Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Process
2.2. Literature Search
2.3. Inclusion and Exclusion Criteria
2.4. Risk of Publication Bias
2.5. Data Extraction
2.6. Data Synthesis
2.7. Statistical Analysis
3. Results
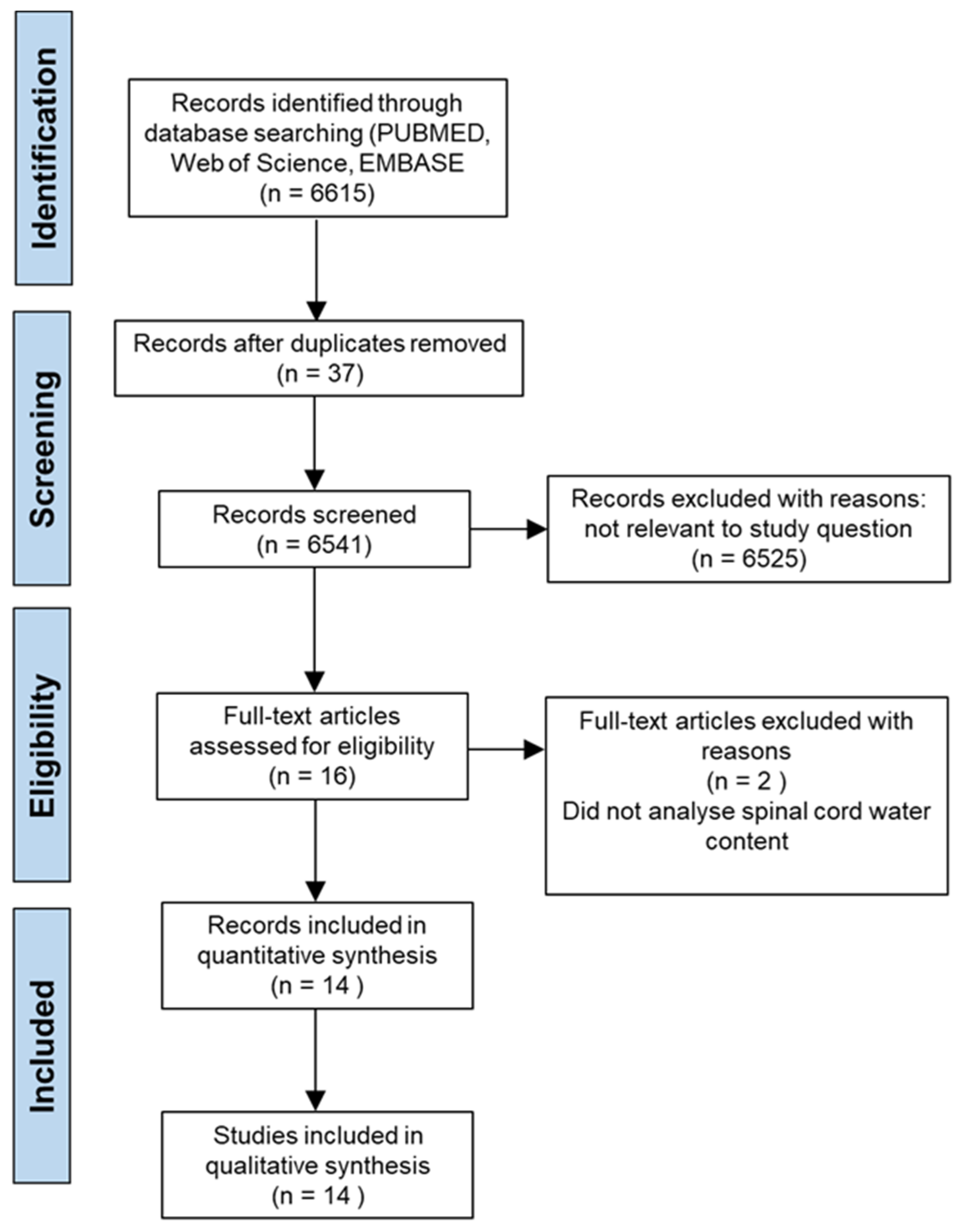
3.1. Study Selection
3.2. Study Characteristics
3.3. Attenuation of Oedema
3.3.1. Results of Individual Studies
3.3.2. Subgroup Analysis
3.3.3. Functional Outcomes
3.4. Meta-Analysis
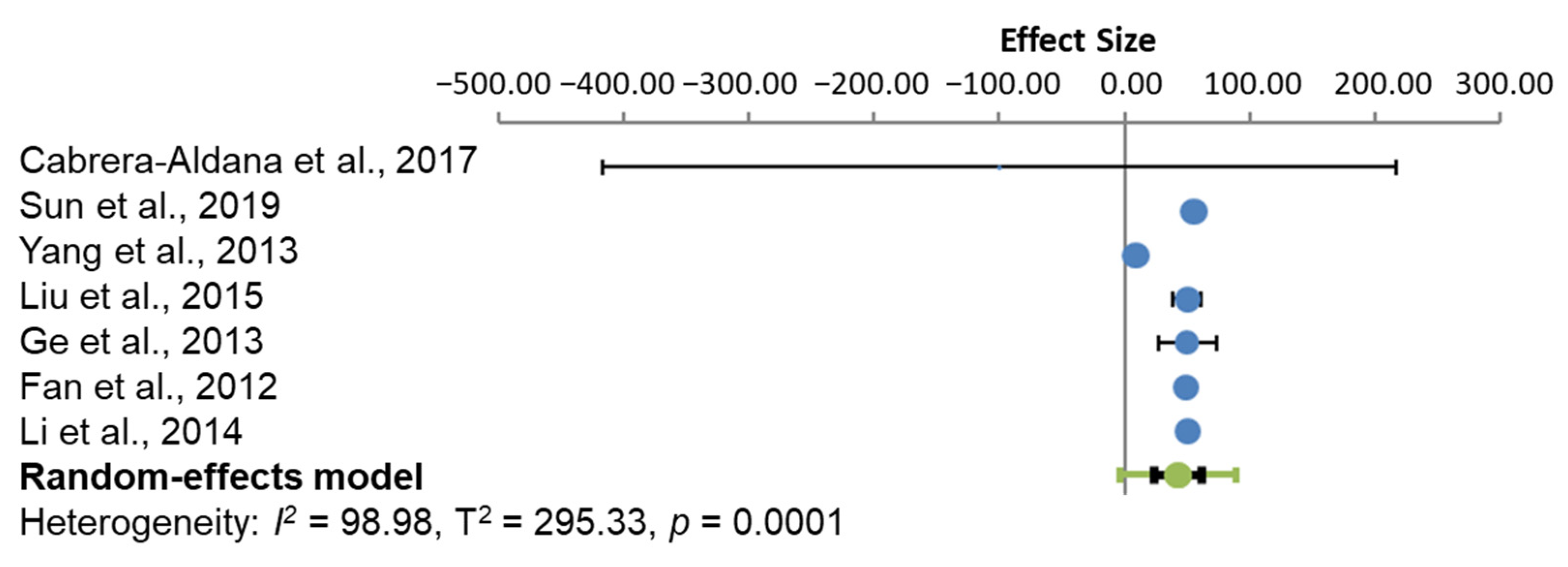
3.4.1. Oedema
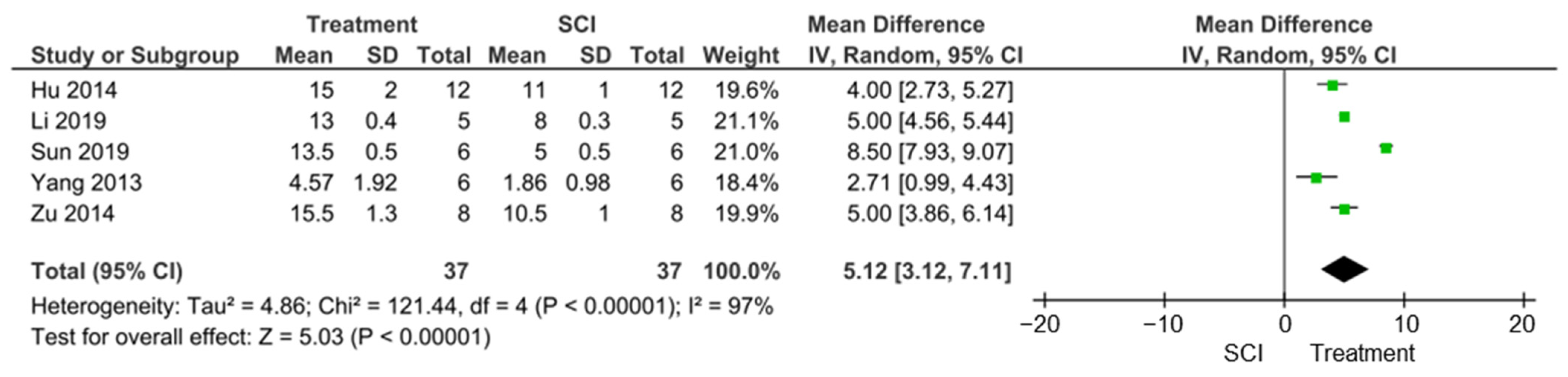
3.4.2. Locomotor Function Improvements
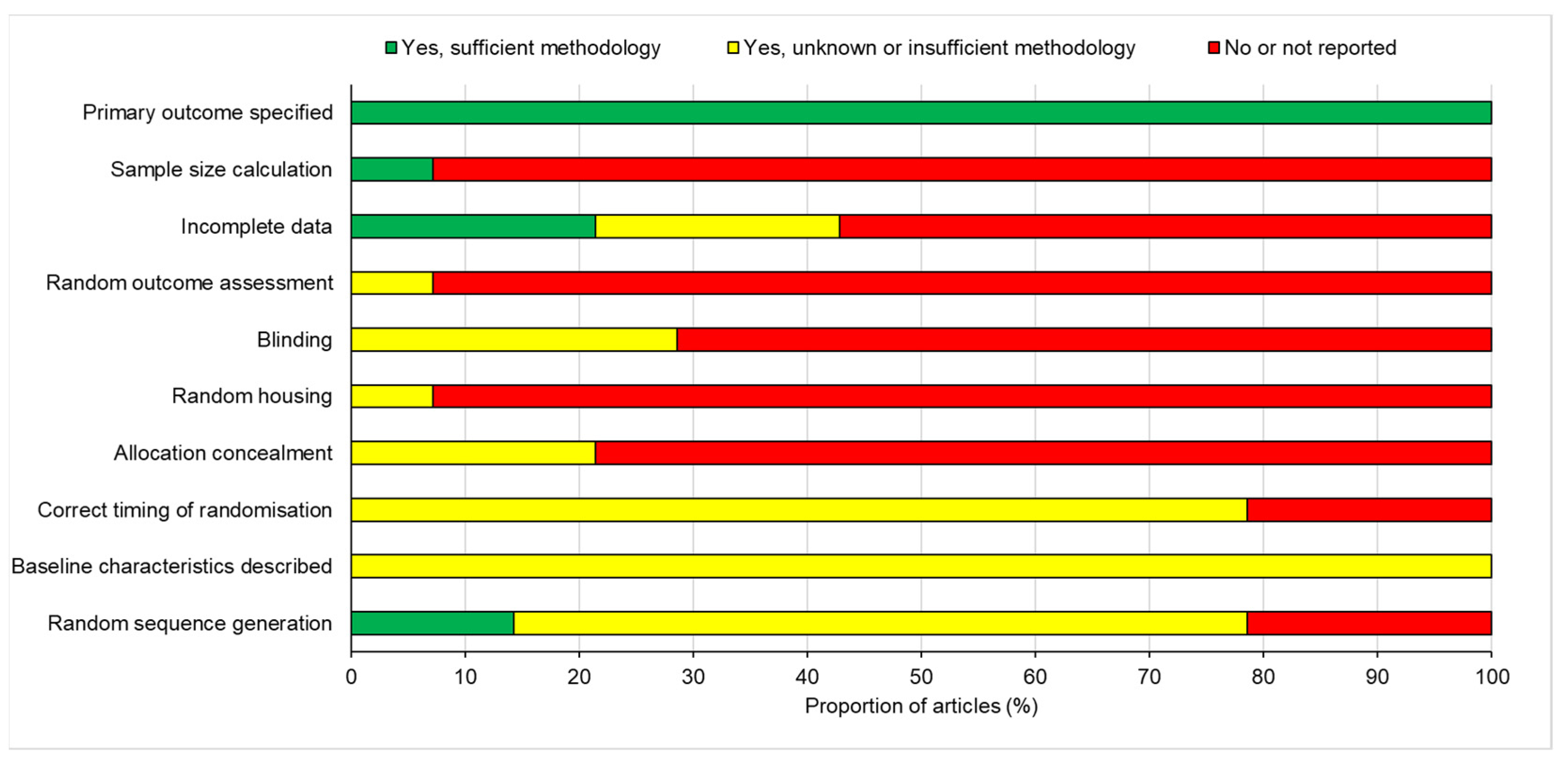
3.5. Risk of Publication Bias
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, Y.; Ding, H.; Zhou, H.; Wei, Z.; Liu, L.; Pan, D.; Feng, S. Epidemiology of worldwide spinal cord injury: A literature review. J. Neurorestoratol. 2017, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Y.; DeVivo, M.J. Changing Demographics and Injury Profile of New Traumatic Spinal Cord Injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 2016, 97, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- McDaid, D.; Park, A.L.; Gall, A.; Purcell, M.; Bacon, M. Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal Cord 2019, 57, 778–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptiste, D.C.; Fehlings, M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma 2006, 23, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T.; Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 2006, 23, 264–280. [Google Scholar] [CrossRef] [Green Version]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020, 43, 425–441. [Google Scholar] [CrossRef]
- Leonard, A.V.; Thornton, E.; Vink, R. The relative contribution of edema and hemorrhage to raised intrathecal pressure after traumatic spinal cord injury. J. Neurotrauma 2015, 32, 397–402. [Google Scholar] [CrossRef]
- Halsey, A.M.; Conner, A.C.; Bill, R.M.; Logan, A.; Ahmed, Z. Aquaporins and Their Regulation after Spinal Cord Injury. Cells 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Faden, A.I.; Chan, P.H.; Longar, S. Alterations in lipid metabolism, Na+,K+-ATPase activity, and tissue water content of spinal cord following experimental traumatic injury. J. Neurochem. 