Mechanisms and Clinical Significance of Tumor Lymphatic Invasion
Abstract
:1. Introduction
2. Lymphatic Intravasation Processes in the Healthy Organism
2.1. Lymphatic Entry by Leukocytes
2.2. Benign Metastasis
3. Local Invasion
4. Mechanisms of Tumor Lymphatic Invasion
4.1. Mechanical Disruption of Endothelial Barriers
4.2. Immune Cell Mimicry
4.3. Tumor-Induced Destabilization of Lymphatic Junctions
4.4. Tumor-Induced Formation of Entry Portals into Lymphatic Vessels
4.5. Role of Bystander Cells in Lymphatic Invasion
5. Clinical Implications of Lymphatic Invasion—From Prognosis to Therapy?
5.1. Prognostic Value of Tumor LVI
5.2. What Is the Relationship between Lymphatic Metastasis and Distant Metastasis?
5.3. Potential Therapeutic Approaches
6. Conclusions and Open Questions
Author Contributions
Funding
Conflicts of Interest
References
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Koh, G.Y. Biological functions of lymphatic vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Koh, G.Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 2018, 215, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; He, Y.; D’Addio, M.; Tacconi, C.; Detmar, M.; Dieterich, L.C. Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLoS Biol. 2020, 18, e3000704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, A.; Hollmén, M.; Dermadi, D.; Pan, J.; Brulois, K.F.; Kaukonen, R.; Lönnberg, T.; Boström, P.; Koskivuo, I.; Irjala, H.; et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 2019, 51, 561–572.e565. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Grosso, R.A.; Takeda, A.; Pan, J.; Bekkhus, T.; Brulois, K.; Dermadi, D.; Nordling, S.; Vanlandewijck, M.; Jalkanen, S.; et al. A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature. Front. Cardiovasc. Med. 2020, 7, 52. [Google Scholar] [CrossRef]
- Jalkanen, S.; Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 2020, 20, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, L.C.; Seidel, C.D.; Detmar, M. Lymphatic vessels: New targets for the treatment of inflammatory diseases. Angiogenesis 2014, 17, 359–371. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Commerford, C.D.; Dieterich, L.C.; He, Y.; Hell, T.; Montoya-Zegarra, J.A.; Noerrelykke, S.F.; Russo, E.; Röcken, M.; Detmar, M. Mechanisms of Tumor-Induced Lymphovascular Niche Formation in Draining Lymph Nodes. Cell Rep. 2018, 25, 3554–3563. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, S.B.; Gsponer, D.; Montoya-Zegarra, J.A.; Schneider, M.; Scholkmann, F.; Tacconi, C.; Noerrelykke, S.F.; Proulx, S.T.; Detmar, M. A Distinct Role of the Autonomic Nervous System in Modulating the Function of Lymphatic Vessels under Physiological and Tumor-Draining Conditions. Cell Rep. 2019, 27, 3305–3314. [Google Scholar] [CrossRef]
- Karnezis, T.; Shayan, R.; Caesar, C.; Roufail, S.; Harris, N.C.; Ardipradja, K.; Zhang, Y.F.; Williams, S.P.; Farnsworth, R.H.; Chai, M.G.; et al. VEGF-D Promotes Tumor Metastasis by Regulating Prostaglandins Produced by the Collecting Lymphatic Endothelium. Cancer Cell 2012, 21, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Dieterich, L.C.; Detmar, M. Multiple roles of lymphatic vessels in tumor progression. Curr. Opin. Immunol. 2018, 53, 7–12. [Google Scholar] [CrossRef]
- Ma, Q.; Dieterich, L.C.; Ikenberg, K.; Bachmann, S.B.; Mangana, J.; Proulx, S.T.; Amann, V.C.; Levesque, M.P.; Dummer, R.; Baluk, P.; et al. Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci. Adv. 2018, 4, eaat4758. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Sugaya, M.; Oka, T.; Blauvelt, A.; Okochi, H.; Sato, S. Lymphatic dysfunction attenuates tumor immunity through impaired antigen presentation. Oncotarget 2015, 6, 18081–18093. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.W.; Wagner, M.; Fankhauser, M.; Steinskog, E.S.; Broggi, M.A.; Spranger, S.; Gajewski, T.F.; Alitalo, K.; Eikesdal, H.P.; Wiig, H.; et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Investig. 2016, 126, 3389–3402. [Google Scholar] [CrossRef]
- Cousin, N.; Cap, S.; Dihr, M.; Tacconi, C.; Detmar, M.; Dieterich, L.C. Lymphatic PD-L1 Expression Restricts Tumor-Specific CD8+ T-cell Responses. Cancer Res. 2021, 81, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Ikenberg, K.; Cetintas, T.; Kapaklikaya, K.; Hutmacher, C.; Detmar, M. Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation. Front. Immunol. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.P.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A. In Sickness and in Health: The Immunological Roles of the Lymphatic System. Int. J. Mol. Sci. 2021, 22, 4458. [Google Scholar] [CrossRef] [PubMed]
