New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies
Abstract
:1. Introduction
1.1. Celiac Disease
1.2. Wheat Allergy (WA)
1.3. Non-Celiac Gluten Sensitivity (NCGS)
1.4. Nickel Allergic Contact Mucositis (Ni ACM)
2. Materials and Methods
2.1. Study Participants
2.2. Group A—Untreated Celiac Disease CD (uCD)
2.3. Group B—Treated Celiac Disease CD (tCD)
2.4. Group C—Wheat Allergy (WA)
2.5. Group D—Non-Celiac Gluten Sensitivity (NCGS)
2.6. Group E—Nickel Allergic Contact Mucositis (Ni ACM)
2.7. Group F—Control Patients (CTRL)
2.8. Esophagogastroduodenoscopy with Biopsy Sampling
2.9. Light Microscopy (LM)
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results
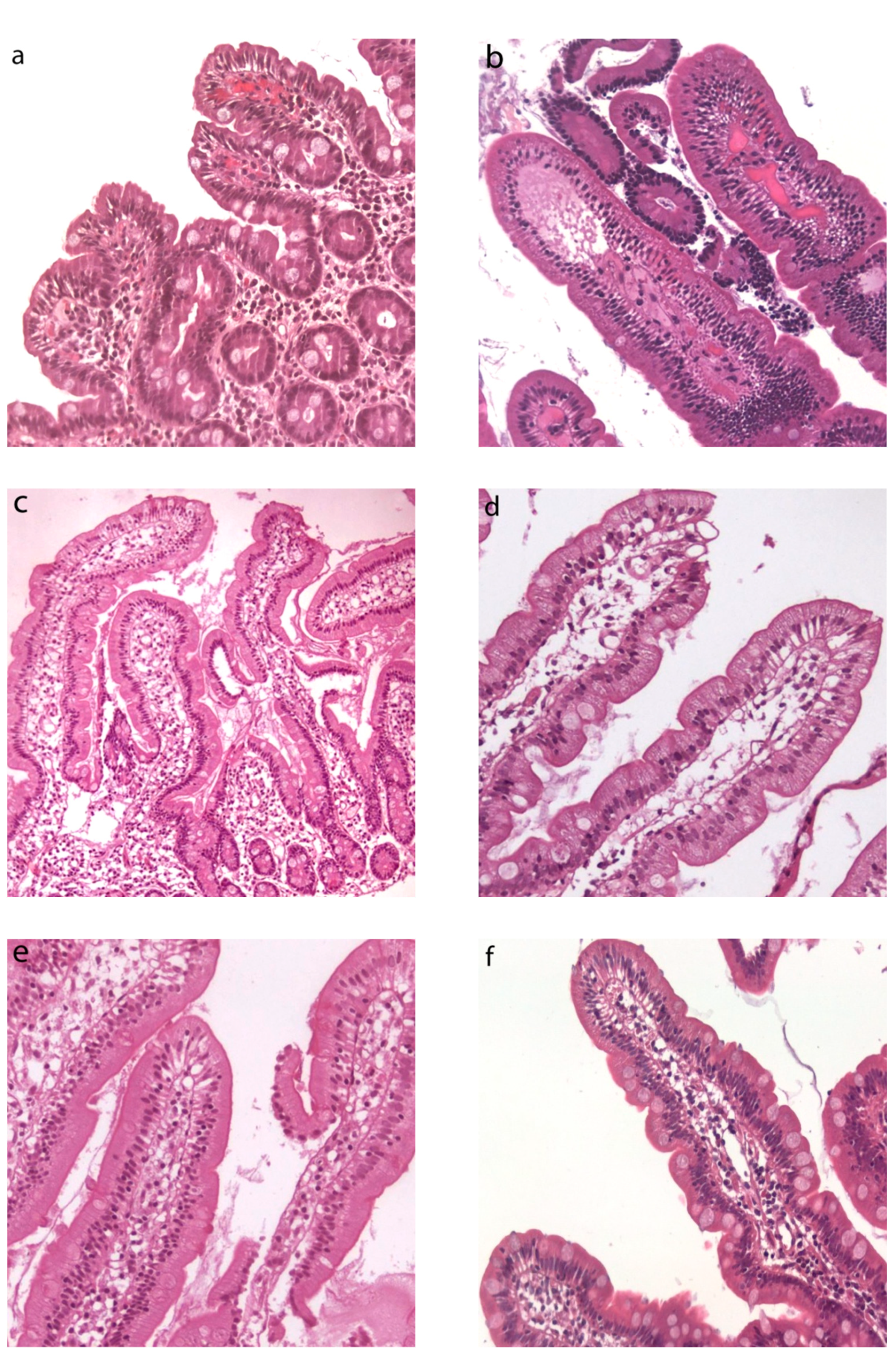
3.1. Light Microscopy Observations
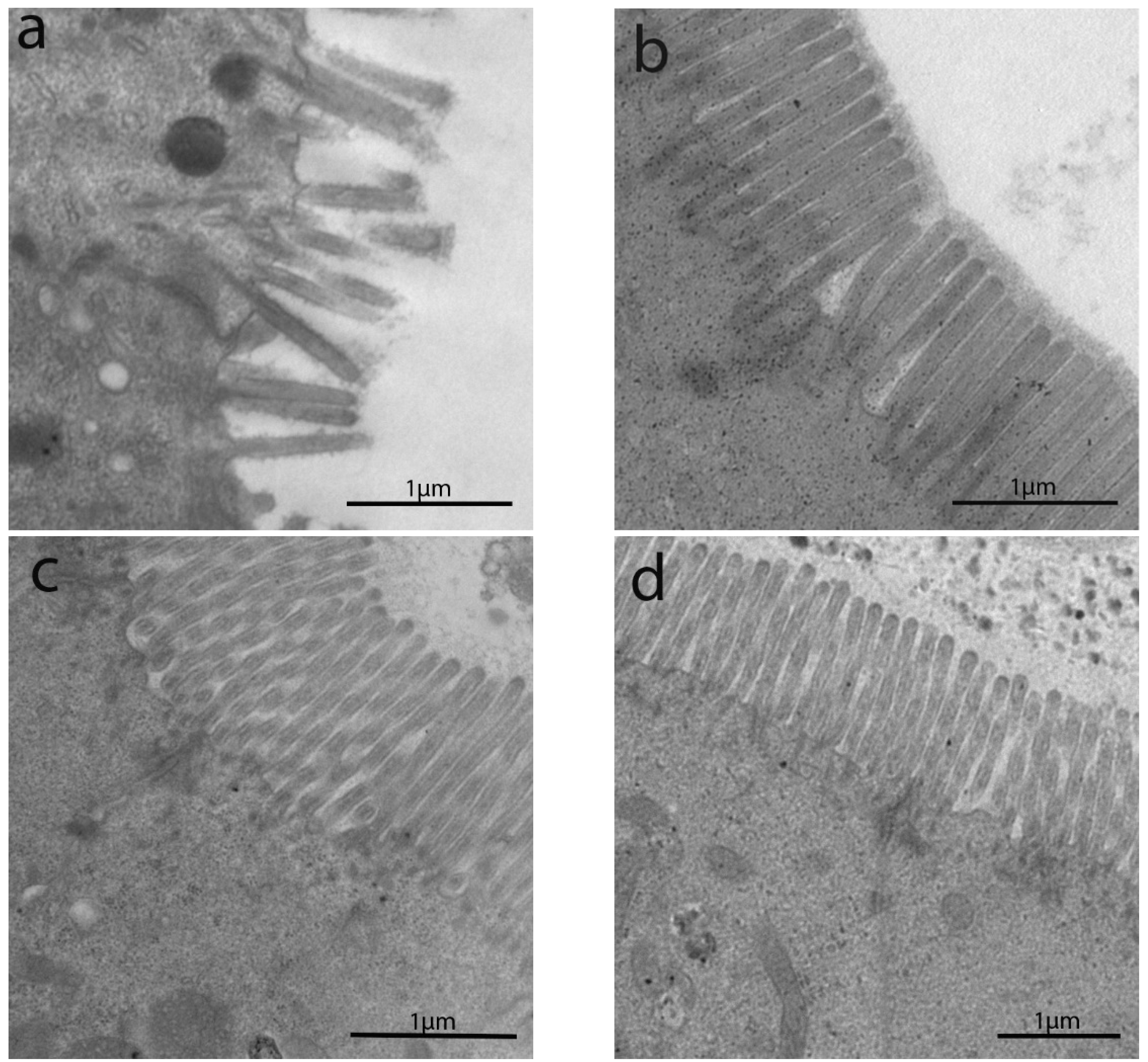
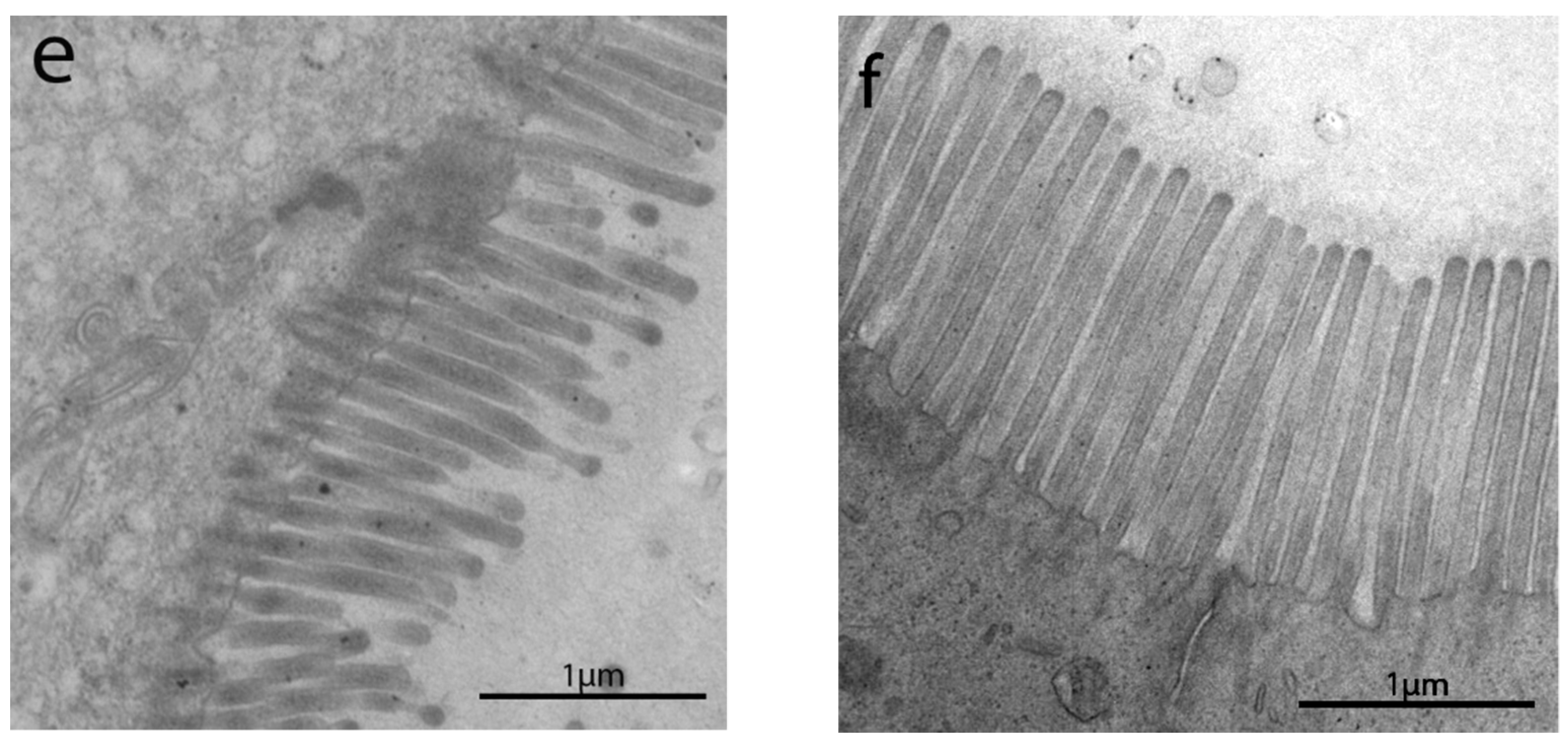
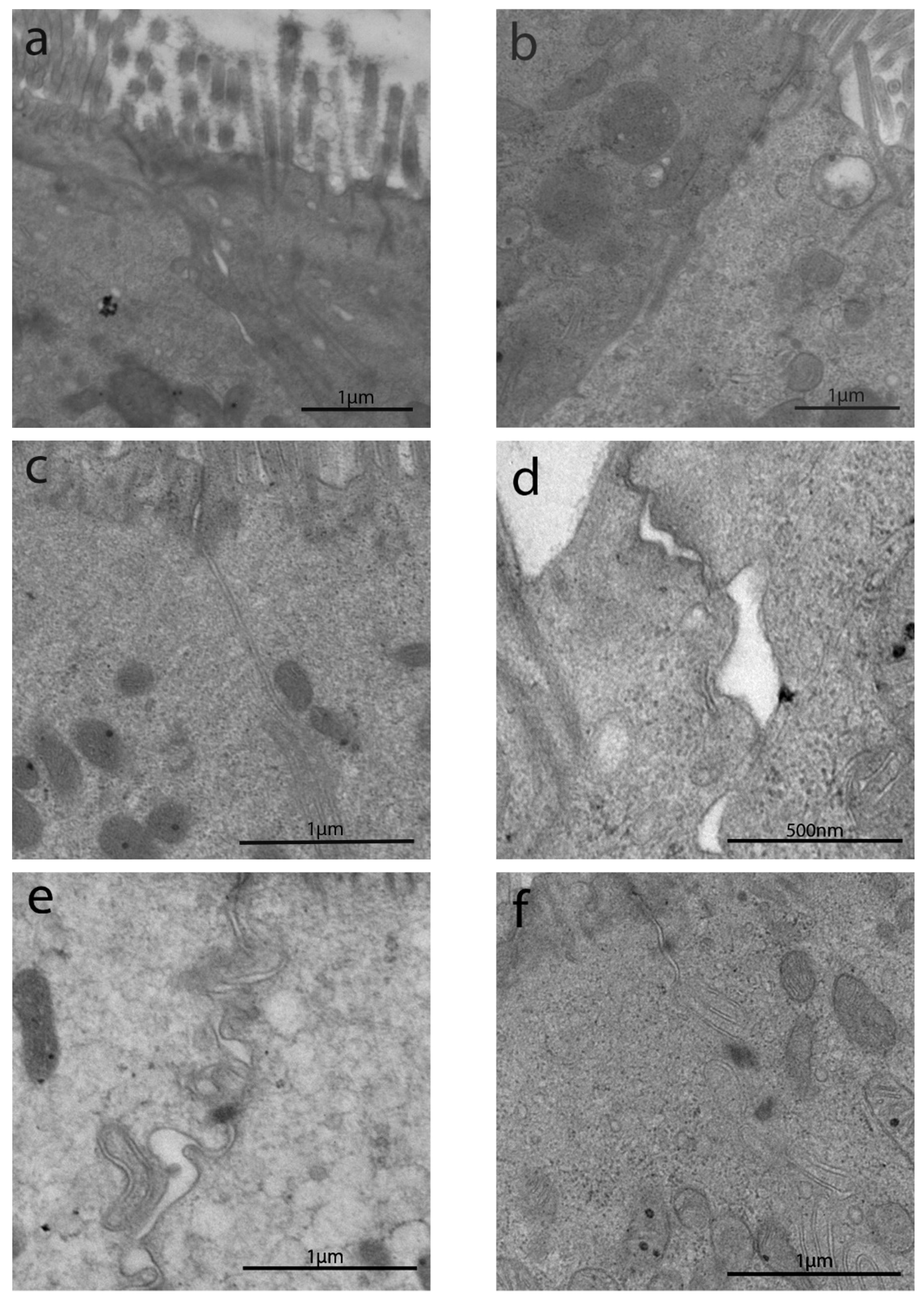
3.2. Transmission Electron Microscopyobservations
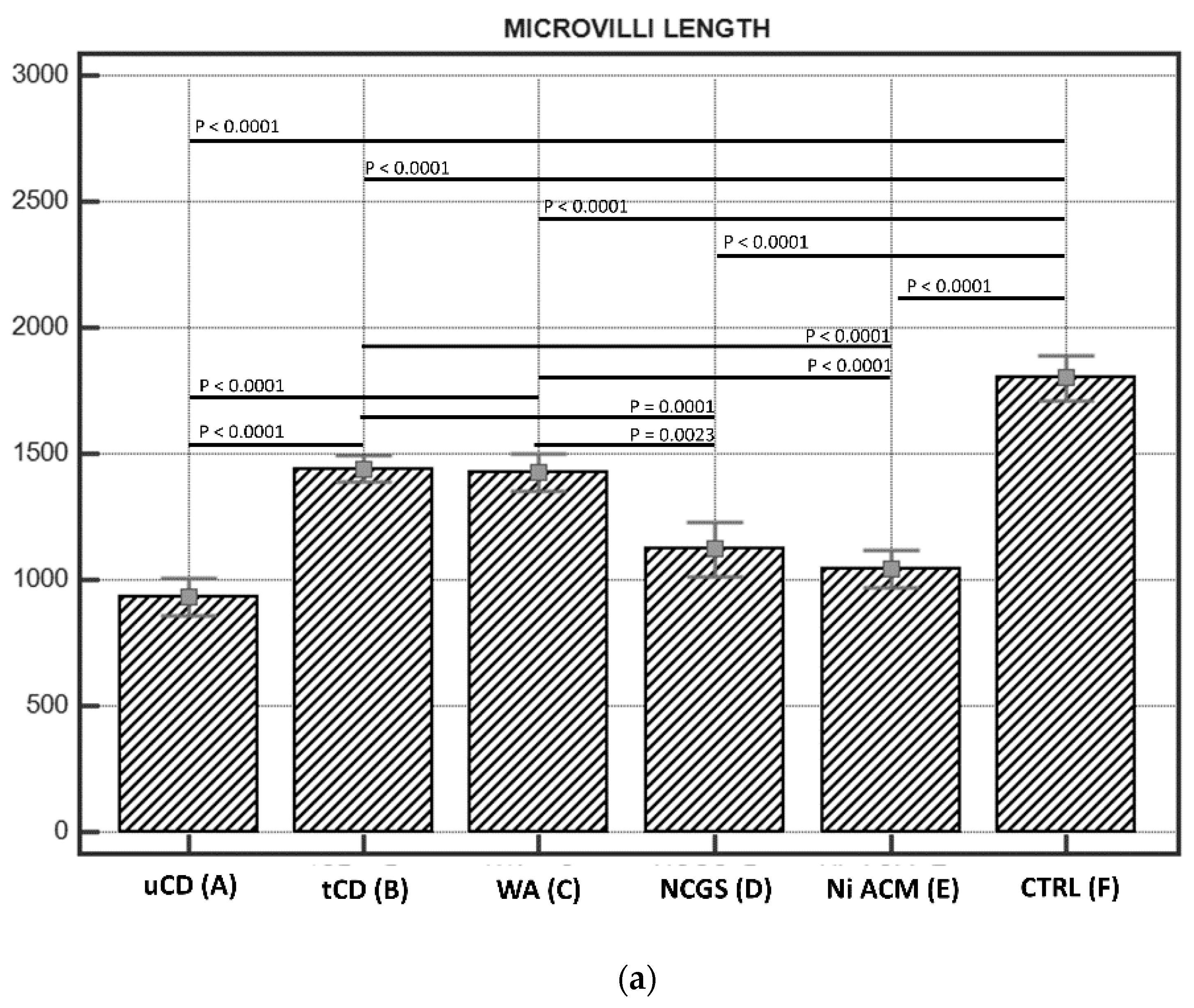
3.2.1. Length of Enterocytes Microvilli
3.2.2. Intermicrovillar Distance in Enterocytes
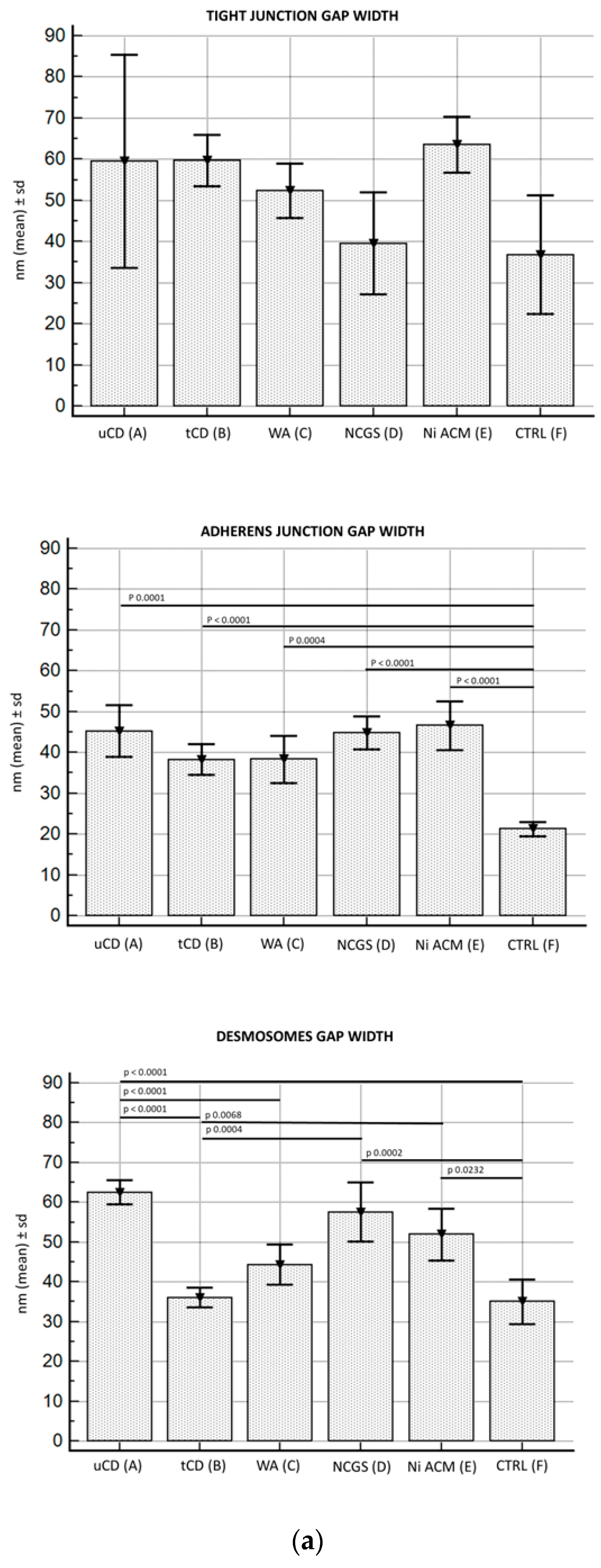
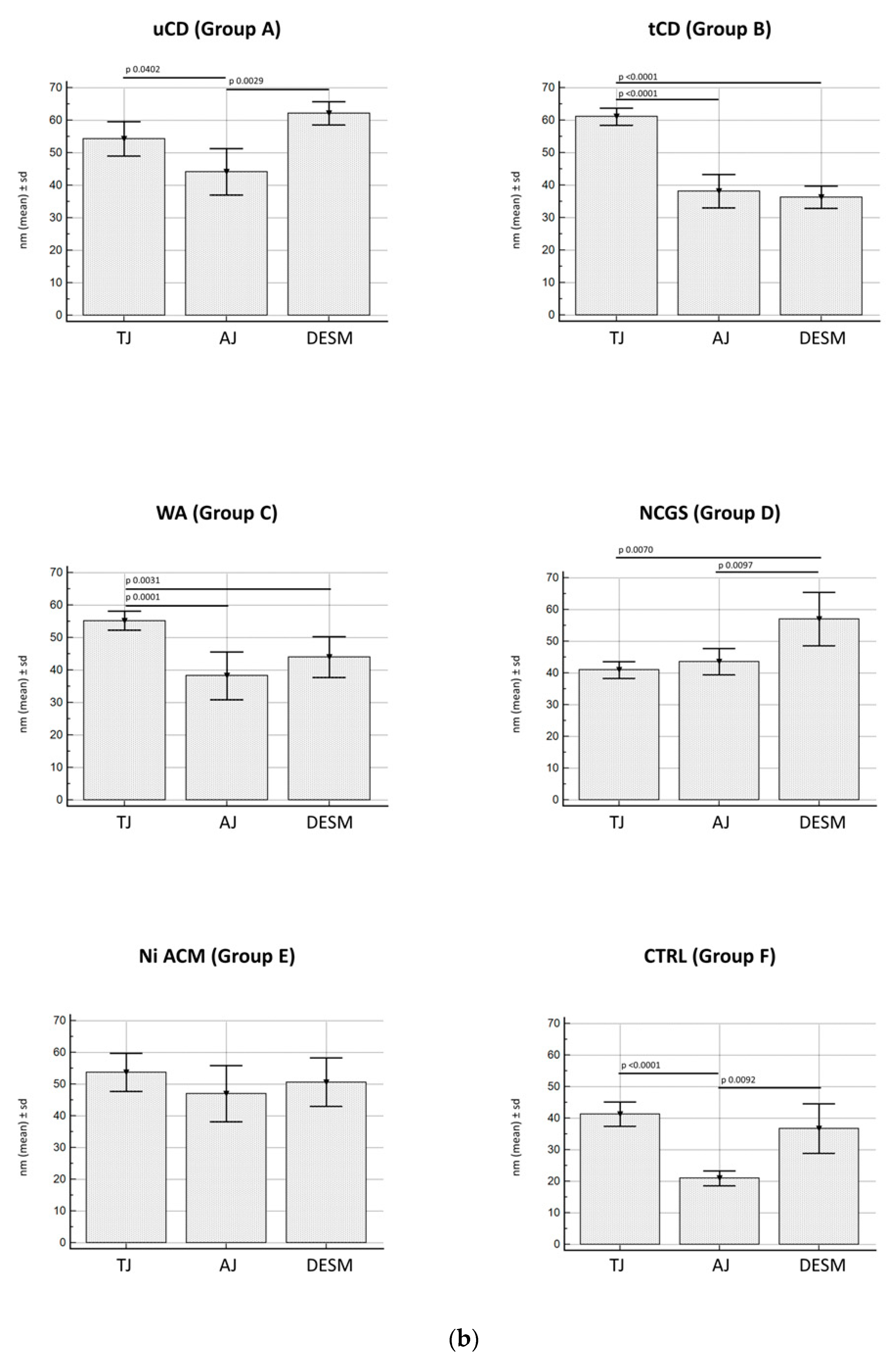
3.2.3. Junctional Complex in Enterocytes
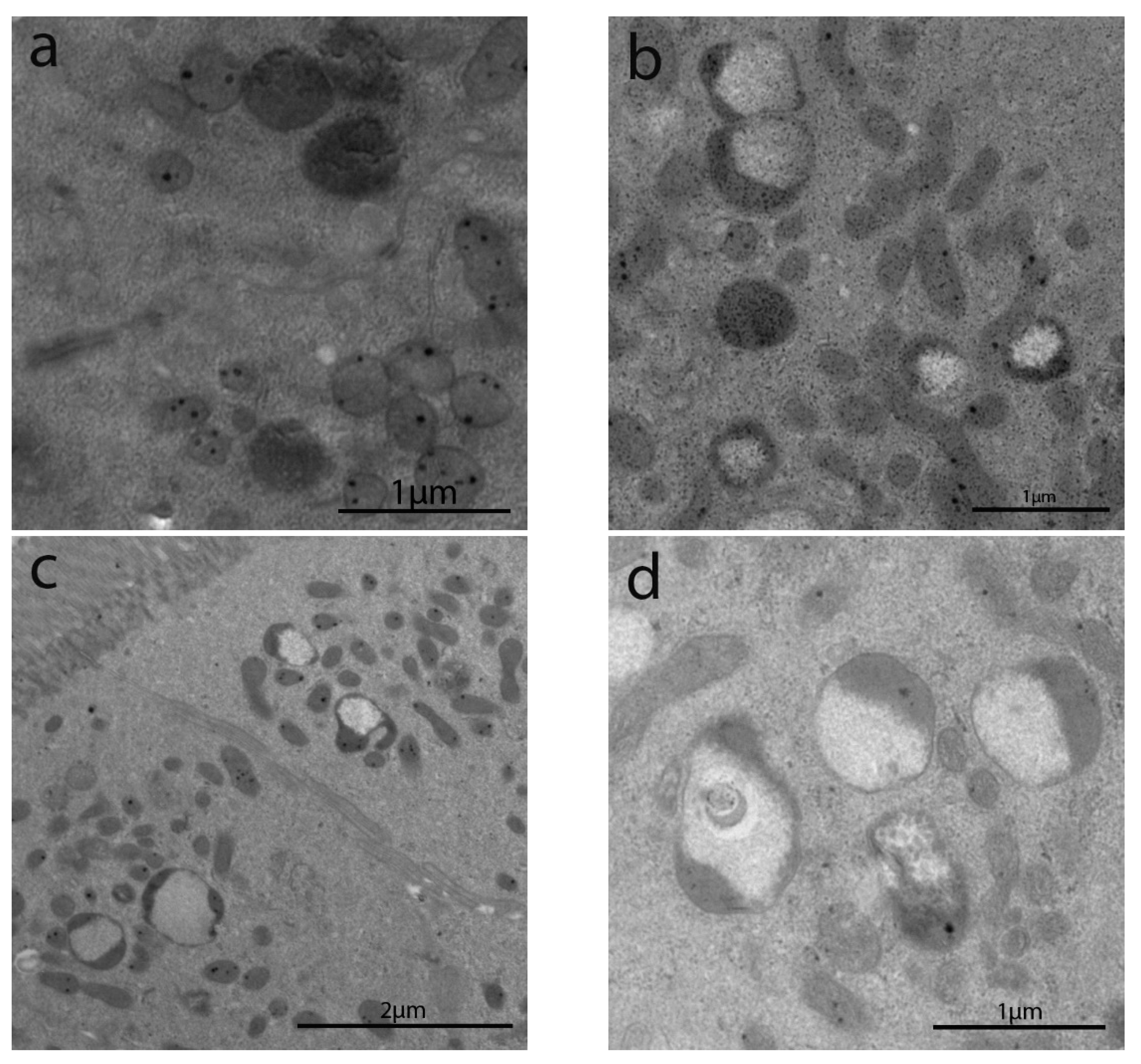
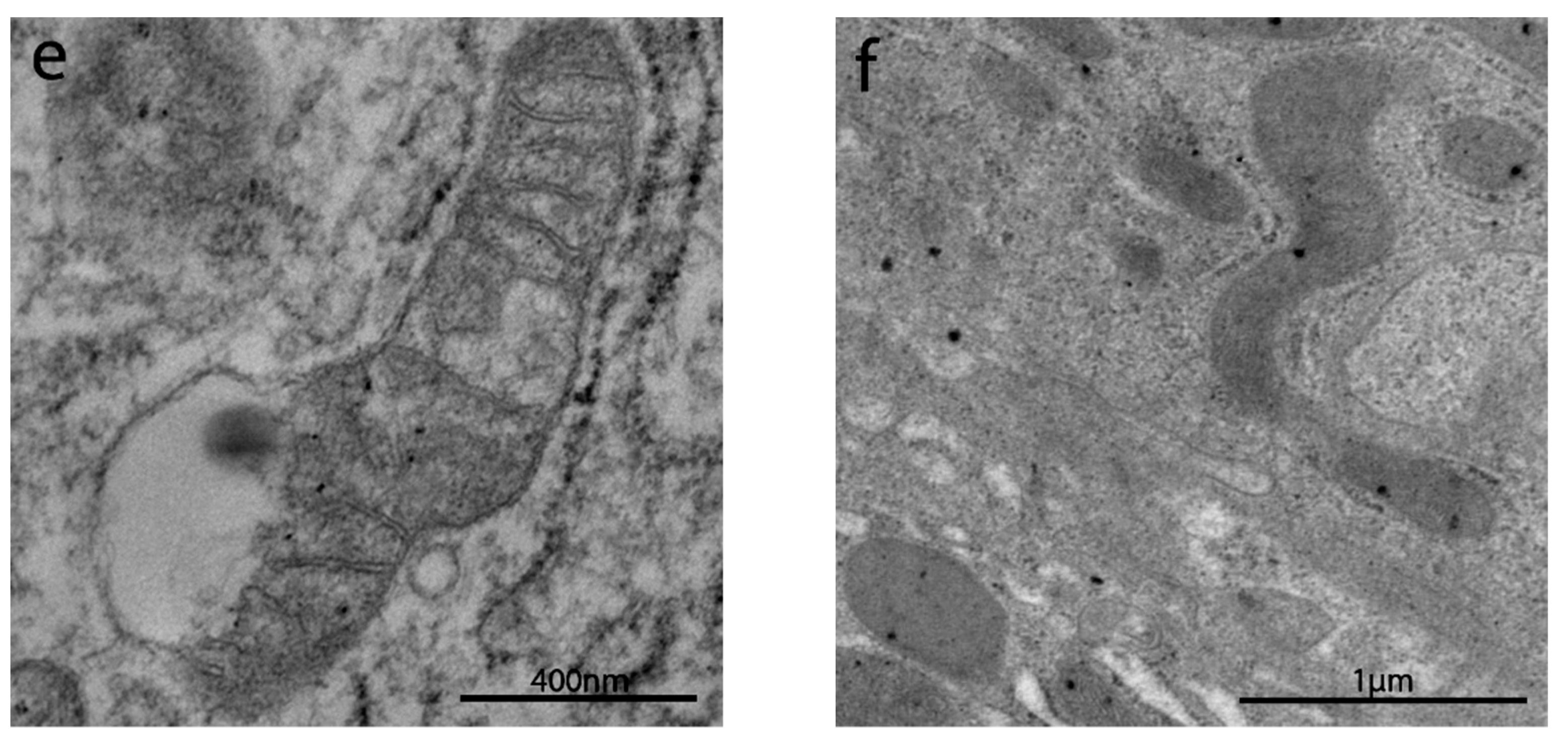
3.2.4. Intracytoplasmic Characteristics of Enterocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiller, R. Irritable bowel syndrome: New insights into symptom mechanisms and advances in treatment. F1000Research 2016, 29, 5. [Google Scholar] [CrossRef]
- Borghini, R.; Donato, G.; Alvaro, D.; Picarelli, A. New insights in IBS-like disorders: Pandora’s box has been opened; a review. Gastroenterol. Hepatol. Bed Bench 2017, 10, 79–89. [Google Scholar]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef]
- Goswami, P.; Das, P.; Verma, A.K.; Prakash, S.; Das, T.K.; Nag, T.C.; Ahuja, V.; Gupta, S.D.; Makharia, G.K. Are alterations of tight junctions at molecular and ultrastructural level different in duodenal biopsies of patients with celiac disease and Crohn’s disease? Virchows Arch. 2014, 465, 521–530. [Google Scholar] [CrossRef]
- Valitutti, F.; Fasano, A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig. Dis. Sci. 2019, 64, 1748–1758. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M.; Fanning, A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 2004, 16, 140–145. [Google Scholar] [CrossRef]
