ray, J.; Fisher, L.J. Isolation, Characterization, and Use of Stem Cells from the CNS. Annu. Rev. Neurosci. 1995, 18, 159–192. [Google Scholar] [CrossRef]
- Sohur, U.S.; Emsley, J.G.; Mitchell, B.D.; Macklis, J.D. Adult Neurogenesis and Cellular Brain Repair with Neural Progenitors, Precursors and Stem Cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1477–1497. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, L.; Liu, Z.; Jiang, Y.; Deng, X. Identification of Neural Stem Cells from Postnatal Mouse Auditory Cortex In Vitro. Stem Cells Dev. 2019, 28, 860–870. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS Stem Cells Express a New Class of Intermediate Filament Protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient Expression of Doublecortin during Adult Neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vellema, M.; Ko, M.-C.; Frankl-Vilches, C.; Gahr, M. What Makes a Marker a Good Marker?. Commentary on Balthazart J and Ball G (2014): Doublecortin Is a Highly Valuable Endogenous Marker of Adult Neurogenesis in Canaries. Brain Behav. Evol. 2014, 84, 1–4. [Google Scholar] [CrossRef]
- Sox2 and Oct-3/4: A Versatile Pair of Master Regulators That Orchestrate the Self-Renewal and Pluripotency of Embryonic Stem Cells by Functioning as Molecular Rheostats. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2794141/ (accessed on 2 June 2020).
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Komitova, M.; Eriksson, P.S. Sox-2 Is Expressed by Neural Progenitors and Astroglia in the Adult Rat Brain. Neurosci. Lett. 2004, 369, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, J.; Dabdoub, A. Atoh1, an Essential Transcription Factor in Neurogenesis and Intestinal and Inner Ear Development: Function, Regulation, and Context Dependency. J. Assoc. Res. Otolaryngol. 2012, 13, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Mason, I. Initiation to End Point: The Multiple Roles of Fibroblast Growth Factors in Neural Development. Nat. Rev. Neurosci. 2007, 8, 583–596. [Google Scholar] [CrossRef]
- Vescovi, A.L.; Reynolds, B.A.; Fraser, D.D.; Weiss, S. BFGF Regulates the Proliferative Fate of Unipotent (Neuronal) and Bipotent (Neuronal/Astroglial) EGF-Generated CNS Progenitor Cells. Neuron 1993, 11, 951–966. [Google Scholar] [CrossRef]
- Gritti, A.; Cova, L.; Parati, E.A.; Galli, R.; Vescovi, A.L. Basic Fibroblast Growth Factor Supports the Proliferation of Epidermal Growth Factor-Generated Neuronal Precursor Cells of the Adult Mouse CNS. Neurosci. Lett. 1995, 185, 151–154. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G.; Calarco, A.; D’Apolito, M.; Petillo, O.; Cipollaro, M.; Fusco, F.R.; Melone, M.A.B. Efficient Cultivation of Neural Stem Cells with Controlled Delivery of FGF-2. Stem Cell Res. 2013, 10, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccarino, F.M.; Schwartz, M.L.; Raballo, R.; Nilsen, J.; Rhee, J.; Zhou, M.; Doetschman, T.; Coffin, J.D.; Wyland, J.J.; Hung, Y.T. Changes in Cerebral Cortex Size Are Governed by Fibroblast Growth Factor during Embryogenesis. Nat. Neurosci. 1999, 2, 246–253. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H. New Nerve Cells for the Adult Brain. Sci. Am. 1999, 280, 48–53. [Google Scholar] [CrossRef]
- Kim, J.H.; Sun, W.; Han, D.W.; Lim, D.-J.; Lee, J. Induced Neural Stem Cells Have Protective Effects on Cortical Neuronal Cells in Vitro. Neurol. Sci 2015, 36, 527–534. [Google Scholar] [CrossRef]
- Maden, M. Retinoic Acid in the Development, Regeneration and Maintenance of the Nervous System. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging Calcium in Neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [Green Version]
- Comparison of In Vitro and In Situ Kd Values for Various Ca2+ Indicators—Table 19.2. Available online: https://www.thermofisher.com/de/de/home/references/molecular-probes-the-handbook/tables/comparison-of-in-vitro-and-in-situ-kd-values-for-various-ca2-indicators.html (accessed on 8 August 2021).