- Arasa, J.; Collado-Diaz, V.; Halin, C. Structure and Immune Function of Afferent Lymphatics and Their Mechanistic Contribution to Dendritic Cell and T Cell Trafficking. Cells 2021, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., III; Dutertre, C.-A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef]
- Lämmermann, T.; Bader, B.L.; Monkley, S.J.; Worbs, T.; Wedlich-Söldner, R.; Hirsch, K.; Keller, M.; Förster, R.; Critchley, D.R.; Fässler, R.; et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 2008, 453, 51–55. [Google Scholar] [CrossRef]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial Dendritic Cell Guidance by Haptotactic Chemokine Gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Worbs, T.; Hammerschmidt, S.I.; Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Saeki, H.; Moore, A.M.; Brown, M.J.; Hwang, S.T. Cutting edge: Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999, 162, 2472–2475. [Google Scholar]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 Coordinates the Primary Immune Response by Establishing Functional Microenvironments in Secondary Lymphoid Organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Ohl, L.; Mohaupt, M.; Czeloth, N.; Hintzen, G.; Kiafard, Z.; Zwirner, J.; Blankenstein, T.; Henning, G.; Förster, R. CCR7 Governs Skin Dendritic Cell Migration under Inflammatory and Steady-State Conditions. Immunity 2004, 21, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czeloth, N.; Bernhardt, G.; Hofmann, F.; Genth, H.; Forster, R. Sphingosine-1-Phosphate Mediates Migration of Mature Dendritic Cells. J. Immunol. 2005, 175, 2960–2967. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Jackson, D.G. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J. Cell Sci. 2013, 126, 5259–5270. [Google Scholar] [CrossRef] [Green Version]
- Kabashima, K.; Shiraishi, N.; Sugita, K.; Mori, T.; Onoue, A.; Kobayashi, M.; Sakabe, J.; Yoshiki, R.; Tamamura, H.; Fujii, N.; et al. CXCL12-CXCR4 Engagement Is Required for Migration of Cutaneous Dendritic Cells. Am. J. Pathol. 2007, 171, 1249–1257. [Google Scholar] [CrossRef] [Green Version]
- Vigl, B.; Aebischer, D.; Nitschké, M.; Iolyeva, M.; Röthlin, T.; Antsiferova, O.; Halin, C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011, 118, 205–215. [Google Scholar] [CrossRef]
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777. [Google Scholar] [CrossRef] [Green Version]
- Arasa, J.; Collado-Diaz, V.; Kritikos, I.; Medina-Sanchez, J.D.; Friess, M.C.; Sigmund, E.C.; Schineis, P.; Hunter, M.C.; Tacconi, C.; Paterson, N.; et al. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Bromley, S.K.; Thomas, S.Y.; Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005, 6, 895–901. [Google Scholar] [CrossRef]
- Debes, G.F.; Arnold, C.N.; Young, A.J.; Krautwald, S.; Lipp, M.; Hay, J.B.; Butcher, E.C. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005, 6, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Ledgerwood, L.G.; Lal, G.; Zhang, N.; Garin, A.; Esses, S.J.; Ginhoux, F.; Merad, M.; Peche, H.; Lira, S.A.; Ding, Y.; et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 2008, 9, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, A.A.L.; Schwab, S.R. Finding a Way Out: S1P Signaling and Immune Cell Migration. Annu. Rev. Immunol. 2020, 38, 759–784. [Google Scholar] [CrossRef]
- McCarthy, S.W.; Palmer, A.A.; Bale, P.M.; Hirst, E. Naevus cells in lymph nodes. Pathology 1974, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bortolani, A.; Barisoni, D.; Scomazzoni, G. Benign “Metastatic” Cellular Blue Nevus. Ann. Plast. Surg. 1994, 33, 426–431. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Kapler, E.S.; Dinges, M.M.; Bastian, B.C.; Yeh, I. Eruptive Spitz nevus, a striking example of benign metastasis. Sci. Rep. 2020, 10, 1621. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, G.; Garrido-Ruiz, M.C.; Rios-Martin, J.J.; Rodriguez-Peralto, J.L. Three Cases of Clear Cell Hidradenoma With “Benign” Lymph Node Involvement. Am. J. Dermatopathol. 2021, 43, e76–e79. [Google Scholar] [CrossRef]
- Pacheco-Rodriguez, G.; Taveira-DaSilva, A.M.; Moss, J. Benign Metastasizing Leiomyoma. Clin. Chest Med. 2016, 37, 589–595. [Google Scholar] [CrossRef]
- Johnson, W.T.; Helwig, E.B. Benign nevus cells in the capsule of lymph nodes. Cancer 1969, 23, 747–753. [Google Scholar] [CrossRef]
- Leblebici, C.; Kelten, C.; Gurel, M.S.; Hacıhasasanoglu, E. Intralymphatic nevus cells in benign nevi. Ann. Diagn. Pathol. 2016, 25, 1–6. [Google Scholar] [CrossRef]
- León, X.; Sancho, F.J.; García, J.; Sañudo, J.R.; Orús, C.; Quer, M. Incidence and Significance of Clinically Unsuspected Thyroid Tissue in Lymph Nodes Found During Neck Dissection in Head and Neck Carcinoma Patients. Laryngoscope 2005, 115, 470–474. [Google Scholar] [CrossRef]