- Visser, J.; Rozing, J.; Sapone, A.; Lammers, K.; Fasano, A. Tight junctions, intestinal permeability, and autoimmunity: Celiac disease and type 1 diabetes paradigms. Ann. N. Y. Acad. Sci. 2009, 1165, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Cukrowska, B.; Sowińska, A.; Bierła, J.B.; Czarnowska, E.; Rybak, A.; Grzybowska-Chlebowczyk, U. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota-Key players in the pathogenesis of celiac disease. World J. Gastroenterol. 2017, 23, 7505–7518. [Google Scholar] [CrossRef]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Picarelli, A.; Borghini, R.; Isonne, C.; Di Tola, M. Reactivity to dietary gluten: New insights into differential diagnosis among gluten-related gastrointestinal disorders. Pol. Arch. Med. Wewn. 2013, 123, 708–712. [Google Scholar] [CrossRef] [Green Version]
- Borghini, R.; Di Tola, M.; Salvi, E.; Isonne, C.; Puzzono, M.; Marino, M.; Donato, G.; Picarelli, A. Impact of gluten-free diet on quality of life in celiac patients. Acta Gastroenterol. Belg. 2016, 79, 447–453. [Google Scholar]
- Shiner, M.; Birbeck, M.S. The microvilli of the small intestinal surface epithelium in coeliac disease and in idiopathic steatorrhoea. Gut 1961, 2, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Serena, G.; D’Avino, P.; Fasano, A. Celiac Disease and Non-celiac Wheat Sensitivity: State of Art of Non-dietary Therapies. Front. Nutr. 2020, 7, 152. [Google Scholar] [CrossRef]
- Picarelli, A.; Borghini, R.; Di Tola, M.; Marino, M.; Urciuoli, C.; Isonne, C.; Puzzono, M.; Porowska, B.; Rumi, G.; Lonardi, S.; et al. Intestinal, Systemic, and Oral Gluten-related Alterations in Patients with Nonceliac Gluten Sensitivity. J. Clin. Gastroenterol. 2016, 50, 849–858. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Carroccio, A.; Giannone, G.; Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; Iacobucci, R.; Porcasi, R.; Catalano, T.; Geraci, G.; et al. Duodenal and Rectal Mucosa Inflammation in Patients with Non-celiacWheatSensitivity. Clin. Gastroenterol. Hepatol. 2019, 17, 682–690.e3. [Google Scholar] [CrossRef] [PubMed]
- Pizzutelli, S. Systemic nickel hypersensitivity and diet: Myth or reality? Eur. Ann. Allergy Clin. Immunol. 2011, 43, 5–18. [Google Scholar] [PubMed]