1987, 48, 1809–1816. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, G. Involvement of aquaporin 4 in astrocyte function and neuropsychiatric disorders. CNS Neurosci. 2014, 20, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Oklinski, M.K.; Skowronski, M.T.; Skowronska, A.; Rutzler, M.; Norgaard, K.; Nieland, J.D.; Kwon, T.H.; Nielsen, S. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 2016, 17, 2050. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.; Xu, W.; Guo, W.; Zhang, M.; Bi, J.; Cao, Y.; Fan, Z.; Li, G. TGN-020 alleviates edema and inhibits astrocyte activation and glial scar formation after spinal cord compression injury in rats. Life Sci. 2019, 222, 148–157. [Google Scholar] [CrossRef]
- Cabrera-Aldana, E.E.; Ruelas, F.; Aranda, C.; Rincon-Heredia, R.; Martinez-Cruz, A.; Reyes-Sanchez, A.; Guizar-Sahagun, G.; Tovar, Y.R.L.B. Methylprednisolone Administration Following Spinal Cord Injury Reduces Aquaporin 4 Expression and Exacerbates Edema. Mediat. Inflamm. 2017, 2017, 4792932. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Li, M.; Ma, X.; Zhang, L.; Song, J.; Lv, C.; He, Y. Inhibiting High Mobility Group Box-1 Reduces Early Spinal Cord Edema and Attenuates Astrocyte Activation and Aquaporin-4 Expression after Spinal Cord Injury in Rats. J. Neurotrauma 2019, 36, 421–435. [Google Scholar] [CrossRef]
- Zu, J.; Wang, Y.; Xu, G.; Zhuang, J.; Gong, H.; Yan, J. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014, 116, 1331–1336. [Google Scholar] [CrossRef]
- Hu, A.M.; Li, J.J.; Sun, W.; Yang, D.G.; Yang, M.L.; Du, L.J.; Gu, R.; Gao, F.; Li, J.; Chu, H.Y.; et al. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord 2015, 53, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, G.; Gao, C.; Shao, G.; Kang, N. Effects of hyperbaric oxygen on MMP-2 and MMP-9 expression and spinal cord edema after spinal cord injury. Life Sci. 2013, 93, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yang, J.; Liu, Y.; Zhou, D.; Hou, M.; Xiang, L. Anti-edema effect of melatonin on spinal cord injury in rats. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015, 159, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, R.; Zhu, Y.; Diao, Y.; Tao, L.; Yuan, W.; Xiong, X.C. Anti-edema effect of epigallocatechin gallate on spinal cord injury in rats. Brain Res. 2013, 1527, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cao, Y.; Zhang, Z.; Wang, Y.; Yu, D.; Zhang, M.; Mei, X.; Lu, G. Effect of aminoguanidine on spinal cord edema of acute spinal cord injury in rats. Chin. J. Reparative Reconstr. Surg. 2012, 26, 984–988. (In Chinese) [Google Scholar]
- Yan, X.; Liu, J.; Wang, X.; Li, W.; Chen, J.; Sun, H. Pretreatment with AQP4 and NKCC1 Inhibitors Concurrently Attenuated Spinal Cord Edema and Tissue Damage after Spinal Cord Injury in Rats. Front. Physiol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, C.; Yonan, J.; Batarseh, R.; Chaar, R.; Jonak, C.R.; Ge, S.; Binder, D.; Rodgers, V.G.J. Implantable Osmotic Transport Device Can Reduce Edema after Severe Contusion Spinal Cord Injury. Front. Bioeng. Biotechnol. 2020, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Qiao, S.; Liu, X.; Liu, C.; Zhu, D.; Su, J.; Wang, Z. Melatonin lowers edema after spinal cord injury. Neural Regen. Res. 2014, 9, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, H.; Liu, J.; Zhu, Q.; Gu, R. Effects of aquaporin 4 and inward rectifier potassium channel 4.1 on medullospinal edema after methylprednisolone treatment to suppress acute spinal cord injury in rats. Acta Cir. Bras. 2018, 33, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S.; Kurachi, Y. Mechanisms of astrocytic K(+) clearance and swelling under high extracellular K(+) concentrations. J. Physiol. Sci. 2016, 66, 127–142. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, M.; Hansen, C.N.; Basso, D.M.; Noble-Haeusslein, L.J. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics 2011, 8, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Noble, L.J.; Donovan, F.; Igarashi, T.; Goussev, S.; Werb, Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002, 22, 7526–7535. [Google Scholar] [CrossRef]
- Kimura, A.; Hsu, M.; Seldin, M.; Verkman, A.S.; Scharfman, H.E.; Binder, D.K. Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann. Neurol. 2010, 67, 794–801. [Google Scholar] [CrossRef]