- Lewis, A.L.; Truong, L.D.; Cagle, P.; Zhai, Q.J. Benign Salivary Gland Tissue Inclusion in a Pulmonary Hilar Lymph Node From a Patient With Invasive Well-Differentiated Adenocarcinoma of the Lung: A Potential Misinterpretation for the Staging of Carcinoma. Int. J. Surg. Pathol. 2011, 19, 382–385. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Karlsson, M.C.; Gonzalez, S.F.; Welin, J.; Fuxe, J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol. Oncol. 2017, 11, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, partial EMT and collective migration: Emerging culprits in metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef]
- Sinha, D.; Saha, P.; Samanta, A.; Bishayee, A. Emerging Concepts of Hybrid Epithelial-to-Mesenchymal Transition in Cancer Progression. Biomolecules 2020, 10, 1561. [Google Scholar] [CrossRef]
- Bronsert, P.; Enderle-Ammour, K.; Bader, M.; Timme, S.; Kuehs, M.; Csanadi, A.; Kayser, G.; Kohler, I.; Bausch, D.; Hoeppner, J.; et al. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. J. Pathol. 2014, 234, 410–422. [Google Scholar] [CrossRef]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Vandamme, N.; Berx, G. From neural crest cells to melanocytes: Cellular plasticity during development and beyond. Cell. Mol. Life Sci. 2019, 76, 1919–1934. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Yoshida, H.; Kunisada, T.; Nishikawa, S.-I. Regulation of E- and P-Cadherin Expression Correlated with Melanocyte Migration and Diversification. Dev. Biol. 1999, 215, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedri, D.; Karras, P.; Landeloos, E.; Marine, J.C.; Rambow, F. Epithelial-to-mesenchymal-like transition events in melanoma. FEBS J. 2021. [Google Scholar] [CrossRef]
- Tatti, O.; Gucciardo, E.; Pekkonen, P.; Holopainen, T.; Louhimo, R.; Repo, P.; Maliniemi, P.; Lohi, J.; Rantanen, V.; Hautaniemi, S.; et al. MMP16 Mediates a Proteolytic Switch to Promote Cell–Cell Adhesion, Collagen Alignment, and Lymphatic Invasion in Melanoma. Cancer Res. 2015, 75, 2083–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Ray, A.; Provenzano, P.P. Aligned forces: Origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr. Opin. Cell Biol. 2021, 72, 63–71. [Google Scholar] [CrossRef]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.Y.; Li, R.X.; Guo, Z. Structural studies of initial lymphatics adjacent to gastric and colonic malignant neoplasms. Lymphology 1999, 32, 70–74. [Google Scholar] [PubMed]
- Niimi, K.; Yoshizawa, M.; Nakajima, T.; Saku, T. Vascular invasion in squamous cell carcinomas of human oral mucosa. Oral Oncol. 2001, 37, 357–364. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.-T.; Hwang, S.T. Expression of CC Chemokine Receptor-7 and Regional Lymph Node Metastasis of B16 Murine Melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Mashino, K.; Sadanaga, N.; Yamaguchi, H.; Tanaka, F.; Ohta, M.; Shibuta, K.; Inoue, H.; Mori, M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002, 62, 2937–2941. [Google Scholar]
- Emmett, M.S.; Lanati, S.; Dunn, D.B.; Stone, O.A.; Bates, D.O. CCR7 Mediates Directed Growth of Melanomas Towards Lymphatics. Microcirculation 2011, 18, 172–182. [Google Scholar] [CrossRef]
- Sperveslage, J.; Frank, S.; Heneweer, C.; Egberts, J.; Schniewind, B.; Buchholz, M.; Bergmann, F.; Giese, N.; Munding, J.; Hahn, S.A.; et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 2012, 131, E371–E381. [Google Scholar] [CrossRef]
- Takanami, I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: Correlation with lymph node metastasis. Int. J. Cancer 2003, 105, 186–189. [Google Scholar] [CrossRef]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weißbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef]
- Kodama, J.; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann. Oncol. 2007, 18, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.T.; Ingman, W.V.; Poltavets, V.; Faulkner, J.W.; Whitfield, R.J.; McColl, S.R.; Kochetkova, M. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene 2016, 35, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, M.F.; Georgoudaki, A.-M.; Lambut, L.; Johansson, J.E.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Almofti, A.; Uchida, D.; Begum, N.M.; Tomizuka, Y.; Iga, H.; Yoshida, H.; Sato, M. The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int. J. Oncol. 2004, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, C.C.; Bahre, R.; Gockel, I.; Müller, A.; Frerichs, K.; Hörner, V.; Teufel, A.; Simiantonaki, N.; Biesterfeld, S.; Wehler, T.; et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br. J. Cancer 2006, 95, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Li, G.; Wu, H.; Sun, K.; Chen, J.; Feng, Y.; Chen, C.; Cai, S.; Xu, J.; et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017, 385, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Kapaklikaya, K.; Cetintas, T.; Proulx, S.T.; Commerford, C.D.; Ikenberg, K.; Bachmann, S.B.; Scholl, J.; Detmar, M. Transcriptional profiling of breast cancer-associated lymphatic vessels reveals VCAM-1 as regulator of lymphatic invasion and permeability. Int. J. Cancer 2019, 145, 2804–2815. [Google Scholar] [CrossRef]