- Borghini, R.; Porpora, M.G.; Casale, R.; Marino, M.; Palmieri, E.; Greco, N.; Donato, G.; Picarelli, A. Irritable Bowel Syndrome-Like Disorders in Endometriosis: Prevalence of Nickel Sensitivity and Effects of a Low-Nickel Diet. An Open-Label Pilot Study. Nutrients 2020, 28, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghini, R.; Puzzono, M.; Rosato, E.; Di Tola, M.; Marino, M.; Greco, F.; Picarelli, A. Nickel-Related Intestinal Mucositis in IBS-Like Patients: Laser Doppler Perfusion Imaging and Oral Mucosa Patch Test in Use. Biol. Trace Elem. Res. 2016, 173, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Di Tola, M.; Marino, M.; Amodeo, R.; Tabacco, F.; Casale, R.; Portaro, L.; Borghini, R.; Cristaudo, A.; Manna, F.; Rossi, A.; et al. Immunologicalcharacterization of the allergiccontactmucositisrelated to the ingestion of nickel-richfoods. Immunobiology 2014, 219, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Vitalone, A.; Di Sotto, A.; Mammola, C.L.; Heyn, R.; Miglietta, S.; Mariani, P.; Sciubba, F.; Passarelli, F.; Nativio, P.; Mazzanti, G. Phytochemicalanalysis and effects on ingestivebehaviour of a Caralluma fimbriata extract. Food Chem. Toxicol. 2017, 108 Pt A, 63–73. [Google Scholar] [CrossRef]
- Nabi, A.; Khalili, M.A.; Talebi, A.R.; Mangoli, E.; Yari, N.; Nottola, S.A.; Miglietta, S.; Taheri, F. In-Vitro Application of Pentoxifylline Preserved Ultrastructure of Spermatozoa After Vitrification in Asthenozoospermic Patients. Urol. J. 2017, 14, 4038–4043. [Google Scholar]
- Sowińska, A.; Morsy, Y.; Czarnowska, E.; Oralewska, B.; Konopka, E.; Woynarowski, M.; Szymańska, S.; Ejmont, M.; Scharl, M.; Bierła, J.B.; et al. Transcriptional and Ultrastructural Analyses Suggest Novel Insightsinto Epithelial Barrier Impairment in Celiac Disease. Cells 2020, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, B.; Baker, R.F. Electron microscope investigation of the striated border of intestinal epithelium. Anat. Rec. 1950, 107, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.H.; Bayless, T.M.; Norton, J.H.; Hendrix, T.R. Celiac disease. A study of the jejunal epithelium before and after a gluten-free diet. N. Engl. J. Med. 1962, 267, 1173–1179. [Google Scholar] [CrossRef]
- Trier, J.S.; Rubin, C.E. Electron microscopy of the small intestine: A review. Gastroenterology 1965, 49, 574–603. [Google Scholar] [CrossRef]