- Zavvarian, M.-M.; Hong, J.; Khazaei, M.; Chio, J.C.T.; Wang, J.; Badner, A.; Fehlings, M.G. The Protein Kinase Inhibitor Midostaurin Improves Functional Neurological Recovery and Attenuates Inflammatory Changes Following Traumatic Cervical Spinal Cord Injury. Biomolecules 2021, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Vandebroek, A.; Yasui, M. Regulation of AQP4 in the Central Nervous System. Int. J. Mol. Sci. 2020, 21, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Ulndreaj, A.; Badner, A.; Fehlings, M.G. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Research 2017, 6, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study ID | Study Location | Rat Strain | Level of SCI | Type of SCI | Therapy | Follow Up Time after SCI | Outcome Measures |
|---|---|---|---|---|---|---|---|
| Kitchen et al. [20] | United Kingdom | Sprague Dawley | T8 | Dorsal column crush | Trifluoperazine (TFP); Calmodulin kinase inhibitor; protein kinase A inhibitor H89 | 72 h, 7 days, 28 days and 6 weeks | Water content (oedema); AQP4 IHC; BSCB breakdown; lesion area; electrophysiology; tape sensing and removal; ladder crossing test. |
| Li et al. [21] | China | Sprague Dawley | T10 | Compression | 2-(nicotinamide)-1,3,4-thiadiazole (TGN-020), | 72 h, 4 weeks | Water content; WB/IF AQP4, GFAP, PCNA, GAP-43 expression; Glial scar formation; neuronal survival; locomotor function. |
| Cabrera-Aldana et al. [22] | Mexico | M/F Long-Evans | T9 | Contusion | Methylprednisolone | 24 h | Water content; AQP4, GFAP expression IHC; BSCB breakdown. |
| Li et al. [33] | China | Sprague Dawley | T6 | Contusion | Methylprednisolone | 8 h, 24 h, 72 h, 7 days | Water content; HE staining; AQP4 expression WB; Motor nerve function. |
| Sun et al. [23] | China | F Sprague Dawley | T10 | Contusion | Ethyl pyruvate (EP); Glycyrrhizin (GL) | 12 h, 24 h, 72 h | Water content; oedema via MRI; AQP4 expression WB/IHC/ELISA; astrocyte expression; TLR4/MyD88 pathway activation; locomotor function. |
| Zu et al. [24] | China | Sprague Dawley | T8 | Contusion | Curcumin | 72 h | Locomotor function; HE; water content; AQP4 expression WB/IHC; astrocyte expression. |
| Hu et al. [25] | China | Sprague Dawley | T10 | Contusion | Myelotomy | 48 h, 4 days, 6 days | Locomotor function; water content; AQP4, AQP9 expression WB. |