- Rebhun, R.B.; Cheng, H.; Gershenwald, J.E.; Fan, D.; Fidler, I.J.; Langley, R.R. Constitutive Expression of the α4 Integrin Correlates with Tumorigenicity and Lymph Node Metastasis of the B16 Murine Melanoma. Neoplasia 2010, 12, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Ammar, A.; Mohammed, R.A.; Salmi, M.; Pepper, M.; Paish, E.C.; Ellis, I.O.; Martin, S.G. Lymphatic expression of CLEVER-1 in breast cancer and its relationship with lymph node metastasis. Anal. Cell. Pathol. 2011, 34, 67–78. [Google Scholar] [CrossRef]
- Clasper, S.; Royston, D.; Baban, D.; Cao, Y.; Ewers, S.; Butz, S.; Vestweber, D.; Jackson, D.G. A Novel Gene Expression Profile in Lymphatics Associated with Tumor Growth and Nodal Metastasis. Cancer Res. 2008, 68, 7293–7303. [Google Scholar] [CrossRef] [Green Version]
- Cuesta-Mateos, C.; Brown, J.R.; Terrón, F.; Muñoz-Calleja, C. Of Lymph Nodes and CLL Cells: Deciphering the Role of CCR7 in the Pathogenesis of CLL and Understanding Its Potential as Therapeutic Target. Front. Immunol. 2021, 12, 662866. [Google Scholar] [CrossRef]
- Till, K.J.; Pettitt, A.R.; Slupsky, J.R. Expression of Functional Sphingosine-1 Phosphate Receptor-1 Is Reduced by B Cell Receptor Signaling and Increased by Inhibition of PI3 Kinase δ but Not SYK or BTK in Chronic Lymphocytic Leukemia Cells. J. Immunol. 2015, 194, 2439–2446. [Google Scholar] [CrossRef] [Green Version]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells: A New Method for the Immunomorphological and Molecular Characterization of Circulating Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Bagnall, J.S.; Byun, S.; Begum, S.; Miyamoto, D.T.; Hecht, V.C.; Maheswaran, S.; Stott, S.L.; Toner, M.; Hynes, R.O.; Manalis, S.R. Deformability of Tumor Cells versus Blood Cells. Sci. Rep. 2015, 5, 18542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmy-Susini, B.; Avraamides, C.J.; Schmid, M.C.; Foubert, P.; Ellies, L.G.; Barnes, L.; Feral, C.; Papayannopoulou, T.; Lowy, A.; Blair, S.L.; et al. Integrin α4β1 Signaling Is Required for Lymphangiogenesis and Tumor Metastasis. Cancer Res. 2010, 70, 3042–3051. [Google Scholar] [CrossRef] [Green Version]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, C.; Correale, C.; Gandelli, A.; Spinelli, A.; Dejana, E.; D’Alessio, S.; Danese, S. Vascular Endothelial Growth Factor C Disrupts the Endothelial Lymphatic Barrier to Promote Colorectal Cancer Invasion. Gastroenterology 2015, 148, 1438–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Y.; Lai, Y.-S.; Chu, P.-Y.; Chan, S.-H.; Wang, L.-H.; Hung, W.-C. Cancer-Derived VEGF-C Increases Chemokine Production in Lymphatic Endothelial Cells to Promote CXCR2-Dependent Cancer Invasion and MDSC Recruitment. Cancers 2019, 11, 1120. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; He, P.; Liu, Y.; He, Y.; Du, Y.; Wu, M.; Zhang, G.; Yang, C.; Gao, F. Hyaluroan-regulated lymphatic permeability through S1P receptors is crucial for cancer metastasis. Med. Oncol. 2015, 32, 381. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Rigby, D.A.; Ferguson, D.J.; Johnson, L.A.; Jackson, D.G. Neutrophils rapidly transit inflamed lymphatic vessel endothelium via integrin-dependent proteolysis and lipoxin-induced junctional retraction. J. Leukoc. Biol. 2015, 98, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Ohuchida, K.; Yonenaga, A.; Sagara, A.; Ando, Y.; Kibe, S.; Takesue, S.; Abe, T.; Endo, S.; Koikawa, K.; et al. S100P regulates the collective invasion of pancreatic cancer cells into the lymphatic endothelial monolayer. Int. J. Oncol. 2019, 55, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Güç, E.; Pollard, J.W. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021, 54, 885–902. [Google Scholar] [CrossRef]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019, 27, 1967–1978. [Google Scholar] [CrossRef] [Green Version]
- Irshad, S.; Flores-Borja, F.; Lawler, K.; Monypenny, J.; Evans, R.; Male, V.; Gordon, P.; Cheung, A.; Gazinska, P.; Noor, F.; et al. RORγt+ Innate Lymphoid Cells Promote Lymph Node Metastasis of Breast Cancers. Cancer Res. 2017, 77, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef]