- Trier, J.S. Structure of the mucosa of the small intestine as it relates to intestinal function. Fed. Proc. 1967, 26, 1391–1404. [Google Scholar] [PubMed]
- Wehman, H.J.; Plantholt, B.A.; Lifshitz, F. Microvillous anomalies induced by various conditions of stress in the small intestine of the rat. Exp. Mol. Pathol. 1972, 17, 296–306. [Google Scholar] [CrossRef]
- Borghini, R.; De Amicis, N.; Bella, A.; Greco, N.; Donato, G.; Picarelli, A. Beneficial Effects of a Low-Nickel Diet on Relapsing IBS-Like and Extraintestinal Symptoms of Celiac Patients during a Proper Gluten-Free Diet: Nickel Allergic Contact Mucositis in Suspected Non-Responsive Celiac Disease. Nutrients 2020, 12, 2277. [Google Scholar] [CrossRef]
- Jing, K.; Lim, K. Why is autophagy important in human diseases? Exp. Mol. Med. 2012, 44, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Klionsky, D.J. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 931–937. [Google Scholar] [CrossRef]
- Huett, A.; Xavier, R.J. Autophagy at the gut interface: Mucosal responses to stress and the consequences for inflammatory bowel diseases. Inflamm. Bowel. Dis. 2010, 16, 152–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.V.; Troncone, R.; Auricchio, S. Gliadin Peptides as Triggers of the Proliferative and Stress/Innate Immune Response of the Celiac Small Intestinal Mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buret, A.G.; Bhargava, A. Modulatory mechanisms of enterocyte apoptosis by viral, bacterial and parasitic pathogens. Crit. Rev. Microbiol. 2014, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shalimar, D.M.; Das, P.; Sreenivas, V.; Gupta, S.D.; Panda, S.K.; Makharia, G.K. Mechanism of villous atrophy in celiac disease: Role of apoptosis and epithelial regeneration. Arch. Pathol. Lab. Med. 2013, 137, 1262–1269. [Google Scholar] [CrossRef]
- Mazzarella, G.; Stefanile, R.; Camarca, A.; Giliberti, P.; Cosentini, E.; Marano, C.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; Sette, A.; et al. Gliadinactivates HLA class I-restricted CD8+ T cells in celiacdiseaseintestinal mucosa and induces the enterocyteapoptosis. Gastroenterology 2008, 134, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhonchal, S.; Nain, C.K.; Prasad, K.K.; Nada, R.; Sharma, A.K.; Sinha, S.K.; Singh, K. Functional and morphological alterations in small intestine mucosa of chronic alcoholics. J. Gastroenterol. Hepatol. 2008, 23 Pt 2, e43–e48. [Google Scholar] [CrossRef]