| Yang et al. [26] | China | M/F Sprague Dawley | T10 | Contusion | Hyperbaric oxygen (HBO) therapy | 24 h, 48 h, 72 h, 5 days | MMP-2, MMP-9, IL-6 and VEGF expression ELISA/WB; water content; locomotor function. |
| Liu et al. [27] | China | M Sprague Dawley | T12 | Compression | Melatonin | 12 h, 24 h, 48 h, 72 h | Water content; AQP4, GFAP expression WB/IHC. |
| Ge et al. [28] | China | M Sprague Dawley | T12 | Compression | Epigallocatechin gallate (EGCG) | 12 h, 24 h, 48 h, 72 h | Water content; AQP4, GFAP expression IHC/WB. |
| Fan et al. [29] | China | Sprague Dawley | NA | NA | Aminoguanidine At 75, 150, 300 mg/kg | 0 h, 12 h, 24 h, 48 h | Water content; BSCB permeability; AQP4 expression. |
| Yan et al. [30] | China | F Sprague Dawley | T12 | Contusion | TGN-020 (AQP4 inhibitor); Bumetanide (NKCC1 antagonist) | 48 h | AQP4, NKCC1 expression WB/IHC; water content; locomotor activity; LDH activity. |
| Hale et al. [31] | United States | F Sprague Dawley | T8 | Contusion | Implantable osmotic transport device | 1 h, 6 h, 12 h, 24 h, 48 h, 72 h, 5 days, 7 days, 14 day, 28 days | Water content. |
| Li et al. [32] | China | F Sprague Dawley | T12 | Compression | Melatonin | 12 h, 24 h, 48 h and 72 h | Water content; AQP4, GFAP IHC/WB. |
| Study ID | Level of Oedema Reported after Treatment (%) | Level of Oedema Attenuated (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kitchen et al. [20] | 72.8 at 72 h | 56.0 at 72 h | |||||||
| 70.4 at 7 days | 108.0 at 7 days | ||||||||
| Li et al. [21] | 73.07 at 72 h | 42.7 at 72 h | |||||||
| Cabrera-Aldana et al. [22] | 77 at 24 h (G) | −100 at 24 h | |||||||
| Li et al. [32] | 65 (G) | 72.2 at 7 days | |||||||
| Sun et al. [23] | EP | GL | EP | GL | |||||
| 74 at 12 h | 75 at 12 h | 55.5 at 12 h | 33.3 at 12 h | ||||||
| 76 at 24 h | 77 at 24 h | 55.5 at 24 h | 44.4 at 24 h | ||||||
| 74.3 at 72 h (G) | 75.5 at 72 h (G) | 67.14 at 72 h | 50 at 72 h | ||||||
| Zu et al. [24] | 76 at 72 h (G) | 80.0 at 72 h | |||||||
| Hu et al. [25] | 76 at 48 h | −9.09 at 48 h | |||||||
| 72 at 4 days | 11.1 at 4 days | ||||||||
| 67 at 6 days (G) | 25 at 6 days | ||||||||
| Yang et al. [26] | 65.70 at 0 h | 165 at 0 h | |||||||
