USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma
Abstract
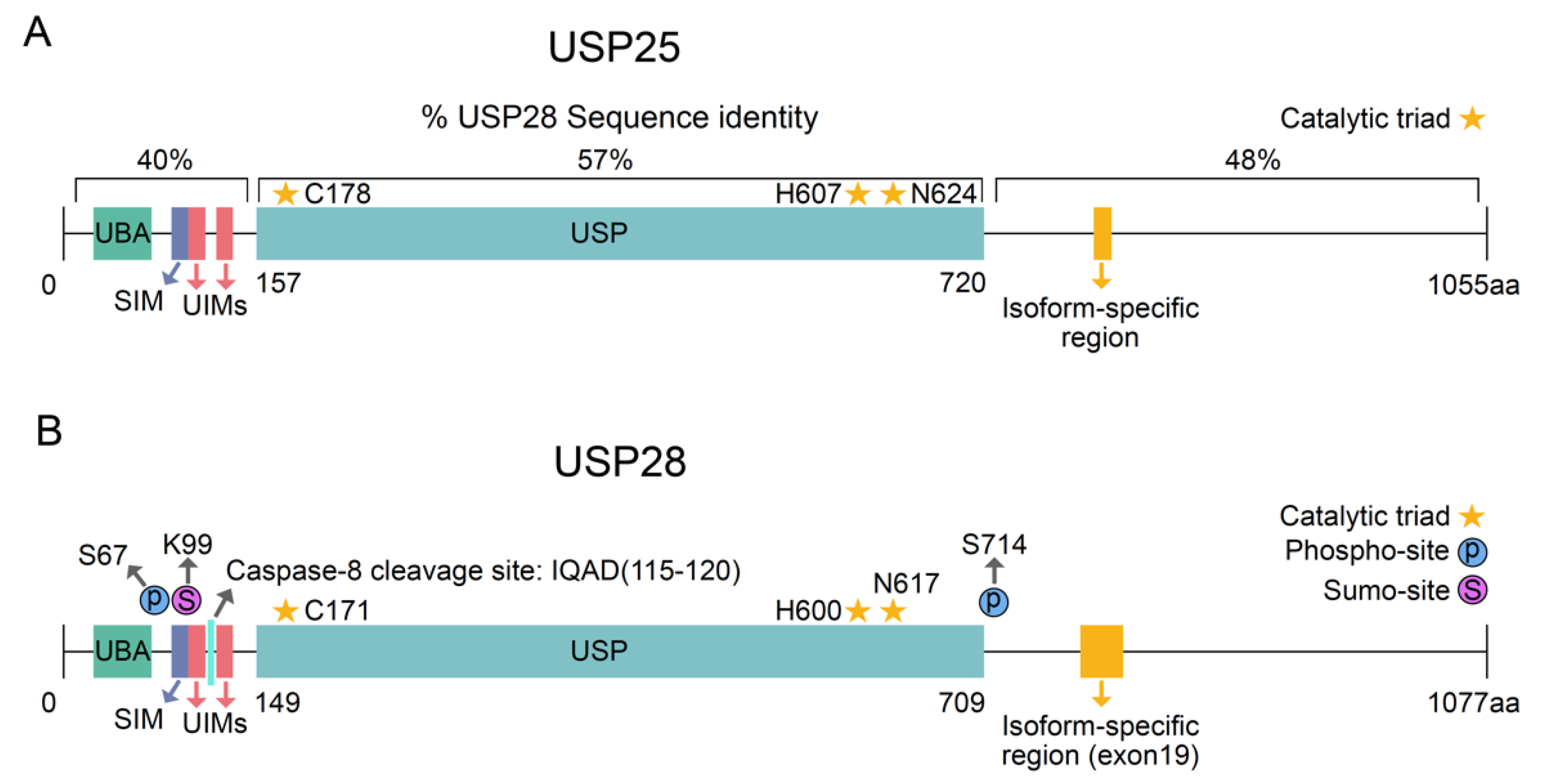
:1. Deubiquitinase USP28
2. Squamous Cell Carcinoma
- ○
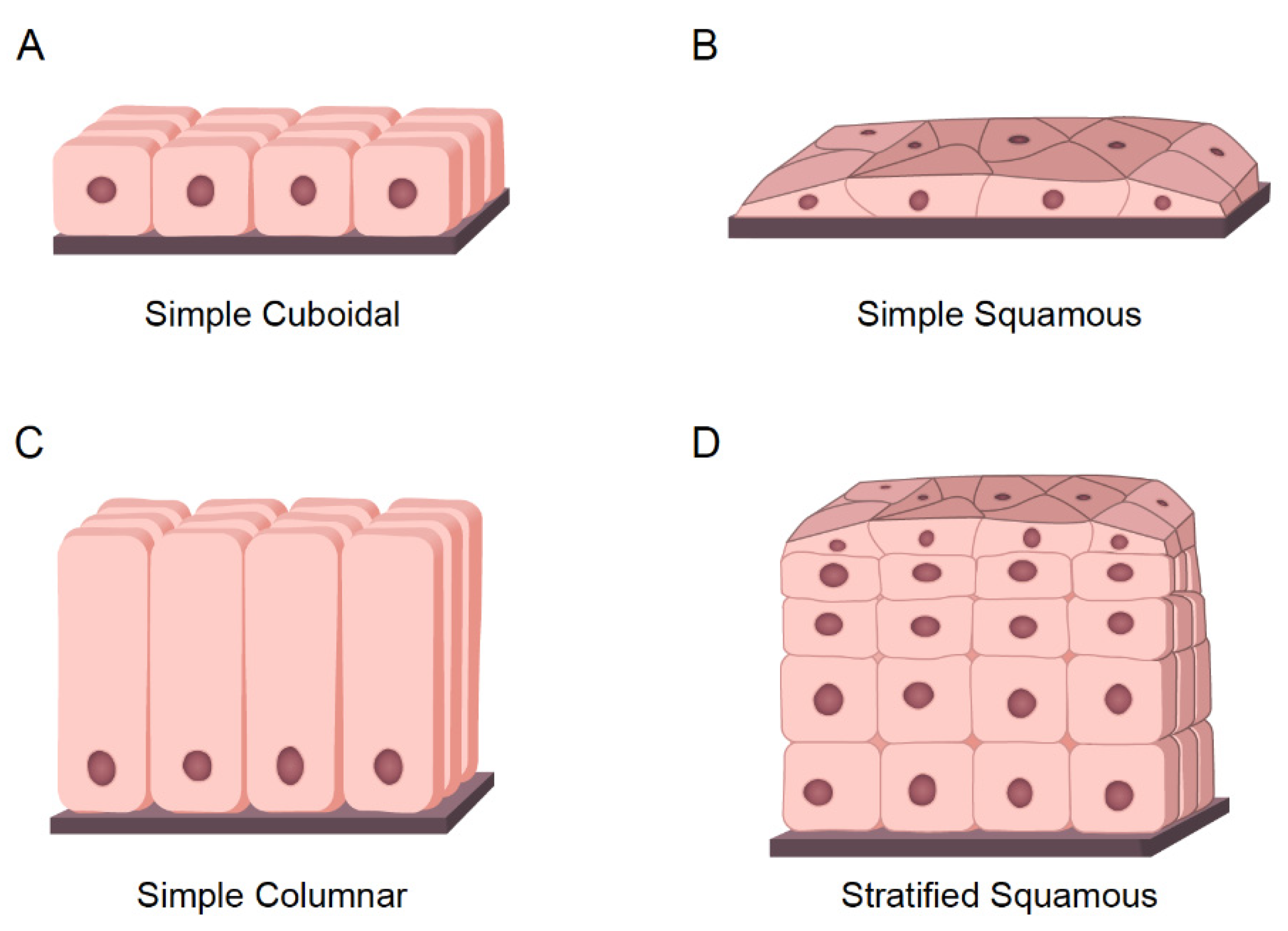
- Cuboidal cells: These are cube-shaped cells with large, spherical central nuclei (Figure 3A). Cuboidal cells provide protection and mechanical support. Notably, they can differentiate and form secretory glands. Kidney or salivary glands are recovered by cuboidal cells upon tissue damage.
