CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and the Preparation of Conditioned Medium (CM)
2.2. Preparation of Apoptotic Cells
2.3. MP Preparation and Flow Cytometry Analysis
2.4. Assess the Phagocytic Engulfment of Apoptotic Cells by Flowcytometric Analysis
2.5. Flow Cytometry Analysis of MFG-E8 Expression on NR8383 Cells
2.6. Measurement of MFG-E8
2.7. Statistical Analysis
3. Results
3.1. Apoptotic Cell-Derived MP Have Significant Pro-Phagocytic Activity on NR8383 Cells
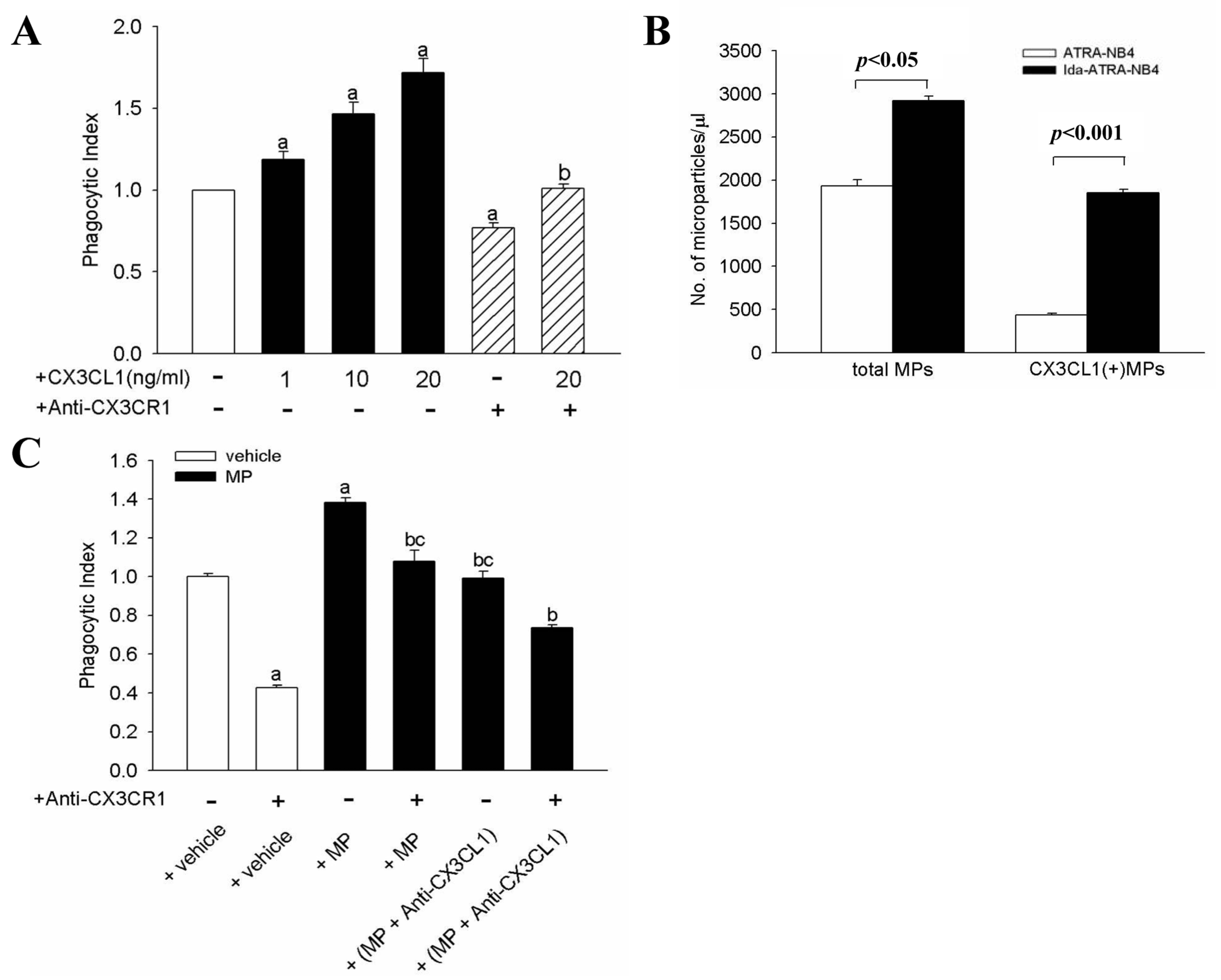
3.2. Apoptotic Cell-Derived CX3CL1(+) MP Enhance Phagocytic Activity of NR8383 Cells