| 85.67 at 24 h | 8.9 at 24 h | ||||||||
| 82.37 at 48 h | 18.7 at 48 h | ||||||||
| 78.02 at 72 h | 28.8 at 72 h | ||||||||
| 72.97 at 5 days | 5.9 at 5 days | ||||||||
| Liu et al. [27] | 72.5 at 12 h | 16.6 at 12 h | |||||||
| 72 at 24 h | 50.0 at 24 h | ||||||||
| 73 at 48 h | 33.3 at 48 h | ||||||||
| 74 at 72 h (G) | 33.3 at 72 h | ||||||||
| Ge et al. [28] | 73 at 12 h | 16.6 at 12 h | |||||||
| 72 at 24 h | 50.0 at 24 h | ||||||||
| 74 at 48 h | 30.0 at 48 h | ||||||||
| 75 at 72 h (G) | 30.8 at 72 h | ||||||||
| Fan et al. [29] | 0 h | 12 h | 24 h | 48 h | 0 h | 12 h | 24 h | 48 h | |
| 78.57 | 79.82 | 81.01 | 80.79 | 75 mg/kg | 16.2 | 26.8 | 12.9 | 37.3 | |
| 78.32 | 78.77 | 79.81 | 79.92 | 150 mg/kg | 37.6 | 62.4 | 48.5 | 58.4 | |
| 78.11 | 79.92 | 81.18 | 81.57 | 300 mg/kg | 55.5 | 23.3 | 8.5 | 18.4 | |
| Yan et al. [30] | BU 74 | BU 33.3 | |||||||
| TGN 73.5 | TGN 44.4 | ||||||||
| BU and TGN 71 at 48 h (G) | BU and TGN 88.8 at 72 h | ||||||||
| Hale et al. [31] | 72.4 at 3 h | 29.03 at 3 h | |||||||
| Li et al. [32] | 72.5 at 12 h | 16.6 at 12 h | |||||||
| 72 at 24 h | 50.0 at 24 h | ||||||||
| 73 at 48 h | 33.3 at 48 h | ||||||||
| 74 at 7 2 h (G) | 38.5 at 72 h | ||||||||
| Study ID | Effect Size, i.e., Attenuation of Oedema (%) | N (Animals) |
|---|---|---|
| Cabrera-Aldana et al. [22] | −100 ± 99.33 | 4 |
| Sun et al. [23] | 55.5 ± 1.52 (EP); 44.4 ± 2.12 (GL) | 6 |
| Yang et al. [26] | 8.85 ± 1.64 | 6 |
| Liu et al. [27] | 50.0 ± 4.08 | 5 |
| Ge et al. [28] | 50.0 ± 8.22 | 5 |
| Fan et al. [29] | 12.87 ± 2.74 (75 mg/kg); 48.54 ± 0.44 (150 mg/kg); 8.48 ± 2.15 (300 mg/kg) | 5 |
| Li et al. [32] | 50.0 ± 2.05 | 5 |
| Study ID | Effect Size, i.e., Attenuation of Oedema (%) | N (Animals) |
|---|---|---|
| Kitchen et al. [20] | 56 ± 13.11 | 4 |
| Li et al. [21] | 42.72 ± 1.69 | 8 |
| Sun et al. [23] | 67.14 ± 7.77 (EP); 50.0 ± 11.64 (GL) | 6 |
| Zu et al. [24] | 80.0 ± 20.55 | 8 |
| Hu et al. [25] | 11.1 ± 4.04 | 5 |
| Yang et al. [26] | 28.79 ± 2.17 | 6 |
| Liu et al. [27] | 33.3 ± 1.04 | 5 |
| Ge et al. [28] | 30.8 ± 7.96 | 5 |
| Li et al. [32] | 38.46 ± 8.76 | 5 |
| Study ID | Effect Size, i.e., Attenuation of Oedema (%) | N (Animals) |
|---|---|---|
| Kitchen et al. [20] | 108.0 ± 10.50 | 4 |