- ○
- Squamous cells: The squamous epithelium is a selective permeable layer formed by a delicate line of thin and flat cells (Figure 3B). The esophagus or oral cavity is covered by squamous epithelial cells.
- ○
- Columnar cells: These cells are taller than squamous or cuboidal cells and present an oval nucleus (Figure 3C). Columnar cells facilitate movement across the epithelial barrier, and some tissues with the columnar epithelium have cells with cilia. Intestine or uterus is covered by columnar cells.
- ○
- Squamous cell carcinoma (SCC): This is also called epidermoid carcinoma and it is composed of squamous cells. As mentioned earlier, squamous cells are flat cells that can be present in many different organs of the body. Accordingly, squamous tumors can be developed in several different tissues such as in the lung, skin, esophagus, prostate, pancreas, cervix, vulva, thyroid, head and neck, penis, and bladder. The precise “cell of origin” for SCC has not yet been identified, and various cellular pools have been suggested for the same [53,57].
- ○
- Adenocarcinoma (ADC): ADC tumors arise from epithelial cells with secretory properties or glandular cells. ADC can be formed in different organs such as the lungs, breasts, esophagus, colon, stomach, and prostate [58]. Carcinomas can be considered adenosquamous (ADSCC) when the tumor is presenting the expression of markers and histopathological features of SCC and ADC. Each type of cell must constitute at least 10% of the tumor bulk to be diagnosed as ADSCC [59].
- ○
- Transitional cell carcinomas: These carcinomas arise from the transitional epithelium, which is a type of stratified epithelium tissue composed of multiple layers of epithelial cells with different morphologies. Transitional carcinomas can be contracted or expanded to adapt themselves to the degree of distension needed by the tissue. Hence, this tumor type usually occurs in organs that form part of the urinary system, such as the bladder [60].
- ○
- Basal cell carcinomas: These tumors originate in the cells of the basal layer, located at the lower part of the epidermis. Basal cell carcinoma is the most common type of skin cancer [61].
2.1. Mutational Landscape of SCC Tumors
2.2. Tp63 in Squamous Cell Carcinoma
2.3. Targeting Tp63 in Squamous Cell Carcinoma
3. USP28: Oncogene or Tumor Suppressor? The Importance of Genetics
3.1. USP28 and p53 Genetic Status
3.2. Are USP28 Substrates Recruited via Another E3-Ligase in FBXW7-Deficient Cells?
4. Inhibitors of USP28 for SCC Cancer Therapy: Progress and Perspective
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Hanpude, P.; Bhattacharya, S.; Dey, A.K.; Maiti, T.K. Deubiquitinating Enzymes in Cellular Signaling and Disease Regulation. IUBMB Life 2015, 67, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the Chains: Structure and Function of the Deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Knobel, P.A.; Stracker, T.H.; Reverter, D. Regulation of USP28 Deubiquitinating Activity by SUMO Conjugation. J. Biol. Chem. 2014, 289, 34838–34850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, R.; Bayés, M.; Francisca Sánchez-Font, M.; González-Angulo, O.; Gonzàlez-Duarte, R.; Marfany, G. Characterization of Alternatively Spliced Products and Tissue-Specific Isoforms of USP28 and USP25. Genome Biol. 2001, 2. [Google Scholar] [CrossRef]
- Liu, B.; Sureda-Gómez, M.; Zhen, Y.; Amador, V.; Reverter, D. A Quaternary Tetramer Assembly Inhibits the Deubiquitinating Activity of USP25. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vlasschaert, C.; Cook, D.; Xia, X.; Gray, D.A. The Evolution and Functional Diversification of the Deubiquitinating Enzyme Superfamily. Genome Biol. Evol. 2017, 9, 558–573. [Google Scholar] [CrossRef] [Green Version]
- Sauer, F.; Klemm, T.; Kollampally, R.B.; Tessmer, I.; Nair, R.K.; Popov, N.; Kisker, C. Differential Oligomerization of the Deubiquitinases USP25 and USP28 Regulates Their Activities. Mol. Cell 2019, 74, 421–435. [Google Scholar] [CrossRef]
- Gersch, M.; Wagstaff, J.L.; Toms, A.v.; Graves, B.; Freund, S.M.V.; Komander, D. Distinct USP25 and USP28 Oligomerization States Regulate Deubiquitinating Activity. Mol. Cell 2019, 74, 436–451. [Google Scholar] [CrossRef]
- Zhang, D.; Zaugg, K.; Mak, T.W.; Elledge, S.J. A Role for the Deubiquitinating Enzyme USP28 in Control of the DNA-Damage Response. Cell 2006, 126, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Lamoliatte, F.; McManus, F.P.; Maarifi, G.; Chelbi-Alix, M.K.; Thibault, P. Uncovering the SUMOylation and Ubiquitylation Crosstalk in Human Cells Using Sequential Peptide Immunopurification. Nat. Commun. 2017, 8, 14109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.; Müller, S. SUMO-Specific Proteases/Isopeptidases: SENPs and Beyond. Genome Biol. 2014, 15, 422. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, K.C.; Marfany, G.; Wu, Y.; Massoumi, R. Putative Role of SUMOylation in Controlling the Activity of Deubiquitinating Enzymes in Cancer. Future Oncol. 2016, 12, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Du, S.C.; Zhu, L.; Wang, Y.X.; Liu, J.; Zhang, D.; Chen, Y.L.; Peng, Q.; Liu, W.; Liu, B. SENP1-Mediated DeSUMOylation of USP28 Regulated HIF-1α Accumulation and Activation during Hypoxia Response 06 Biological Sciences 0601 Biochemistry and Cell Biology. Cancer Cell Int. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Wu, J.; Sokirniy, I.; Nguyen, P.; Bregnard, T.; Weinstock, J.; Mattern, M.; Bezsonova, I.; Hancock, W.W.; et al. Active Site-Targeted Covalent Irreversible Inhibitors of USP7 Impair the Functions of Foxp3+ T-Regulatory Cells by Promoting Ubiquitination of Tip60. PLoS ONE 2017, 12, e0189744. [Google Scholar] [CrossRef] [Green Version]