- Beilstein, F.; Carrière, V.; Leturque, A.; Demignot, S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp. Cell Res. 2016, 340, 172–179. [Google Scholar] [CrossRef]
- Demignot, S.; Beilstein, F.; Morel, E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: Key players in intestinal physiology and metabolic disorders. Biochimie 2014, 96, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrone, T.; Magrone, M.; Jirillo, E. Mast Cells as a Double Edged Sword in Immunity: Disorders of Mast Cell Activation and Therapeutic Management. Second of Two Parts. Endocr. Metab. Immune Disord. Drug Targets 2019, 20, 670–686. [Google Scholar] [CrossRef]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Frossi, B.; De Carli, M.; Calabrò, A. Coeliac Disease and Mast Cells. Int. J. Mol. Sci. 2019, 20, 3400. [Google Scholar] [CrossRef] [Green Version]
- Hiatt, R.B.; Katz, L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am. J. Gastroenterol. 1962, 37, 541–545. [Google Scholar]
- Robles, A.; Perez Ingles, D.; Myneedu, K.; Deoker, A.; Sarosiek, I.; Zuckerman, M.J.; Schmulson, M.J.; Bashashati, M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2019, 31, e13718. [Google Scholar] [CrossRef] [Green Version]
- Vanheel, H.; Vicario, M.; Boesmans, W.; Vanuytsel, T.; Salvo-Romero, E.; Tack, J.; Farré, R. Activation of Eosinophils and Mast Cells in Functional Dyspepsia: An Ultrastructural Evaluation. Sci. Rep. 2018, 8, 5383. [Google Scholar] [CrossRef] [PubMed]
- Giancola, F.; Volta, U.; Repossi, R.; Latorre, R.; Beeckmans, D.; Carbone, F.; Van den Houte, K.; Bianco, F.; Bonora, E.; Gori, A.; et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten/wheat sensitivity. Neurogastroenterol. Motil. 2020, 5, e13814. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinbara, M.; Bando, K.; Shiraishi, D.; Kuroishi, T.; Nagai, Y.; Ohtsu, H.; Takano-Yamamoto, T.; Sugawara, S.; Endo, Y. Mast cell histamine-mediated transient inflammation following exposure to nickel promotes nickel allergy in mice. Exp. Dermatol. 2016, 25, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.L.; Smith, V.H.; King, C.M. Type 1 and type IV hypersensitivity to nickel. Australas. J. Dermatol. 