- Mezawa, Y.; Orimo, A. Phenotypic heterogeneity, stability and plasticity in tumor-promoting carcinoma-associated fibroblasts. FEBS J. 2021. in print. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tu, G.; Liu, Z.; Liu, M. Cancer-associated fibroblasts: A multifaceted driver of breast cancer progression. Cancer Lett. 2015, 361, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.P.; Duncan, L.M.; Kraft, S. Lymphatic invasion and angiotropism in primary cutaneous melanoma. Lab. Investig. 2017, 97, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.L.; Teerenstra, S.; De Wilt, J.H.; Cunningham, C.; Nagtegaal, I.D. Predicting lymph node metastasis in pT1 colorectal cancer: A systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013, 45, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasgow, S.C.; Bleier, J.I.; Burgart, L.J.; Finne, C.O.; Lowry, A.C. Meta-analysis of Histopathological Features of Primary Colorectal Cancers that Predict Lymph Node Metastases. J. Gastrointest. Surg. 2012, 16, 1019–1028. [Google Scholar] [CrossRef]
- Pastushenko, I.; Vermeulen, P.B.; Carapeto, F.; Van den Eynden, G.; Rutten, A.; Ara, M.; Dirix, L.Y.; Van Laere, S. Blood microvessel density, lymphatic microvessel density and lymphatic invasion in predicting melanoma metastases: Systematic review and meta-analysis. Br. J. Dermatol. 2014, 170, 66–77. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Gong, M.; Wen, L.; Liao, C.; Zou, L. High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer 2017, 17, 335. [Google Scholar] [CrossRef] [Green Version]
- Schoppmann, S.F.; Bayer, G.; Aumayr, K.; Taucher, S.; Geleff, S.; Rudas, M.; Kubista, E.; Hausmaninger, H.; Samonigg, H.; Gnant, M.; et al. Prognostic Value of Lymphangiogenesis and Lymphovascular Invasion in Invasive Breast Cancer. Ann. Surg. 2004, 240, 306–312. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Martin, S.G.; Gill, M.S.; Green, A.R.; Paish, E.C.; Ellis, I.O. Improved Methods of Detection of Lymphovascular Invasion Demonstrate That It is the Predominant Method of Vascular Invasion in Breast Cancer and has Important Clinical Consequences. Am. J. Surg. Pathol. 2007, 31, 1825–1833. [Google Scholar] [CrossRef]
- El-Gohary, Y.M.; Metwally, G.; Saad, R.S.; Robinson, M.J.; Mesko, T.; Poppiti, R.J. Prognostic Significance of Intratumoral and Peritumoral Lymphatic Density and Blood Vessel Density in Invasive Breast Carcinomas. Am. J. Clin. Pathol. 2008, 129, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-C.; Ni, X.-J.; Li, Y.; Dai, M.; Yuan, Z.-X.; Zhu, Y.-Y.; Luo, C.-Y. Peritumoral lymphangiogenesis induced by vascular endothelial growth factor C and D promotes lymph node metastasis in breast cancer patients. World J. Surg. Oncol. 2012, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Van der Schaft, D.W.; Pauwels, P.; Hulsmans, S.; Zimmermann, M.; van de Poll-Franse, L.V.; Griffioen, A.W. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett. 2007, 254, 128–136. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Ellis, I.O.; Elsheikh, S.; Paish, E.C.; Martin, S.G. Lymphatic and angiogenic characteristics in breast cancer: Morphometric analysis and prognostic implications. Breast Cancer Res. Treat. 2009, 113, 261–273. [Google Scholar] [CrossRef]
- Tateishi, Y.; Nakanishi, Y.; Taniguchi, H.; Shimoda, T.; Umemura, S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod. Pathol. 2010, 23, 1068–1072. [Google Scholar] [CrossRef]
- Akagi, Y.; Adachi, Y.; Ohchi, T.; Kinugasa, T.; Shirouzu, K. Prognostic impact of lymphatic invasion of colorectal cancer: A single-center analysis of 1,616 patients over 24 years. Anticancer Res. 2013, 33, 2965–2970. [Google Scholar] [PubMed]
- Nishida, T.; Egashira, Y.; Akutagawa, H.; Fujii, M.; Uchiyama, K.; Shibayama, Y.; Hirose, Y. Predictors of Lymph Node Metastasis in T1 Colorectal Carcinoma: An immunophenotypic analysis of 265 patients. Dis. Colon Rectum 2014, 57, 905–915. [Google Scholar] [CrossRef]
- Lee, Y.J.; Huh, J.W.; Shin, J.K.; Park, Y.A.; Cho, Y.B.; Kim, H.C.; Yun, S.H.; Lee, W.Y. Risk factors for lymph node metastasis in early colon cancer. Int. J. Color. Dis. 2020, 35, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Schlemmer, A.; Rehak, P.; Vieth, M.; Hoefler, G.; Langner, C. Intramural and extramural vascular invasion in colorectal cancer: Prognostic significance and quality of pathology reporting. Cancer 2012, 118, 628–638. [Google Scholar] [CrossRef]
- Iida, S.; Hasegawa, H.; Okabayashi, K.; Moritani, K.; Mukai, M.; Kitagawa, Y. Risk Factors for Postoperative Recurrence in Patients with Pathologically T1 Colorectal Cancer. World J. Surg. 2012, 36, 424–430. [Google Scholar] [CrossRef]
- Tokodai, K.; Narimatsu, H.; Nishida, A.; Takaya, K.; Hara, Y.; Kawagishi, N.; Hashizume, E.; Ohuchi, N. Risk factors for recurrence in stage II/III colorectal cancer patients treated with curative surgery: The impact of postoperative tumor markers and an infiltrative growth pattern. J. Surg. Oncol. 2016, 114, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Leijssen, L.G.J.; Dinaux, A.M.; Kinutake, H.; Bordeianou, L.G.; Berger, D.L. Do Stage I Colorectal Cancers with Lymphatic Invasion Require a Different Postoperative Approach? J. Gastrointest. Surg. 2019, 23, 1884–1892. [Google Scholar] [CrossRef]