| Li et al. [33] | 72.2 ± 11.25 | 5 |
| Study ID | Outcomes |
|---|---|
| Kitchen et al. [20] | Decreased AQP4 IHC in astrocyte end-feet; suppressed BSCB breakdown; reduced lesion area; increased CAP, CAP amplitudes and CAP areas; improved tape sensing and removal times; improved ladder crossing performance. |
| Li et al. [21] | Decreased AQP4 expression at 72 h; decreased proliferation of astrocytes at 72 h; decreased glial scar formation at 4 weeks; inhibited loss of neurones at 4 weeks; improved locomotor function (BBB scale) at 4 weeks. |
| Cabrera-Aldana et al. [22] | No improvement in motor outcome following MP; increased impairment of BSCB; increased spinal cord tissue water content following MP; MP decreased AQP4. |
| Li et al. [33] | Increase in motor neurone function; decrease in oedema volume; reduced haemorrhagic area; decreased AQP4 expression. |
| Sun et al. [23] | Increase HMGB1 expression following SCI; EP/ GL inhibits HMGB1 expression; improved locomotor function; reduced oedema using MRI; decreased astrocyte expression; reduced AQP4 expression; reduced TLR4/MyD88 pathway activation. |
| Zu et al. [24] | Improved motor dysfunction; decreased overexpression of AQP4; decrease in astrocyte expression; decrease in activation of JAK/STAT pathway; decreased traumatic manifestations in tissue HE staining. |
| Hu et al. [25] | Improved locomotor function; decreased AQP4 and AQP9 expression 4 days and 6 days. |
| Yang et al. [26] | Decreased MMP-2, MMP-9 and IL-6 expression at 48 h, 72 h and 5 days; increased VEGF; Improved locomotor function. |
| Liu et al. [27] | Decreased AQP4 expression; decreased GFAP expression. |
| Ge et al. [28] | Decreased AQP4 expression at 4 h and 72 h in IHC; decreased GFAP expression 24 h and 72 h. |
| Fan et al. [29] | Decreased BSCB permeability; decreased AQP4 levels at 24 h and 48 h |
| Yan et al. [30] | Decreased tissue destruction; decrease loss of dendrites; decrease in LDH activity; AQP4 and NKCC1 functionally interact with each other. |
| Hale et al. [31] | Only studied spinal cord water content. |
| Li et al. [32] | Decreased AQP4 expression; decreased GFAP expression. |
| Study ID | Functional Test | Level of Injury | Type of Injury | Outcome of Functional Test |