2010, 51, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Sugita, Y.; Nakajima, H.; Tanaka, S.; Askenase, P.W. Elicitation of nickel sulfate (NiSO4)-specific delayed-type hypersensitivity requires early-occurring and early-acting, NiSO4-specific DTH-initiating cells with an unusual mixed phenotype for an antigen-specific cell. Cell Immunol. 1995, 161, 244–255. [Google Scholar] [CrossRef] [PubMed]
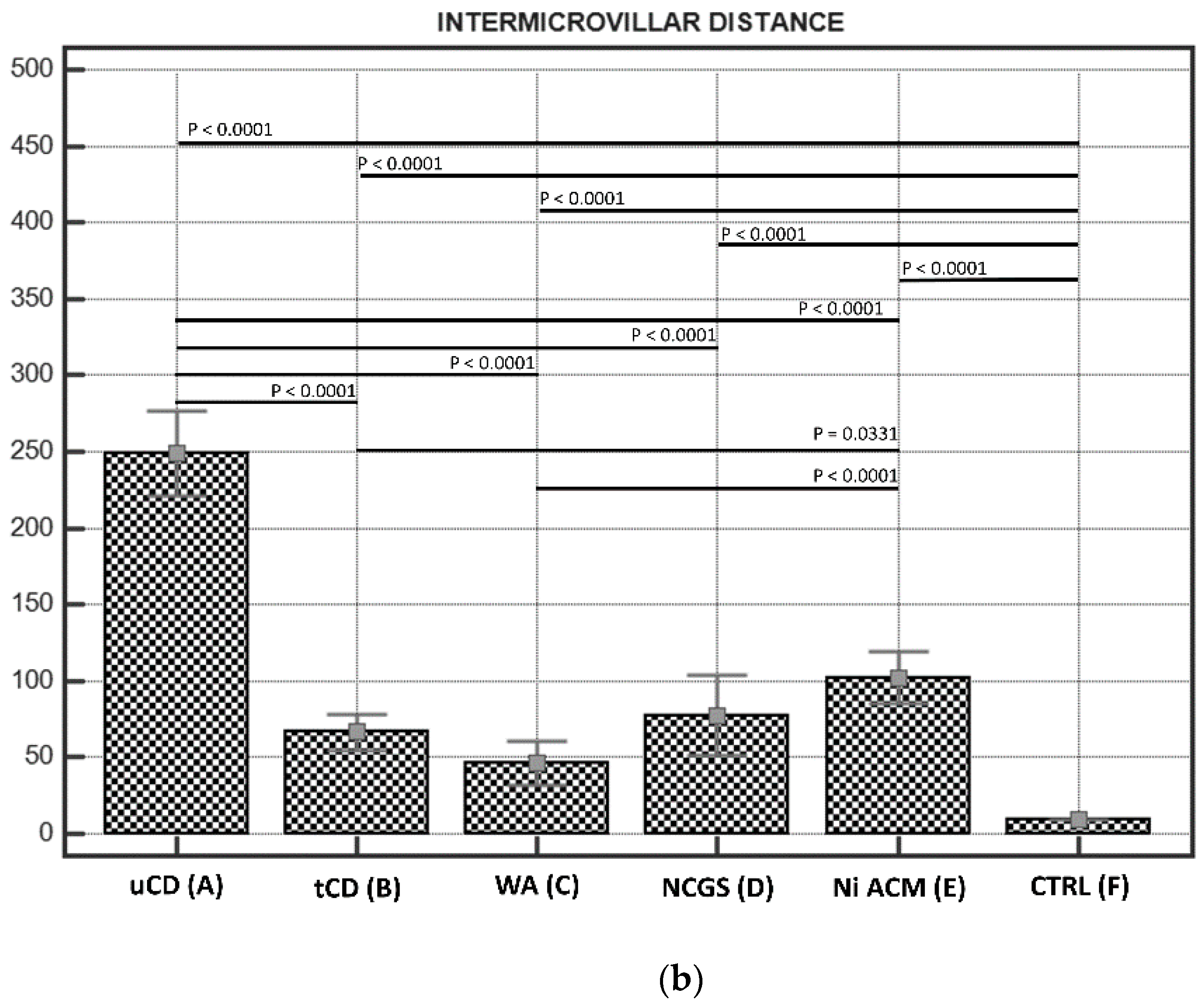



| Groups | Villous:Crypt Ratio | Crypts | IELs/100 ECs | Marsh-Oberhuber Classification |
|---|---|---|---|---|
| (A) uCD | ||||
| pt n.1 | 1:3 | hyperplasia | ≥25 | 3 C |
| pt n.2 | 1:2 | hyperplasia | ≥25 | 3 B |
| pt n.3 | 1:3 | hyperplasia | ≥25 | 3 C |
| pt n.4 | 1:2 | hyperplasia | ≥25 | 3 A |
| pt n.5 | 1:2 | hyperplasia | ≥25 | 3 B |
| (B) tCD | ||||
| pt n.1 | 3:1 | normal | <25 | 0 |
| pt n.2 | 3:1 | normal | ≥25 | 1 |
| pt n.3 | 3:1 | normal | <25 | 0 |
| pt n.4 | 2:1 | hyperplasia | ≥25 | 2 |
| pt n.5 | 2:1 | normal | ≥25 | 1 |
| (C) WA | ||||
| pt n.1 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| pt n.2 | 3:1 | normal | <25 | 0 |
| pt n.3 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| pt n.4 | 3:1 | normal | <25 | 0 |
| pt n.5 | 3:1 | normal | <25 | 0 |
| (D) NCGS | ||||
| pt n.1 | 3:1 | normal | <25 | 0 |
| pt n.2 | 3:1 | normal | <25 | 0 |
| pt n.3 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| pt n.4 | 3:1 | normal | <25 | 0 |
| pt n.5 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| (E) Ni ACM | ||||
| pt n.1 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| pt n.2 | 3:1 | normal | <25 | 0 |
| pt n.3 | 3:1 | normal | <25 | 0 |
| pt n.4 | 3:1 | normal | <25 | 0 |
| pt n.5 | 3:1 (with dismorphyc villi) | normal | ≥25 | 1 |
| (F) CTRL | ||||
| pt n.1 | 3:1 | normal | <25 | 0 |
| pt n.2 | 3:1 | normal | <25 | 0 |
| pt n.3 | 3:1 | normal | <25 | 0 |
| pt n.4 | 3:1 | normal | ≥25 | 1 |
| pt n.5 | 3:1 | normal | <25 | 0 |
| Transmission Electron Microscopy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Gender | Age | Symptoms | Microvilli | JC | Auto Phagy | Apo Ptosis | Altered Mitochondria | Lipid Drops | Chylomicrons | Mast Cells | ||
| Length | Distance | Regularity | |||||||||||
| (A) uCD | 931.00 ± 30 * | 249 ± 10 * | |||||||||||
| pt n.1 | F | 20 | diarrhea, anemia | atrophic/irregular and loosely packed | see graph | ++ | - | - | - | - | ++ | ||