- De Leon, M.L.; Schoetz, D.J., Jr.; Coller, J.A.; Veidenheimer, M.C. Colorectal cancer: Lahey Clinic experience, 1972−1976. An analysis of prognostic indicators. Dis. Colon Rectum 1987, 30, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Borda, F.; Jimenez, F.J.; Martinez-Peñuela, J.M.; Larrinaga, B. Multivariate analysis of prognostic factors in resected colorecta I cancer: A new prognostic index. Eur. J. Gastroenterol. Hepatol. 1998, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Takanami, I. Lymphatic microvessel density using D2-40 is associated with nodal metastasis in non-small cell lung cancer. Oncol. Rep. 2006, 15, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, Y.; Nakamura, H.; Kitamura, Y.; Taniguchi, Y.; Araki, K.; Shomori, K.; Horie, Y.; Kurozawa, Y.; Ito, H.; Hayashi, K. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol. Int. 2007, 57, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, D.J.; Harpole, D.H., Jr.; Godleski, J.; Herndon, J.E., 2nd; Shieh, D.B.; Richards, W.; Blanco, R.; Xu, H.J.; Strauss, G.M.; Sugarbaker, D.J. Molecular pathologic substaging in 244 stage I non-small-cell lung cancer patients: Clinical implications. J. Clin. Oncol. 1998, 16, 2468–2477. [Google Scholar] [CrossRef]
- Kato, T.; Ishikawa, K.; Aragaki, M.; Sato, M.; Okamoto, K.; Ishibashi, T.; Kaji, M. Angiolymphatic invasion exerts a strong impact on surgical outcomes for stage I lung adenocarcinoma, but not non-adenocarcinoma. Lung Cancer 2012, 77, 394–400. [Google Scholar] [CrossRef]
- Al-Alao, B.S.; Gately, K.; Nicholson, S.; McGovern, E.; Young, V.K.; O’Byrne, K.J. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc. Thorac. Ann. 2014, 22, 55–64. [Google Scholar] [CrossRef]
- Matsuura, N.; Go, T.; Fujiwara, A.; Nakano, T.; Nakashima, N.; Tarumi, S.; Chang, S.S.; Yokomise, H. Lymphatic invasion is a cause of local recurrence after wedge resection of primary lung cancer. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Hanagiri, T.; Takenaka, M.; Oka, S.; Shigematsu, Y.; Nagata, Y.; Shimokawa, H.; Uramoto, H.; Yamada, S.; Tanaka, F. Prognostic Significance of Lymphovascular Invasion for Patients with Stage I Non-Small Cell Lung Cancer. Eur. Surg. Res. 2011, 47, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.F.; Bohn, B.A.; Uzunoglu, F.G.; Reeh, M.; Quaas, A.; Grob, T.J.; Perez, D.; Kutup, A.; Bockhorn, M.; Izbicki, J.R.; et al. Lymphatic invasion predicts survival in patients with early node-negative non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2013, 146, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Masuda, R.; Kijima, H.; Nito, M.; Wada, A.; Matsuzaki, T.; Ikoma, Y.; Nakazato, K.; Masuda, D.; Tanaka, M.; Kobayashi, H.; et al. Lymphatic invasion is a significant indicator of poor patient prognosis in lung squamous cell carcinoma. Mol. Med. Rep. 2017, 15, 2067–2073. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Pan, J.; Li, X.Q.; Wang, X.Y.; Tang, C.; Xuan, M. Intratumoral lymphangiogenesis in oral squamous cell carcinoma and its clinicopathological significance. J. Oral Pathol. Med. 2008, 37, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Min, J.-Y.; So, Y.K.; Ko, Y.-H.; Jeong, H.-S.; Son, Y.-I.; Baek, C.H. Correlation between lymphatic vessel density and regional metastasis in squamous cell carcinoma of the tongue. Head Neck 2010, 32, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Kao, H.-K.; Hsu, C.-L.; Huang, J.-J.; Lee, L.-Y.; Huang, Y.; Browne, T.; Tsang, N.-M.; Chang, Y.-L.; Chang, K.-P. Evaluation of Lymphatic and Vascular Invasion in Relation to Clinicopathological Factors and Treatment Outcome in Oral Cavity Squamous Cell Carcinoma. Medicine 2015, 94, e1510. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kubota, A.; Yokose, T.; Furukawa, M.; Matsushita, T.; Oridate, N. Association between pathological invasion patterns and late lymph node metastases in patients with surgically treated clinical No early oral tongue carcinoma. Head Neck 2020, 42, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.N.; Elkins, T.; Roberts, D.; Byers, R.M. Squamous cell carcinoma of the tongue in young adults: Increasing incidence and factors that predict treatment outcomes. Otolaryngol. Head Neck Surg. 2000, 122, 44–51. [Google Scholar] [CrossRef]
- Mochiki, M.; Sugasawa, M.; Nibu, K.; Asai, M.; Nakao, K.; Asakage, T. Prognostic factors for hypopharyngeal cancer: A univariate and multivariate study of 142 cases. Acta Oto Laryngol. 2007, 127, 136–144. [Google Scholar] [CrossRef]