- Mistry, H.; Hsieh, G.; Buhrlage, S.J.; Huang, M.; Park, E.; Cuny, G.D.; Galinsky, I.; Stone, R.M.; Gray, N.S.; D’Andrea, A.D.; et al. Small-Molecule Inhibitors of Usp1 Target Id1 Degradation in Leukemic Cells. Mol. Cancer Ther. 2013, 12, 2651–2662. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Garcia, C.; Hartmann, O.; Reissland, M.; Braun, F.; Fischer, T.; Walz, S.; Schülein-Völk, C.; Eilers, U.; Ade, C.P.; Calzado, M.A.; et al. Maintaining Protein Stability of ∆Np63 via USP 28 Is Required by Squamous Cancer Cells. EMBO Mol. Med. 2020, 12, e11101. [Google Scholar] [CrossRef]
- Müller, I.; Strozyk, E.; Schindler, S.; Beissert, S.; Oo, H.Z.; Sauter, T.; Lucarelli, P.; Raeth, S.; Hausser, A.; al Nakouzi, N.; et al. Cancer Cells Employ Nuclear Caspase-8 to Overcome the P53-Dependent G2/M Checkpoint through Cleavage of USP28. Mol. Cell 2020, 77, 970–984. [Google Scholar] [CrossRef]
- Cao, C.; Vasilatos, S.N.; Bhargava, R.; Fine, J.L.; Oesterreich, S.; Davidson, N.E.; Huang, Y. Functional Interaction of Histone Deacetylase 5 (HDAC5) and Lysine-Specific Demethylase 1 (LSD1) Promotes Breast Cancer Progression. Oncogene 2017, 36, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.W.; Fang, M.; Park, S.M.; Hutchinson, L.; Green, M.R. A KRAS-Directed Transcriptional Silencing Pathway That Mediates the CpG Island Methylator Phenotype. eLife 2014, 2014, e02313. [Google Scholar] [CrossRef]
- Diefenbacher, M.E.; Popov, N.; Blake, S.M.; Schülein-Völk, C.; Nye, E.; Spencer-Dene, B.; Jaenicke, L.A.; Eilers, M.; Behrens, A. The Deubiquitinase USP28 Controls Intestinal Homeostasis and Promotes Colorectal Cancer. J. Clin. Investig. 2014, 124, 3407–3418. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Ma, C.; Chen, S.; Dang, J.; Cheng, X.; Zhu, D. Reverse the down Regulation of MiR-92b-3p by Hypoxia Can Suppress the Proliferation of Pulmonary Artery Smooth Muscle Cells by Targeting USP28. Biochem. Biophys. Res. Commun. 2018, 503, 3064–3077. [Google Scholar] [CrossRef]
- Zhang, J.F. MicroRNA-216b Suppresses the Cell Growth of Hepatocellular Carcinoma by Inhibiting Ubiquitin-Specific Peptidase 28 Expression. Kaohsiung J. Med. Sci. 2020, 36, 423–428. [Google Scholar] [CrossRef]
- Chen, B.; Sang, Y.; Song, X.; Zhang, D.; Wang, L.; Zhao, W.; Liang, Y.; Zhang, N.; Yang, Q. Exosomal MiR-500a-5p Derived from Cancer-Associated Fibroblasts Promotes Breast Cancer Cell Proliferation and Metastasis through Targeting USP28. Theranostics 2021, 11, 3932–3947. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Li, Y.; Lu, H.; Li, Z.; Han, X. MiR-3940-5p Functions as a Tumor Suppressor in Non-Small Cell Lung Cancer Cells by Targeting Cyclin D1 and Ubiquitin Specific Peptidase-28. Transl. Oncol. 2017, 10, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Popov, N.; Wanzel, M.; Madiredjo, M.; Zhang, D.; Beijersbergen, R.; Bernards, R.; Moll, R.; Elledge, S.J.; Eilers, M. The Ubiquitin-Specific Protease USP28 Is Required for MYC Stability. Nat. Cell Biol. 2007, 9, 765–774. [Google Scholar] [CrossRef]
- Cremona, C.A.; Sancho, R.; Diefenbacher, M.E.; Behrens, A. Fbw7 and Its Counteracting Forces in Stem Cells and Cancer: Oncoproteins in the Balance. Semin. Cancer Biol. 2016, 36, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Rossi, M.; D’Alessandra, Y.; de Simone, M.; Lopardo, T.; Haupt, Y.; Alsheich-Bartok, O.; Anzi, S.; Shaulian, E.; Calabrò, V.; et al. MDM2 and Fbw7 Cooperate to Induce P63 Protein Degradation Following DNA Damage and Cell Differentiation. J. Cell Sci. 2010, 123, 2423–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spruck, C.H.; Strohmaier, H.; Sangfelt, O.; Müller, H.M.; Hubalek, M.; Müller-Holzner, E.; Marth, C.; Widschwendter, M.; Reed, S.I. HCDC4 Gene Mutations in Endometrial Cancer. Cancer Res. 2002, 62, 62. [Google Scholar]
- Sailo, B.L.; Banik, K.; Girisa, S.; Bordoloi, D.; Fan, L.; Halim, C.E.; Wang, H.; Kumar, A.P.; Zheng, D.; Mao, X.; et al. FBXW7 in Cancer: What Has Been Unraveled Thus Far? Cancers 2019, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.H.; Bellon, M.; Nicot, C. FBXW7: A Critical Tumor Suppressor of Human Cancers. Mol. Cancer 2018, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Diefenbacher, M.E.; Chakraborty, A.; Blake, S.M.; Mitter, R.; Popov, N.; Eilers, M.; Behrens, A. Usp28 Counteracts Fbw7 in Intestinal Homeostasis and Cancer. Cancer Res. 2015, 75, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Schülein-Völk, C.; Wolf, E.; Zhu, J.; Xu, W.; Taranets, L.; Hellmann, A.; Jänicke, L.A.; Diefenbacher, M.E.; Behrens, A.; Eilers, M.; et al. Dual Regulation of Fbw7 Function and Oncogenic Transformation by Usp28. Cell Rep. 2014, 9, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, Z.; Zhang, L.; Yang, Z.; Chen, X.; Luo, J.; Zhou, Z.; Mei, X.; Yu, X.; Shao, Z.; et al. Targeting Deubiquitinase USP28 for Cancer Therapy. Cell Death Dis. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohgaki, M.; Hakem, A.; Halaby, M.J.; Bohgaki, T.; Li, Q.; Bissey, P.A.; Shloush, J.; Kislinger, T.; Sanchez, O.; Sheng, Y.; et al. The E3 Ligase PIRH2 Polyubiquitylates CHK2 and Regulates Its Turnover. Cell Death Differ. 2013, 20, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Knobel, P.A.; Belotserkovskaya, R.; Galanty, Y.; Schmidt, C.K.; Jackson, S.P.; Stracker, T.H. USP28 Is Recruited to Sites of DNA Damage by the Tandem BRCT Domains of 53BP1 but Plays a Minor Role in Double-Strand Break Metabolism. Mol. Cell. Biol. 2014, 34, 2062–2074. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Yoshimoto, C.; Yamada, Y.; Sudo, T.; Kobayashi, H. The HNF-1β-USP28-Claspin Pathway Upregulates DNA Damage-Induced Chk1 Activation in Ovarian Clear Cell Carcinoma. Oncotarget 2018, 9, 17512–17522. [Google Scholar] [CrossRef] [Green Version]
- Weili, Z.; Zhikun, L.; Jianmin, W.; Qingbao, T. Knockdown of USP28 Enhances the Radiosensitivity of Esophageal Cancer Cells via the C-Myc/Hypoxia-Inducible Factor-1 Alpha Pathway. J. Cell. Biochem. 2019, 120, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Popov, N.; Herold, S.; Llamazares, M.; Schülein, C.; Eilers, M. Fbw7 and Usp28 Regulate Myc Protein Stability in Response to DNA Damage. Cell Cycle 2007, 6, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Yang, X.H.; Kang, T.; Zhao, Y.; Wang, C.; Evers, B.M.; Zhou, B.P. The Deubiquitinase USP28 Stabilizes LSD1 and Confers Stem-Cell-like Traits to Breast Cancer Cells. Cell Rep. 2013, 5, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Flügel, D.; Görlach, A.; Kietzmann, T. GSK-3β Regulates Cell Growth, Migration, and Angiogenesis via Fbw7 and USP28-Dependent Degradation of HIF-1α. Blood 2012, 119, 1292–1301. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Han, H.; Sun, Q.; Liu, K.; Lin, N.; Xu, C.; Zhao, Z.; Zhao, W. USP28 Regulates Deubiquitination of Histone H2A and Cell Proliferation. Exp. Cell Res. 2019, 379, 11–18. [Google Scholar] [CrossRef]
- Meitinger, F.; Anzola, J.v.; Kaulich, M.; Richardson, A.; Stender, J.D.; Benner, C.; Glass, C.K.; Dowdy, S.F.; Desai, A.; Shiau, A.K.; et al. 53BP1 and USP28 Mediate P53 Activation and G1 Arrest after Centrosome Loss or Extended Mitotic Duration. J. Cell Biol. 2016, 214, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.S.; Mazo, G.; Das, T.; Goodman, J.; Kim, M.; O’Rourke, B.P.; Izquierdo, D.; Tsou, M.F.B. 53BP1 and USP28 Mediate P53- Dependent Cell Cycle Arrest in Response to Centrosome Loss and Prolonged Mitosis. eLife 2016, 5, e16270. [Google Scholar] [CrossRef] [PubMed]
- Lambrus, B.G.; Daggubati, V.; Uetake, Y.; Scott, P.M.; Clutario, K.M.; Sluder, G.; Holland, A.J. A USP28-53BP1-P53-P21 Signaling Axis Arrests Growth after Centrosome Loss or Prolonged Mitosis. J. Cell Biol. 2016, 214, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Feng, X.; Song, H.; Zhang, Z.; Shen, A.; Qiu, X. Deubiquitinase USP28 Inhibits Ubiquitin Ligase KLHL2-Mediated Uridine-Cytidine Kinase 1 Degradation and Confers Sensitivity to 5’-Azacytidine-Resistant Human Leukemia Cells. Theranostics 2020, 10, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, Z.; Wang, J.; Chen, W.; Huang, J. Ubiquitin-Specific Peptidase 28 Enhances STAT3 Signaling and Promotes Cell Growth in Non-Small-Cell Lung Cancer. OncoTargets Ther. 2019, 12, 1603–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, S.; Das, S.; Kim, D.H.; Chandrasekaran, A.P.; Hong, S.H.; Kim, K.S.; Ramakrishna, S. The Stability and Oncogenic Function of LIN28A Are Regulated by USP28. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2019, 1865, 599–610. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Xu, X.; Zhang, L.; Pan, Y.; Chen, D. USP28 Promotes Aerobic Glycolysis of Colorectal Cancer by Increasing Stability of FOXC1. Acta Biochim. Pol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, Z.; Hua, X.; Huang, M.; Xu, J.; Li, J.; Huang, H.; Cohen, M.; Huang, C. Isorhapontigenin (ISO) Inhibits Stem Cell-like Properties and Invasion of Bladder Cancer Cell by Attenuating CD44 Expression. Cell. Mol. Life Sci. 2020, 77, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, G.; Hua, X.; Li, Y.; Yan, H.; Che, X.; Tian, Z.; Liufu, H.; Huang, C.; Li, J.; et al. CD44s Is a Crucial ATG7 Downstream Regulator for Stem-like Property, Invasion, and Lung Metastasis of Human Bladder Cancer (BC) Cells. Oncogene 2019, 38, 3301–3315. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the Cells of Origin of Squamous Cell Carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef]