| pt n.2 | F | 63 | constipation, epigastralgia, heartburn | atrophic/irregular and loosely packed | ++ | - | - | - | - | + | |||
| pt n.3 | M | 34 | Abdominal swelling, diarrhea | atrophic/irregular and loosely packed | ++ | - | + | - | - | - | |||
| pt n.4 | F | 25 | abdominal pain, swelling, heartburn, asthenia | atrophic/irregular and loosely packed | + | - | - | - | - | ++ | |||
| pt n.5 | F | 41 | abdominal pain, swelling, nausea, vomiting, headache | atrophic/irregular and loosely packed | ++ | - | - | - | - | + | |||
| (B) tCD | 1439.10 ± 69 | 66.55 ± 7 | |||||||||||
| pt n.1 | F | 51 | asymptomatic | regular butlooselypacked | See graph | ++ | - | + | - | - | - | ||
| pt n.2 | M | 39 | acid regurgitation, headache | regular and tightly-packed | ++ | - | - | - | - | - | |||
| pt n.3 | F | 21 | asymptomatic | regular and tightly-packed | + | - | - | - | - | - | |||
| pt n.4 | F | 31 | asymptomatic | regular and tightly-packed | ++ | - | - | - | - | - | |||
| pt n.5 | M | 64 | abdominal swelling, flatulence | regular and tightly-packed | + | - | - | - | - | - | |||
| (C) WA | 1427.09 ± 71 | 46.23 ± 8 | |||||||||||
| pt n.1 | F | 23 | diarrhea, abdominal pain, swelling, anemia, headache | regular and packed | see graph | ++ | - | + | - | + | - | ||
| pt n.2 | F | 28 | abdominal pain, diarrhea | regular and packed | ++ | - | + | - | + | + | |||
| pt n.3 | F | 41 | diarrhea, abdominal swelling, atopic dermatitis | regular and tightly-packed | ++ | - | - | - | + | + | |||
| pt n.4 | M | 65 | diarrhea, abdominal bloating, asthenia, headache | regular and packed | ++ | - | + | - | + | + | |||
| pt n.5 | M | 33 | Abdominal swelling, asthenia | regular and tightly-packed | ++ | - | + | - | - | + | |||
| (D) NCGS | 1120.69 ± 60 | 77.59 ± 12 | |||||||||||
| pt n.1 | F | 52 | abdominal pain, swelling, diarrhea, asthenia | regular and packed | see graph | + | + | + | + | - | - | ||
| pt n.2 | M | 35 | abdominal pain, swelling | regular and packed | + | + | + | - | - | - | |||
| pt n.3 | F | 38 | heartburn, nausea, headache, foggy mind | regular and loosely-packed | ++ | + | + | + | - | - | |||
| pt n.4 | F | 28 | abdominal pain, swelling, asthenia, alopecia | regular and packed | + | - | + | + | - | - | |||
| pt n.5 | F | 22 | abdominal pain, swelling, diarrhea | regular and tightly-packed | ++ | + | - | + | - | - | |||