- Casal, D.; Carmo, L.; Melancia, T.; Zagalo, C.; Cid, O.; Rosa-Santos, J. Lip cancer: A 5-year review in a tertiary referral centre. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 2040–2045. [Google Scholar] [CrossRef]
- Bertolli, E.; de Macedo, M.P.; Pinto, C.A.; Damascena, A.S.; Molina, A.S.; Ueno, P.S.; Duprat Neto, J.P. Evaluation of Melanoma Features and Their Relationship with Nodal Disease: The Importance of the Pathological Report. Tumori J. 2015, 101, 501–505. [Google Scholar] [CrossRef]
- Donizy, P.; Kaczorowski, M.; Halon, A.; Leskiewicz, M.; Matkowski, R. Lymphangioinvasion in routine H&E staining is strongly associated with poor clinical outcome in lymph node-negative cutaneous melanoma patients. Folia Histochem. Cytobiol. 2016, 54, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, A.P.; Mochel, M.C.; Muzikansky, A.; Duncan, L.M.; Kraft, S. Lymphatic invasion predicts sentinel lymph node metastasis and adverse outcome in primary cutaneous melanoma. J. Cutan. Pathol. 2017, 44, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Lee, W.J. Lymphatic invasion in acral and non-acral melanoma: A comparative, clinicoprognostic study of primary cutaneous melanoma according to tumour site. Pathology 2020, 52, 670–675. [Google Scholar] [CrossRef]
- Xu, X.; Gimotty, P.A.; Guerry, D.; Karakousis, G.; Van Belle, P.; Liang, H.; Montone, K.; Pasha, T.; Ming, M.E.; Acs, G.; et al. Lymphatic invasion revealed by multispectral imaging is common in primary melanomas and associates with prognosis. Hum. Pathol. 2008, 39, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statius Muller, M.G.; van Leeuwen, P.A.; de Lange-De Klerk, E.S.; van Diest, P.J.; Pijpers, R.; Ferwerda, C.C.; Vuylsteke, R.J.; Meijer, S. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with Stage I or II cutaneous melanoma. Cancer 2001, 91, 2401–2408. [Google Scholar] [CrossRef]
- Vuylsteke, R.J.; van Leeuwen, P.A.; Muller, M.G.; Gietema, H.A.; Kragt, D.R.; Meijer, S. Clinical Outcome of Stage I/II Melanoma Patients After Selective Sentinel Lymph Node Dissection: Long-Term Follow-Up Results. J. Clin. Oncol. 2003, 21, 1057–1065. [Google Scholar] [CrossRef]
- Borgstein, P.J.; Meijer, S.; Van Diest, P.J. Are Locoregional Cutaneous Metastases in Melanoma Predictable? Ann. Surg. Oncol. 1999, 6, 315–321. [Google Scholar] [CrossRef]
- Merchant, N.B.; Guillem, J.G.; Paty, P.B.; Enker, W.E.; Minsky, B.D.; Quan, S.H.; Wong, D.; Cohen, A.M. T3N0 rectal cancer: Results following sharp mesorectal excision and no adjuvant therapy. J. Gastrointest. Surg. 1999, 3, 642–647. [Google Scholar] [CrossRef]
- Fujita, S.; Yamamoto, S.; Akasu, T.; Moriya, Y. Outcome of patients with clinical stage II or III rectal cancer treated without adjuvant radiotherapy. Int. J. Color. Dis. 2008, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.-W.; Lehmann, P.; et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 757–767. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 2006, 213, 146–158. [Google Scholar] [CrossRef]
- Cochran, A.J.; Huang, R.-R.; Lee, J.; Itakura, E.; Leong, S.P.; Essner, R. Tumour–induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006, 6, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Molodtsov, A.K.; Khatwani, N.; Vella, J.L.; Lewis, K.A.; Zhao, Y.; Han, J.; Sullivan, D.E.; Searles, T.G.; Preiss, N.K.; Shabaneh, T.B.; et al. Resident memory CD8+ T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity 2021, 54, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.R.; Kedrin, D.; Seano, G.; Gautier, O.; Meijer, E.F.J.; Jones, D.; Chin, S.-M.; Kitahara, S.; Bouta, E.M.; Chang, J.; et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018, 359, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Hognas, G.; Annala, M.; et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef]
- Hong, M.K.; Macintyre, G.; Wedge, D.C.; Van Loo, P.; Patel, K.; Lunke, S.; Alexandrov, L.B.; Sloggett, C.; Cmero, M.; Marass, F.; et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun. 2015, 6, 6605. [Google Scholar] [CrossRef] [PubMed]
- Naxerova, K.; Reiter, J.G.; Brachtel, E.; Lennerz, J.K.; Van De Wetering, M.; Rowan, A.; Cai, T.; Clevers, H.; Swanton, C.; Nowak, M.A.; et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017, 357, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, L.; Xu, T.; Xue, R.; Yu, L.; Zhu, Y.; Wu, Y.; Zhang, Q.; Li, D.; Shen, S.; et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 2020, 11, 1993. [Google Scholar] [CrossRef]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2021, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann. N. Y. Acad. Sci. 2021, 1499, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rajantie, I.; Pajusola, K.; Jeltsch, M.; Holopainen, T.; Ylä-Herttuala, S.; Harding, T.; Jooss, K.; Takahashi, T.; Alitalo, K. Vascular Endothelial Cell Growth Factor Receptor 3–Mediated Activation of Lymphatic Endothelium Is Crucial for Tumor Cell Entry and Spread via Lymphatic Vessels. Cancer Res. 2005, 65, 4739–4746. [Google Scholar] [CrossRef] [Green Version]