- Gibb, E.A.; Enfield, K.S.S.; Tsui, I.F.L.; Chari, R.; Lam, S.; Alvarez, C.E.; Lam, W.L. Deciphering Squamous Cell Carcinoma Using Multidimensional Genomic Approaches. J. Ski. Cancer 2011, 2011, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Berman, J.J. Tumor Taxonomy for the Developmental Lineage Classification of Neoplasms. BMC Cancer 2004, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, F.; Ma, W.; Hiscock, T.W.; Mosaliganti, K.R.; Tentner, A.R.; Brakke, K.A.; Rannou, N.; Gelas, A.; Souhait, L.; Swinburne, I.A.; et al. Interplay of Cell Shape and Division Orientation Promotes Robust Morphogenesis of Developing Epithelia. Cell 2014, 159, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamate, W.; Baad, R.; Vibhute, N.; Belgaumi, U.; Kadashetti, V.; Gugwad, S. Trending Speculations of Tumor-Initiating Cells in Squamous Cell Cancers of Head and Neck. Int. J. Stem Cells 2017, 10, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.W.; Chen, L.; Huang, T.; Zhang, N.; Kong, X.Y.; Cai, Y.D. Classifying Ten Types of Major Cancers Based on Reverse Phase Protein Array Profiles. PLoS ONE 2015, 10, e0123147. [Google Scholar] [CrossRef]
- Li, C.; Lu, H. Adenosquamous Carcinoma of the Lung. OncoTargets Ther. 2018, 11, 4829–4835. [Google Scholar] [CrossRef] [Green Version]
- Al-Husseini, M.J.; Kunbaz, A.; Saad, A.M.; Santos, J.V.; Salahia, S.; Iqbal, M.; Alahdab, F. Trends in the Incidence and Mortality of Transitional Cell Carcinoma of the Bladder for the Last Four Decades in the USA: A SEER-Based Analysis. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.M.; Furniss, M.; Mackay-Wiggan, J.M. Basal Cell Carcinoma: An Evidence-Based Treatment Update. Am. J. Clin. Dermatol. 2014, 15, 197–216. [Google Scholar] [CrossRef]
- Conde, E.; Angulo, B.; Redondo, P.; Toldos, O.; García-García, E.; Suárez-Gauthier, A.; Rubio-Viqueira, B.; Marrón, C.; García-Luján, R.; Sánchez-Céspedes, M.; et al. The Use of P63 Immunohistochemistry for the Identification of Squamous Cell Carcinoma of the Lung. PLoS ONE 2010, 5, e12209. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Shin, H.C.; Shin, K.C.; Ro, J.Y. Best Immunohistochemical Panel in Distinguishing Adenocarcinoma from Squamous Cell Carcinoma of Lung: Tissue Microarray Assay in Resected Lung Cancer Specimens. Ann. Diagn. Pathol. 2013, 17, 85–90. [Google Scholar] [CrossRef]
- Khayyata, S.; Yun, S.; Pasha, T.; Jian, B.; McGrath, C.; Yu, G.; Gupta, P.; Baloch, Z. Value of P63 and CK5/6 in Distinguishing Squamous Cell Carcinoma from Adenocarcinoma in Lung Fine-Needle Aspiration Specimens. Diagn. Cytopathol. 2009, 37, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Ishii, G.; Nagai, K.; Atsumi, N.; Fujii, S.; Yamada, A.; Yamane, Y.; Hishida, T.; Nishimura, M.; Yoshida, J.; et al. Expression of Podoplanin, CD44, and P63 in Squamous Cell Carcinoma of the Lung. Cancer Sci. 2009, 100, 2054–2059. [Google Scholar] [CrossRef]
- Hartmann, O.; Reissland, M.; Maier, C.R.; Fischer, T.; Prieto-Garcia, C.; Baluapuri, A.; Schwarz, J.; Schmitz, W.; Garrido-Rodriguez, M.; Pahor, N.; et al. Implementation of CRISPR/Cas9 Genome Editing to Generate Murine Lung Cancer Models That Depict the Mutational Landscape of Human Disease. Front. Cell Dev. Biol. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2008; Volume 83. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Voet, D.; Lawrence, M.S.; Voet, D.; Jing, R.; Cibulskis, K.; Sivachenko, A.; Stojanov, P.; McKenna, A.; Lander, E.S.; et al. Comprehensive Genomic Characterization of Squamous Cell Lung Cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Sos, M.L.; Thomas, R.K. Genetic Insight and Therapeutic Targets in Squamous-Cell Lung Cancer. Oncogene 2012, 31, 4811–4814. [Google Scholar] [CrossRef] [Green Version]
- Smardova, J.; Liskova, K.; Ravcukova, B.; Malcikova, J.; Hausnerova, J.; Svitakova, M.; Hrabalkova, R.; Zlamalikova, L.; Stano-Kozubik, K.; Blahakova, I.; et al. Complex Analysis of the P53 Tumor Suppressor in Lung Carcinoma. Oncol. Rep. 2016, 35, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Amlani, L.; Bellile, E.; Spector, M.; Smith, J.; Brenner, C.; Rozek, L.; Nguyen, A.; Zarins, K.; Thomas, D.; McHugh, J.; et al. Expression of P53 and Prognosis in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC). Int. J. Cancer Clin. Res. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, E.J.; Diefenbacher, M.E.; Nelson, J.K.; Sancho, R.; Pucci, F.; Chakraborty, A.; Moreno, P.; Annibaldi, A.; Liccardi, G.; Encheva, V.; et al. LUBAC Determines Chemotherapy Resistance in Squamous Cell Lung Cancer. J. Exp. Med. 2019, 216, 450–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokobori, T.; Mimori, K.; Iwatsuki, M.; Ishii, H.; Tanaka, F.; Sato, T.; Toh, H.; Sudo, T.; Iwaya, T.; Tanaka, Y.; et al. Copy Number Loss of FBXW7 Is Related to Gene Expression and Poor Prognosis in Esophageal Squamous Cell Carcinoma. Int. J. Oncol. 2012, 41, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, H.; Nagata, M.; Yoshida, R.; Matsuoka, Y.; Hirosue, A.; Kawahara, K.; Sakata, J.; Nakashima, H.; Kojima, T.; Toya, R.; et al. FBXW7 Expression Affects the Response to Chemoradiotherapy and Overall Survival among Patients with Oral Squamous Cell Carcinoma: A Single-Center Retrospective Study. Tumor Biol. 2017, 39, 39. [Google Scholar] [CrossRef] [Green Version]
- Bass, A.J.; Watanabe, H.; Mermel, C.H.; Yu, S.; Perner, S.; Verhaak, R.G.; Kim, S.Y.; Wardwell, L.; Tamayo, P.; Gat-Viks, I.; et al. SOX2 Is an Amplified Lineage-Survival Oncogene in Lung and Esophageal Squamous Cell Carcinomas. Nat. Genet. 2009, 41, 1238–1242. [Google Scholar] [CrossRef]