|---|---|---|---|---|
| Kitchen et al. [20] | Tape sensing and removal test and ladder crossing test | T8 | DC crush | Sensory and locomotor function returns to sham-treated levels by 3 weeks after treatment. |
| Li et al. [21] | BBB | T10 | Compression | 8 ± 0.3 (SCI) vs. 13 ± 0.4 (TGN) at 28 days |
| Li et al. [33] | Motor nerve function score (MNFS; Tarlov scores) | T6 | Contusion | Significantly improved MNFS scores at 3 and 7 days after treatment (81 and 95% of control, respectively) |
| Sun et al. [33] | BBB and inclined plane (IP) test | T10 | Contusion | BBB: 5 ± 0.5 (SCI) vs. 13.5 ± 0.5 (EP); 5 (SCI) and 7 (GL) at 14 days IP: 37 ± 0.05 (SCI) vs. 47.5 ± 0.05 (EP): 37 ± 0.05 (SCI) vs. 41 ± 0.5 (GL) at 14 days |
| Zu et al. [24] | BBB | T8 | Contusion | 10.5 ± 1 (SCI) vs. 15.5 ± 1.3 (curcumin) at 14 days |
| Hu et al. [25] | BBB and incline plane (IP) tests | T10 | Contusion | BBB: 11 ± 1 (SCI) vs. 15 ± 2 (myelotomy); IP: 39 ± 1 (SCI) vs. 48 ± 1.7 (myelotomy) |
| Yang et al. [26] | BBB | T10 | Contusion | 1.86 ± 0.98 (SCI) vs. 4.57 ± 1.92 (HBO) at 5 days |
| Study ID | BBB Scores | n | Percentage Reduction in AQP4 Protein | ||
|---|---|---|---|---|---|
| SCI | SCI + Treatment | Effect Size | |||
| Li et al. [22] | 8 ± 0.3 | 13 ± 0.4 | 5 | 5 | 67 ± 6 |
| Sun et al. [33] | 5 ± 0.5 | 13.5 ± 0.5 | 8.5 | 6 | N/R |
| Zu et al. [24] | 10.5 ± 1 | 15.5 ± 1.3 | 5 | 8 | 53 ± 10 |
| Hu et al. [25] | 11 ± 1 | 15 ± 2 | 4 | 12 | 73 ± 5 |
| Yang et al. [26] | 1.86 ± 0.98 * | 4.57 ± 1.92 * | 2.71 | 6 | N/R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masterman, E.; Ahmed, Z. Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cells 2021, 10, 2682. https://doi.org/10.3390/cells10102682
Masterman E, Ahmed Z. Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cells. 2021; 10(10):2682. https://doi.org/10.3390/cells10102682
Chicago/Turabian StyleMasterman, Emma, and Zubair Ahmed. 2021. "Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis" Cells 10, no. 10: 2682. https://doi.org/10.3390/cells10102682
APA StyleMasterman, E., & Ahmed, Z. (2021). Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cells, 10(10), 2682. https://doi.org/10.3390/cells10102682