- Tada, M.; Takeuchi, A.; Hashizume, M.; Kitamura, K.; Kano, M. A Highly Sensitive Fluorescent Indicator Dye for Calcium Imaging of Neural Activity in Vitro and in Vivo. Eur. J. Neurosci. 2014, 39, 1720–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnhold, S.; Andressen, C.; Angelov, D.N.; Vajna, R.; Volsen, S.G.; Hescheler, J.; Addicks, K. Embryonic Stem-cell Derived Neurones Express a Maturation Dependent Pattern of Voltage-gated Calcium Channels and Calcium-binding Proteins. Int. J. Dev. Neurosci. 2000, 18, 201–212. [Google Scholar] [CrossRef]
- Bando, Y.; Irie, K.; Shimomura, T.; Umeshima, H.; Kushida, Y.; Kengaku, M.; Fujiyoshi, Y.; Hirano, T.; Tagawa, Y. Control of Spontaneous Ca2+ Transients Is Critical for Neuronal Maturation in the Developing Neocortex. Cereb Cortex 2016, 26, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prada, J.; Sasi, M.; Martin, C.; Jablonka, S.; Dandekar, T.; Blum, R. An Open Source Tool for Automatic Spatiotemporal Assessment of Calcium Transients and Local “signal-Close-to-Noise” Activity in Calcium Imaging Data. PLoS Comput. Biol. 2018, 14, e1006054. [Google Scholar] [CrossRef] [Green Version]
- Lowery, L.A.; Vactor, D.V. The Trip of the Tip: Understanding the Growth Cone Machinery. Nat. Rev. Mol. Cell Biol. 2009, 10, 332–343. [Google Scholar] [CrossRef]
- Blankenship, A.G.; Feller, M.B. Mechanisms Underlying Spontaneous Patterned Activity in Developing Neural Circuits. Nat. Rev. Neurosci. 2010, 11, 18–29. [Google Scholar] [CrossRef]
- Yamamoto, N.; López-Bendito, G. Shaping Brain Connections through Spontaneous Neural Activity: Shaping Brain Connections through Spontaneous Neural Activity. Eur. J. Neurosci. 2012, 35, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Domenichini, F.; Terrié, E.; Arnault, P.; Harnois, T.; Magaud, C.; Bois, P.; Constantin, B.; Coronas, V. Store-Operated Calcium Entries Control Neural Stem Cell Self-Renewal in the Adult Brain Subventricular Zone. Stem Cells 2018, 36, 761–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lnenicka, G.A.; Arcaro, K.F.; Calabro, J.M. Activity-Dependent Development of Calcium Regulation in Growing Motor Axons. J. Neurosci. 1998, 18, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Dent, E.W.; Kalil, K. Spontaneous Calcium Transients in Developing Cortical Neurons Regulate Axon Outgrowth. J. Neurosci. 2003, 23, 927–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewenstein, Y.; Yarom, Y.; Sompolinsky, H. The Generation of Oscillations in Networks of Electrically Coupled Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 8095–8100. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, U.; Mohanan, P.P.; Almeida, A.F.; Abraham, G.; Khan, M.Y.; Gaurav, K.; Mane, A.; Vikas, S.; Jain, M.; Meel, B. Effectiveness and Effect on Renal Parameters of Amlodipine vs. Other Dihydropyridine Calcium Channel Blockers in Patients with Essential Hypertension: Retrospective Observational Study Based on Real-World Evidence from Electronic Medical Records. Cardiol. Ther. 2021. [Google Scholar] [CrossRef]
- Zongfang, Z.; Wenjing, L.; Zhaomin, C.; Lei, Z. Therapeutic Effect of Piracetam with Nimodipine on Vascular Dementia after Cerebral Infarction. Pak. J. Pharm. Sci. 2020, 33, 2405–2411. [Google Scholar]
- Cecchini, G.; Scaglione, A.; Allegra Mascaro, A.L.; Checcucci, C.; Conti, E.; Adam, I.; Fanelli, D.; Livi, R.; Pavone, F.S.; Kreuz, T. Cortical Propagation Tracks Functional Recovery after Stroke. PLoS Comput. Biol. 2021, 17, e1008963. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Seth, B.; Agarwal, S.; Yadav, A.; Karmakar, M.; Gupta, S.K.; Choubey, V.; Sharma, A.; Chaturvedi, R.K. Ethosuximide Induces Hippocampal Neurogenesis and Reverses Cognitive Deficits in an Amyloid-β Toxin-Induced Alzheimer Rat Model via the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/β-Catenin Pathway. J. Biol. Chem. 2015, 290, 28540–28558. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voelker, J.; Voelker, C.; Engert, J.; Goemann, N.; Hagen, R.; Rak, K. Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells. Cells 2021, 10, 2802. https://doi.org/10.3390/cells10102802
Voelker J, Voelker C, Engert J, Goemann N, Hagen R, Rak K. Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells. Cells. 2021; 10(10):2802. https://doi.org/10.3390/cells10102802
Chicago/Turabian StyleVoelker, Johannes, Christine Voelker, Jonas Engert, Nikolas Goemann, Rudolf Hagen, and Kristen Rak. 2021. "Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells" Cells 10, no. 10: 2802. https://doi.org/10.3390/cells10102802
APA StyleVoelker, J., Voelker, C., Engert, J., Goemann, N., Hagen, R., & Rak, K. (2021). Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells. Cells, 10(10), 2802. https://doi.org/10.3390/cells10102802