- Weiss, J.; Sos, M.L.; Seidel, D.; Peifer, M.; Zander, T.; Heuckmann, J.M.; Ullrich, R.T.; Menon, R.; Maier, S.; Soltermann, A.; et al. Frequent and Focal FGFR1 Amplification Associates with Therapeutically Tractable FGFR1 Dependency in Squamous Cell Lung Cancer. Sci. Transl. Med. 2010, 2, 62ra93. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J. Reduced Lung Cancer Mortality with Low-Dose Computed Tomographic Screening. Community Oncol. 2011, 8, 441–442. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.U.; Koo, S.H.; Kwon, K.C.; Park, J.W.; Kim, J.M. Identification of Novel Candidate Target Genes, Including EPHB3, MASP1 and SST at 3q26.2-Q29 in Squamous Cell Carcinoma of the Lung. BMC Cancer 2009, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Dötsch, V.; Bernassola, F.; Coutandin, D.; Candi, E.; Melino, G. P63 and P73, the Ancestors of P53. Cold Spring Harb. Perspect. Biol. 2010, 2, a004887. [Google Scholar] [CrossRef]
- Vanbokhoven, H.; Melino, G.; Candi, E.; Declercq, W. P63, a Story of Mice and Men. J. Investig. Dermatol. 2011, 131, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; George, A.L.; Sakakibara, N.; Mahmood, K.; Ponnamperuma, R.M.; King, K.E.; Weinberg, W.C. Molecular Mechanisms of P63-Mediated Squamous Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 3590. [Google Scholar] [CrossRef] [Green Version]
- Melino, G.; Lu, X.; Gasco, M.; Crook, T.; Knight, R.A. Functional Regulation of P73 and P63: Development and Cancer. Trends Biochem. Sci. 2003, 28, 663–670. [Google Scholar] [CrossRef]
- Armstrong, S.R.; Wu, H.; Wang, B.; Abuetabh, Y.; Sergi, C.; Leng, R.P. The Regulation of Tumor Suppressor P63 by the Ubiquitin-Proteasome System. Int. J. Mol. Sci. 2016, 17, 2041. [Google Scholar] [CrossRef] [Green Version]
- Rocco, J.W.; Ellisen, L.W. P63 and P73: Life and Death in Squamous Cell Carcinoma. Cell Cycle 2006, 5, 936–940. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Torkelson, J.L.; Shankar, G.; Pattison, J.M.; Zhen, H.H.; Fang, F.; Duren, Z.; Xin, J.; Gaddam, S.; et al. TFAP2C- and P63-Dependent Networks Sequentially Rearrange Chromatin Landscapes to Drive Human Epidermal Lineage Commitment. Cell Stem Cell 2019, 24, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Li, X.; Zhang, Y.; Guo, Y.; Zhou, J.; Gao, K.; Dai, J.; Hu, G.; Lv, L.; Du, J. The MicroRNA Feedback Regulation of P63 in Cancer Progression. Oncotarget 2015, 6, 8434–8453. [Google Scholar] [CrossRef] [PubMed]
- Gatti, V.; Bongiorno-Borbone, L.; Fierro, C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Peschiaroli, A. P63 at the Crossroads between Stemness and Metastasis in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyes, W.M.; Pecoraro, M.; Aranda, V.; Vernersson-Lindahl, E.; Li, W.; Vogel, H.; Guo, X.; Garcia, E.L.; Michurina, T.v.; Enikolopov, G.; et al. Δnp63α Is an Oncogene That Targets Chromatin Remodeler Lsh to Drive Skin Stem Cell Proliferation and Tumorigenesis. Cell Stem Cell 2011, 8, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, M.R.; Wilson, C.; Ory, B.; Rothenberg, S.M.; Faquin, W.; Mills, A.A.; Ellisen, L.W. FGFR2 Signaling Underlies P63 Oncogenic Function in Squamous Cell Carcinoma. J. Clin. Investig. 2013, 123, 3525–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerville, T.D.D.; Xu, Y.; Miyabayashi, K.; Tiriac, H.; Cleary, C.R.; Maia-Silva, D.; Milazzo, J.P.; Tuveson, D.A.; Vakoc, C.R. TP63-Mediated Enhancer Reprogramming Drives the Squamous Subtype of Pancreatic Ductal Adenocarcinoma. Cell Rep. 2018, 25, 1741–1755. [Google Scholar] [CrossRef] [Green Version]
- Rocco, J.W.; Leong, C.O.; Kuperwasser, N.; DeYoung, M.P.; Ellisen, L.W. P63 Mediates Survival in Squamous Cell Carcinoma by Suppression of P73-Dependent Apoptosis. Cancer Cell 2006, 9, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallant-Behm, C.L.; Ramsey, M.R.; Bensard, C.L.; Nojek, I.; Tran, J.; Liu, M.; Ellisen, L.W.; Espinosa, J.M. ΔNp63α Represses Anti-Proliferative Genes via H2A.Z Deposition. Genes Dev. 2012, 26, 2325–2336. [Google Scholar] [CrossRef] [Green Version]
- Gallant-Behm, C.L.; Espinosa, J.M. Δnp63α Utilizes Multiple Mechanisms to Repress Transcription in Squamous Cell Carcinoma Cells. Cell Cycle 2013, 12, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallant-Behm, C.L.; Espinosa, J.M. How Does Δnp63α Drive Cancer? Epigenomics 2013, 5, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Bretz, A.C.; Gittler, M.P.; Charles, J.P.; Gremke, N.; Eckhardt, I.; Mernberger, M.; Mandic, R.; Thomale, J.; Nist, A.; Wanzel, M.; et al. Δnp63 Activates the Fanconi Anemia DNA Repair Pathway and Limits the Efficacy of Cisplatin Treatment in Squamous Cell Carcinoma. Nucleic Acids Res. 2016, 44, 3204–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.L.; Sengupta, S.; Gurdziel, K.; Bell, G.W.; Jacks, T.; Flores, E.R. P63 and P73 Transcriptionally Regulate Genes Involved in DNA Repair. PLoS Genet. 2009, 5, e1000680. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting Transcription Factors in Cancer—From Undruggable to Reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.v.