- Roberts, N.; Kloos, B.; Cassella, M.; Podgrabinska, S.; Persaud, K.; Wu, Y.; Pytowski, B.; Skobe, M. Inhibition of VEGFR-3 Activation with the Antagonistic Antibody More Potently Suppresses Lymph Node and Distant Metastases than Inactivation of VEGFR-2. Cancer Res. 2006, 66, 2650–2657. [Google Scholar] [CrossRef] [Green Version]
- Sennino, B.; Ishiguro-Oonuma, T.; Schriver, B.J.; Christensen, J.G.; McDonald, D.M. Inhibition of c-Met Reduces Lymphatic Metastasis in RIP-Tag2 Transgenic Mice. Cancer Res. 2013, 73, 3692–3703. [Google Scholar] [CrossRef] [Green Version]
- Gengenbacher, N.; Singhal, M.; Mogler, C.; Hai, L.; Milde, L.; Pari, A.A.A.; Besemfelder, E.; Fricke, C.; Baumann, D.; Gehrs, S.; et al. Timed Ang2-Targeted Therapy Identifies the Angiopoietin–Tie Pathway as Key Regulator of Fatal Lymphogenous Metastasis. Cancer Discov. 2021, 11, 424–445. [Google Scholar] [CrossRef]
- Kretschy, N.; Teichmann, M.; Kopf, S.; Atanasov, A.G.; Saiko, P.; Vonach, C.; Viola, K.; Giessrigl, B.; Huttary, N.; Raab, I.; et al. In vitro inhibition of breast cancer spheroid-induced lymphendothelial defects resembling intravasation into the lymphatic vasculature by acetohexamide, isoxsuprine, nifedipin and proadifen. Br. J. Cancer 2013, 108, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.H.; Brenner, S.; Huttary, N.; Li, Y.; Atanasov, A.G.; Dirsch, V.M.; Holzner, S.; Stadler, S.; Riha, J.; Krieger, S.; et al. 12(S)-HETE increases intracellular Ca2+ in lymph-endothelial cells disrupting their barrier function in vitro; stabilization by clinical drugs impairing calcium supply. Cancer Lett. 2016, 380, 174–183. [Google Scholar] [CrossRef] [PubMed]
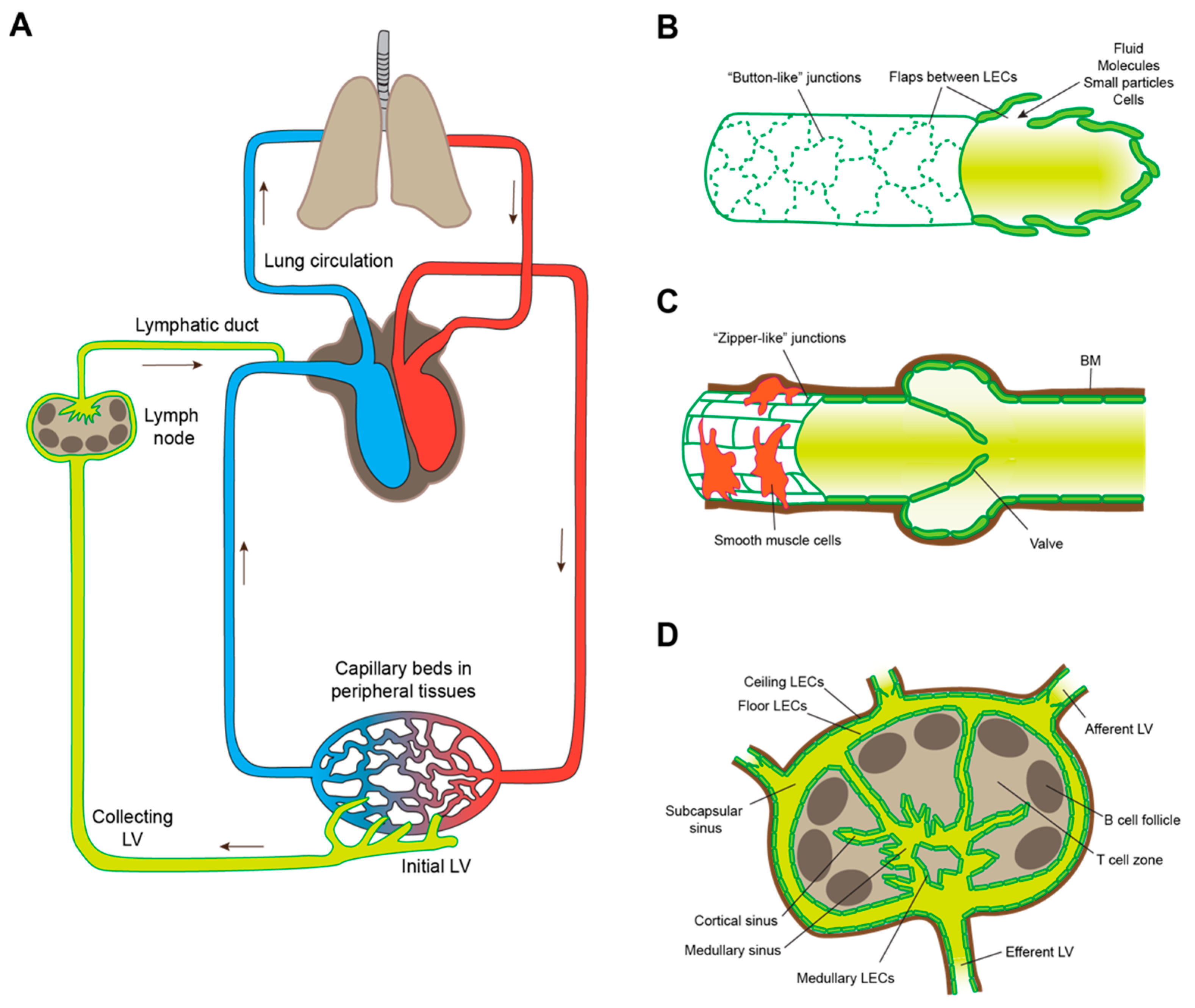

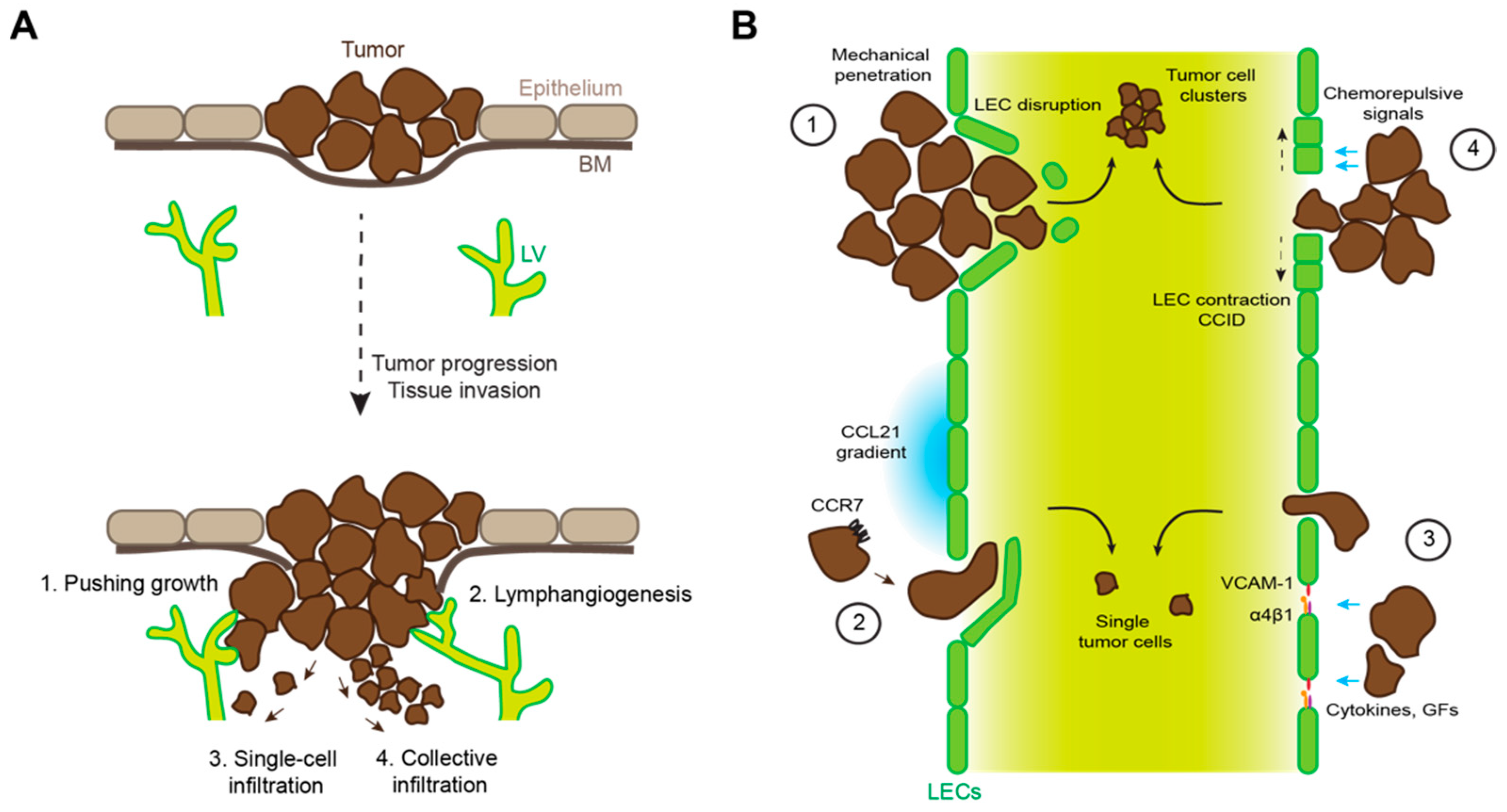
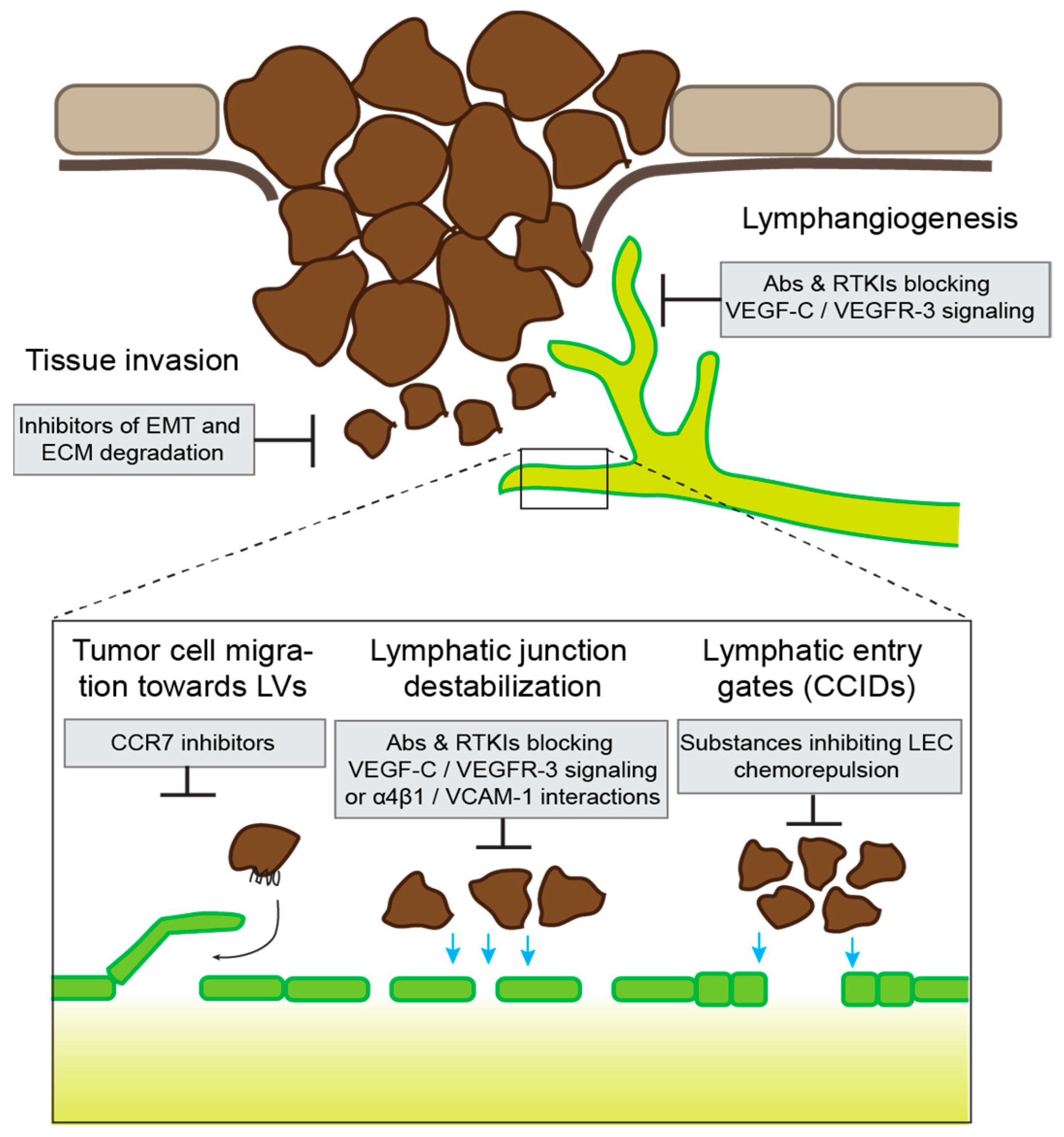
| Process | Role in LVI | Other Functions |
|---|---|---|
| EMT | Increased invasiveness and tissue infiltration of tumor cells. | Essential for ontogeny, wound healing, etc. |
| LEC-derived chemokine gradients. | Guidance of disseminating tumor cells towards/into initial lymphatic vessels. | Guidance of DCs and other immune cells towards/into initial lymphatic vessels. |
| Destabilization of lymphatic endothelial junctions. | Promotion of tumor trans-endothelial migration into the lymphatic lumen. | Unknown. Potentially involved in the regulation of lymphatic transport in inflammatory conditions. |
| Formation of entry gates (CCIDs) by LEC-repulsive signals. | Promotion of tumor cell cluster entry into lymphatic vessels. | Lymphatic entry of neutrophils in inflammatory conditions. |
| Cancer Type | LN Metastasis | Recurrence | Survival |
|---|---|---|---|
| Breast cancer | Schoppmann et al. [108] Mohammed et al. [109] El Gohary et al. [110] Zhao et al. [111] | Schoppmann et al. [108] Mohammed et al. [109] El Gohary et al. [110] Van der Schaft et al. [112] 1 | Schoppmann et al. [108] Mohammed et al. [109] El Gohary et al. [110] Mohammed et al. [113] |
| Colorectal cancer | Tateishi et al. [114] Akagi et al. [115] Nishida et al. [116] Lee et al. [117] | Betge et al. [118] Iida et al. [119] Tokodai et al. [120] Leijssen et al. [121] | De Leon et al. [122] Guerra et al. [123] Akagi et al. [115] Betge et al. [118] |
| Lung cancer | Takanami, [124] Adachi et al. [125] | Kwiatkowski et al. [126] Kato et al. [127] Al-Alao et al. [128] Matsuura et al. [129] | Hanagiri et al. [130] Nentwich et al. [131] Masuda et al. [132] |
| SCC 2 of head and neck | Zhao et al. [133] Chung et al. [134] Adel et al. [135] | Hori et al. [136] | Myers et al. [137] 3 Mochiki et al. [138] Adel et al. [135] Casai et al. [139] |
| Melanoma | Bertolli et al. [140] Donizy et al. [141] Moy et al. [142] Jung et al. [143] | Donizy et al. [141] Moy et al. [142] Xu et al. [144] Statius Muller et al. [145] | Donizy et al. [141] Xu et al. [144] Vuylsteke et al. [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, N.; Dieterich, L.C. Mechanisms and Clinical Significance of Tumor Lymphatic Invasion. Cells 2021, 10, 2585. https://doi.org/10.3390/cells10102585
Fujimoto N, Dieterich LC. Mechanisms and Clinical Significance of Tumor Lymphatic Invasion. Cells. 2021; 10(10):2585. https://doi.org/10.3390/cells10102585
Chicago/Turabian StyleFujimoto, Noriki, and Lothar C. Dieterich. 2021. "Mechanisms and Clinical Significance of Tumor Lymphatic Invasion" Cells 10, no. 10: 2585. https://doi.org/10.3390/cells10102585
APA StyleFujimoto, N., & Dieterich, L. C. (2021). Mechanisms and Clinical Significance of Tumor Lymphatic Invasion. Cells, 10(10), 2585. https://doi.org/10.3390/cells10102585