| (E) Ni ACM | 1042.29 ± 42 | 102.30 ± 10 | |||||||||||
| pt n.1 | M | 33 | abdominal pain, diarrhea, heartburn | irregular and packed | see graph | ++ | + | + | + | - | + | ||
| pt n.2 | F | 21 | abdominal pain, swelling, constipation | regular and packed | + | - | + | - | - | - | |||
| pt n.3 | F | 30 | abdominal pain, swelling | irregular and packed | ++ | + | + | + | - | + | |||
| pt n.4 | F | 45 | abdominal pain, swelling, constipation | irregular and packed | ++ | + | + | + | - | + | |||
| pt n.5 | F | 52 | abdominal pain, swelling, diarrhea, asthenia, anemia | irregular and packed | ++ | + | + | + | - | + | |||
| (F) CTRL | 1799.75 ± 49 | 9.6 ± 1 | |||||||||||
| pt n.1 | F | 20 | GERD and dyspepsia | regular and tightly-packed | see graph | - | - | - | - | - | - | ||
| pt n.2 | F | 30 | GERD and dyspepsia | regular and tightly-packed | - | - | - | - | - | - | |||
| pt n.3 | M | 45 | GERD and dyspepsia | regular and tightly-packed | - | - | - | - | - | + | |||
| pt n.4 | F | 59 | GERD and dyspepsia | regular and tightly-packed | - | - | - | - | - | - | |||
| pt n.5 | M | 43 | GERD and dyspepsia | regular and packed | + | - | - | - | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglietta, S.; Borghini, R.; Relucenti, M.; Sorrentino, V.; Chen, R.; Li, X.; Fazi, F.; Donato, G.; Familiari, G.; Petrozza, V.; et al. New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies. Cells 2021, 10, 2593. https://doi.org/10.3390/cells10102593
Miglietta S, Borghini R, Relucenti M, Sorrentino V, Chen R, Li X, Fazi F, Donato G, Familiari G, Petrozza V, et al. New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies. Cells. 2021; 10(10):2593. https://doi.org/10.3390/cells10102593
Chicago/Turabian StyleMiglietta, Selenia, Raffaele Borghini, Michela Relucenti, Veronica Sorrentino, Rui Chen, Xiaobo Li, Francesco Fazi, Giuseppe Donato, Giuseppe Familiari, Vincenzo Petrozza, and et al. 2021. "New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies" Cells 10, no. 10: 2593. https://doi.org/10.3390/cells10102593
APA StyleMiglietta, S., Borghini, R., Relucenti, M., Sorrentino, V., Chen, R., Li, X., Fazi, F., Donato, G., Familiari, G., Petrozza, V., & Picarelli, A. (2021). New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies. Cells, 10(10), 2593. https://doi.org/10.3390/cells10102593