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the “undruggable” Cancer Targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.H. Targeting Transcription Factors for Cancer Treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shaik, S.; Dai, X.; Wu, Q.; Zhou, X.; Wang, Z.; Wei, W. Targeting the Ubiquitin Pathway for Cancer Treatment. Biochim. Et Biophys. Acta-Rev. Cancer 2015, 1855, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating Enzymes and Drug Discovery: Emerging Opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, Q.; Xue, J.; Zhao, Y.; Qin, S. Ubiquitin-Specific Protease 28 Is Overexpressed in Human Glioblastomas and Contributes to Glioma Tumorigenicity by Regulating MYC Expression. Exp. Biol. Med. 2016, 241, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Lambrus, B.G.; Holland, A.J. A New Mode of Mitotic Surveillance. Trends Cell Biol. 2017, 27, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuella-Martin, R.; Oliveira, C.; Lockstone, H.E.; Snellenberg, S.; Grolmusova, N.; Chapman, J.R. 53BP1 Integrates DNA Repair and P53-Dependent Cell Fate Decisions via Distinct Mechanisms. Mol. Cell 2016, 64, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Como, C.J.; Gaiddon, C.; Prives, C. P73 Function Is Inhibited by Tumor-Derived P53 Mutants in Mammalian Cells. Mol. Cell. Biol. 1999, 19, 1438–1449. [Google Scholar] [CrossRef] [Green Version]
- Parrales, A.; Iwakuma, T. Targeting Oncogenic Mutant P53 for Cancer Therapy. Front. Oncol. 2015, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.W. DNA Damage-Induced Activation of P53 by the Checkpoint Kinase Chk2. Science 2000, 287, 1824–1827. [Google Scholar] [CrossRef]
- Taranets, L.; Zhu, J.; Xu, W.; Popov, N. Fbw7 and Usp28–Enemies and Allies. Mol. Cell. Oncol. 2015, 2, e995041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, R.P.; Lin, Y.; Ma, W.; Wu, H.; Lemmers, B.; Chung, S.; Parant, J.M.; Lozano, G.; Hakem, R.; Benchimol, S. Pirh2, a P53-Induced Ubiquitin-Protein Ligase, Promotes P53 Degradation. Cell 2003, 112, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Qian, Y.; Yan, W.; Chen, X. Pirh2 E3 Ubiquitin Ligase Modulates Keratinocyte Differentiation through P63. J. Investig. Dermatol. 2013, 133, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Hakem, A.; Bohgaki, M.; Lemmers, B.; Tai, E.; Salmena, L.; Matysiak-Zablocki, E.; Jung, Y.S.; Karaskova, J.; Kaustov, L.; Duan, S.; et al. Role of Pirh2 in Mediating the Regulation of P53 and C-Myc. PLoS Genet. 2011, 7, e1002360. [Google Scholar] [CrossRef]
- Halaby, M.J.; Hakem, R.; Hakem, A. Pirh2: An E3 Ligase with Central Roles in the Regulation of Cell Cycle, DNA Damage Response, and Differentiation. Cell Cycle 2013, 12, 2733–2737. [Google Scholar] [CrossRef] [Green Version]
- Gregoire-Mitha, S.; Gray, D.A. What Deubiquitinating Enzymes, Oncogenes, and Tumor Suppressors Actually Do: Are Current Assumptions Supported by Patient Outcomes? BioEssays 2021, 43, e2000269. [Google Scholar] [CrossRef]
- Henderson, M.J.; Munoz, M.A.; Saunders, D.N.; Clancy, J.L.; Russell, A.J.; Williams, B.; Pappin, D.; Kum, K.K.; Jackson, S.P.; Sutherland, R.L.; et al. EDD Mediates DNA Damage-Induced Activation of CHK2. J. Biol. Chem. 2006, 281, 39990–40000. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Lin, W.C. EDD Inhibits ATM-Mediated Phosphorylation of P53. J. Biol. Chem. 2011, 286, 14972–14982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schukur, L.; Zimmermann, T.; Niewoehner, O.; Kerr, G.; Gleim, S.; Bauer-Probst, B.; Knapp, B.; Galli, G.G.; Liang, X.; Mendiola, A.; et al. Identification of the HECT E3 Ligase UBR5 as a Regulator of MYC Degradation Using a CRISPR/Cas9 Screen. Sci. Rep. 2020, 10, 20044. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, Y.; Prada, M.L.; Mohan, A.K.; Gupta, A.; Jaiswal, A.; Sharma, M.; Merisaari, J.; Haikala, H.M.; Talvinen, K.; et al. UBR5 Is Coamplified with MYC in Breast Tumors and Encodes an Ubiquitin Ligase That Limits MYC-Dependent Apoptosis. Cancer Res. 2020, 80, 1414–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, R.F.; Iconomou, M.; Watts, C.K.W.; Saunders, D.N. Functional Roles of the E3 Ubiquitin Ligase UBR5 in Cancer. Mol. Cancer Res. 2015, 13, 1523–1532. [Google Scholar] [CrossRef] [Green Version]
- Vita, M.; Henriksson, M. The Myc Oncoprotein as a Therapeutic Target for Human Cancer. Semin. Cancer Biol. 2006, 16, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Eilers, M. Transcriptional Regulation and Transformation by Myc Proteins. Nat. Rev. Mol. Cell Biol. 2005, 6, 635–645. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, T.; Li, Z.; Sun, K.; Fu, Y.; Cheng, T.; Guo, J.; Yu, B.; Shi, X.; Liu, H. Discovery of [1,2,3]Triazolo[4,5-d]Pyrimidine Derivatives as Highly Potent, Selective, and Cellularly Active USP28 Inhibitors. Acta Pharm. Sin. B 2020, 10, 1476–1491. [Google Scholar] [CrossRef]
- Wrigley, J.D.; Gavory, G.; Simpson, I.; Preston, M.; Plant, H.; Bradley, J.; Goeppert, A.U.; Rozycka, E.; Davies, G.; Walsh, J.; et al. Identification and Characterization of Dual Inhibitors of the USP25/28 Deubiquitinating Enzyme Subfamily. ACS Chem. Biol. 2017, 12, 3113–3125. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Meng, Q.; Ding, Y.; Xiong, M.; Zhu, M.; Yang, Y.; Su, H.; Gu, L.; Xu, Y.; Shi, L.; et al. USP28 and USP25 Are Downregulated by Vismodegib in Vitro and in Colorectal Cancer Cell Lines. FEBS J. 2021, 288, 1325–1342. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, D.; Ghosh, A.; Saha, S. Computational Approach to Target USP28 for Regulating Myc. Comput. Biol. Chem. 2020, 85, 107208. [Google Scholar] [CrossRef] [PubMed]
- Bushman, J.W.; Donovan, K.A.; Schauer, N.J.; Liu, X.; Hu, W.; Varca, A.C.; Buhrlage, S.J.; Fischer, E.S. Proteomics-Based Identification of DUB Substrates Using Selective Inhibitors. Cell Chem. Biol. 2021, 28, 78–87. [Google Scholar] [CrossRef]
- Wang, X.M.; Yang, C.; Zhao, Y.; Xu, Z.G.; Yang, W.; Wang, P.; Lin, D.; Xiong, B.; Fang, J.Y.; Dong, C.; et al. The Deubiquitinase USP25 Supports Colonic Inflammation and Bacterial Infection and Promotes Colorectal Cancer. Nat. Cancer 2020, 1, 811–825. [Google Scholar] [CrossRef]
- Ruiz, E.J.; Pinto-Fernandez, A.; Turnbull, A.P.; Lan, L.; Charlton, T.M.; Scott, H.C.; Damianou, A.; Vere, G.; Riising, E.M.; da Costa, C.; et al. USP28 Deletion and Small Molecule Inhibition Destabilises C-Myc and Elicits Regression of Squamous Cell Lung Carcinoma. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, D.; Wang, D.; Yang, L.; Zhao, C.; Huang, X. Emerging Roles of Ubiquitin-Specific Protease 25 in Diseases. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Rudolph, J.; Settleman, J.; Malek, S. Emerging Trends in Cancer Drug Discovery—From Drugging the “Undruggable” to Overcoming Resistance. Cancer Discov. 2021, 11, 815–821. [Google Scholar] [CrossRef]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. Protacs: Great Opportunities for Academia and Industry. Signal Transduct. Target. Ther. 2019, 4, 1–33. [Google Scholar] [CrossRef] [Green Version]





| Regulator: | Positive or Negative Regulation: | Effect on USP28: | REF.: |
|---|---|---|---|
| ATM | Positive | Phosphorylates USP28 increasing its activity | [10] |
| CASPASE 8 | Negative | Cleaves and inactivates USP28 | [18] |
| SENP1 | Positive | Desumoylates USP28 increasing its activity | [14] |
| HDAC5 | Positive | Increases USP28 stability blocking its polyubiquitination | [19] |
| miR-92b-3p | Negative | Reduces USP28 translation | [22] |
| miR-216b | Negative | Reduces USP28 translation | [23] |
| mi-R3940-5p | Negative | Reduces USP28 translation | [25] |
| miR-500a-5p | Negative | Reduces USP28 translation | [24] |
| Circ-FBXW7 | Negative | Reduces USP28 substrate recognition decreasing its activity | [26] |
| c-JUN | Positive | Increases USP28 transcription | [20] |
| c-MYC | Positive | Increases USP28 transcription | [21] |
| Target: | USP28 Effect: | USP28 Cancer Function: | REF.: |
|---|---|---|---|
| c-MYC | Increases its stability | Oncoprotein | [21,27,33,34,39,40] |
| FBXW7 | Increases its stability | Tumor suppressor | [34] |
| c-JUN | Increases its stability | Oncoprotein | [21,33,34] |
| NOTCH1 | Increases its stability | Oncoprotein | [21,33,34] |
| CLASPIN | Increases its stability | Oncoprotein or tumor suppressor dependent on cellular context | [38] |
| CHK2 | Increases its stability | Tumor suppressor | [10,36] |
| 53BP1 | Increases its stability | Tumor suppressor | [10] |
| MDC1 | Increases its stability | Tumor suppressor | [10] |
| LSD1 | Increases its stability | Oncoprotein | [19,41] |
| HIF-1a | Increases its stability | Oncoprotein or tumor suppressor dependent on cellular context | [14,42] |
| CCNE | Increases its stability | Oncoprotein | [21,33,34] |
| H2A | Enhances transcriptional activation | Tumor suppressor | [43] |
| ∆Np63 | Increases its stability | Oncoprotein | [17] |
| p53 | Increases its stability | Tumor suppressor | [18,44,45,46] |
| UCK1 | Increases its stability | Tumor suppressor | [47] |
| STAT3 | Increases its stability | Oncoprotein | [48] |
| ZNF304 | Increases its stability | Oncoprotein | [20] |
| LIN28A | Increases its stability | Oncoprotein | [49] |
| FOXC1 | Increases its stability | Oncoprotein | [50] |
| MTOR | Increases its stability | Oncoprotein | [34] |
| MCL1 | Increases its stability | Oncoprotein | [34] |
| CD44 | Increases its stability | Oncoprotein | [51,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Garcia, C.; Tomašković, I.; Shah, V.J.; Dikic, I.; Diefenbacher, M. USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma. Cells 2021, 10, 2652. https://doi.org/10.3390/cells10102652
Prieto-Garcia C, Tomašković I, Shah VJ, Dikic I, Diefenbacher M. USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma. Cells. 2021; 10(10):2652. https://doi.org/10.3390/cells10102652
Chicago/Turabian StylePrieto-Garcia, Cristian, Ines Tomašković, Varun Jayeshkumar Shah, Ivan Dikic, and Markus Diefenbacher. 2021. "USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma" Cells 10, no. 10: 2652. https://doi.org/10.3390/cells10102652
APA StylePrieto-Garcia, C., Tomašković, I., Shah, V. J., Dikic, I., & Diefenbacher, M. (2021). USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma. Cells, 10(10), 2652. https://doi.org/10.3390/cells10102652