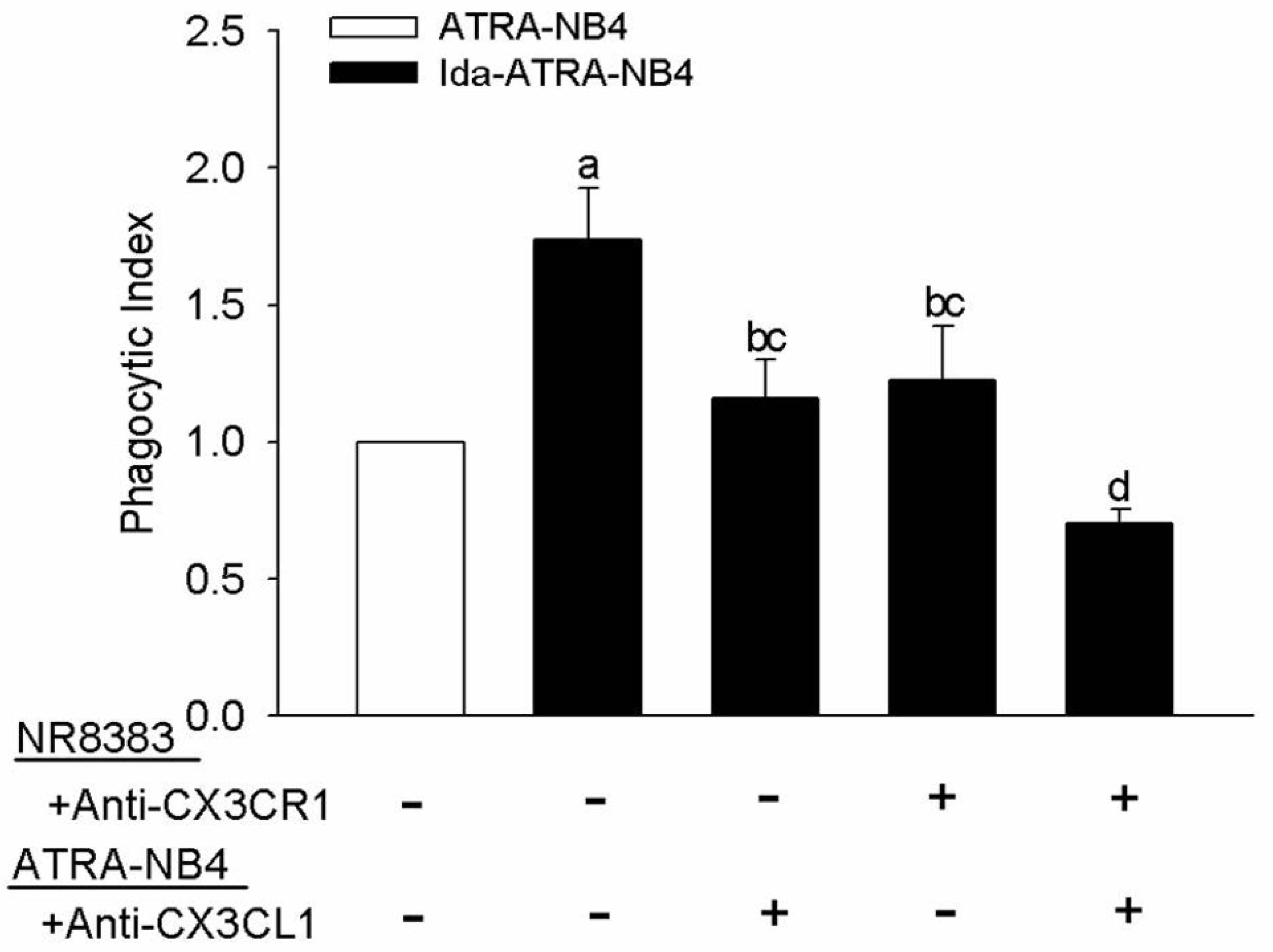
3.3. Surface CX3CL1 on Ida-ATRA-NB4 Cells Enhances NR8383 Cells’ Phagocytic Activity
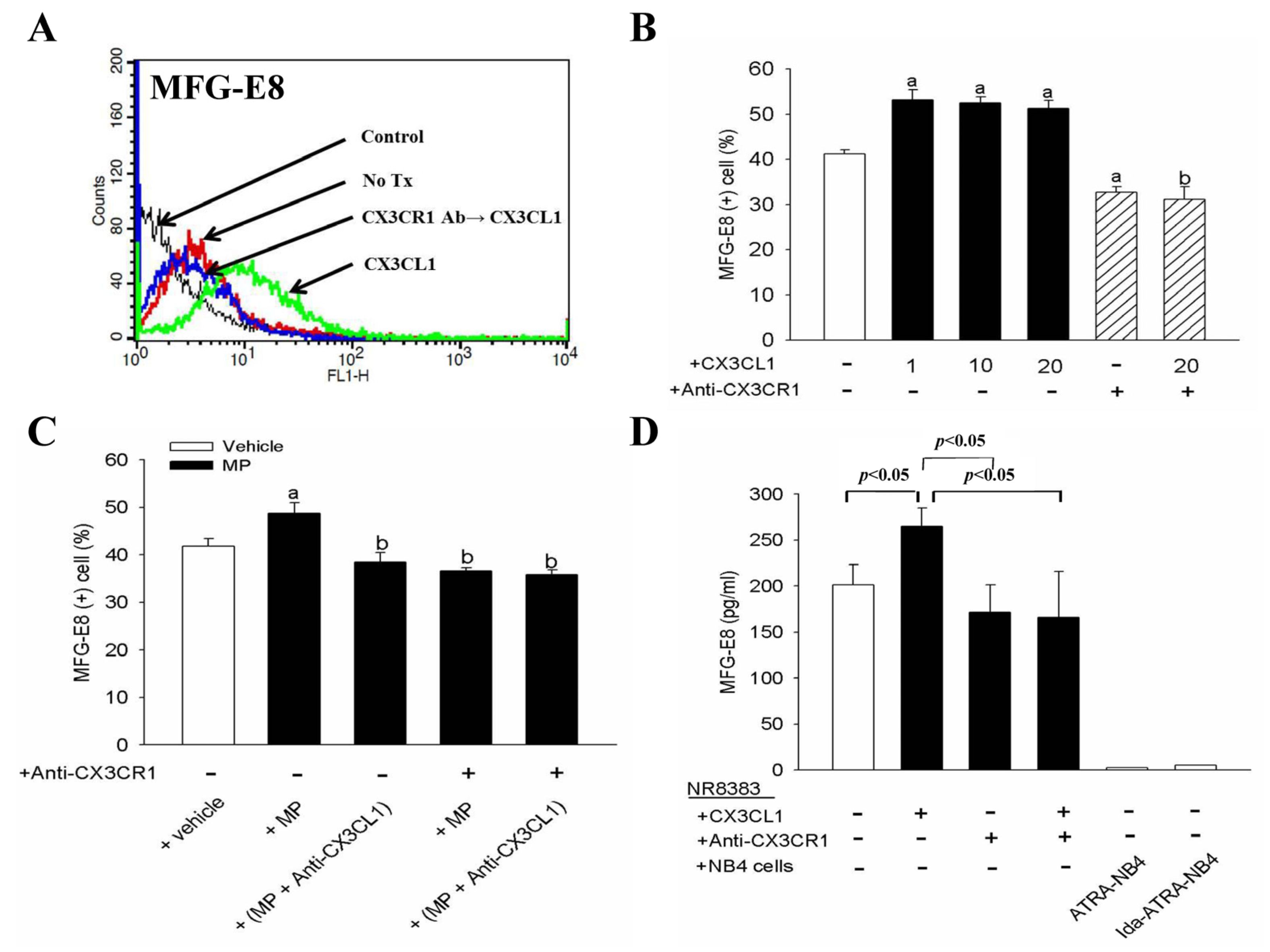
3.4. CX3CL1 and Apoptotic Cell-Derived CX3CL1(+) MP Enhances NR8383 Cells in Surface Expression and Release of MFG-E8
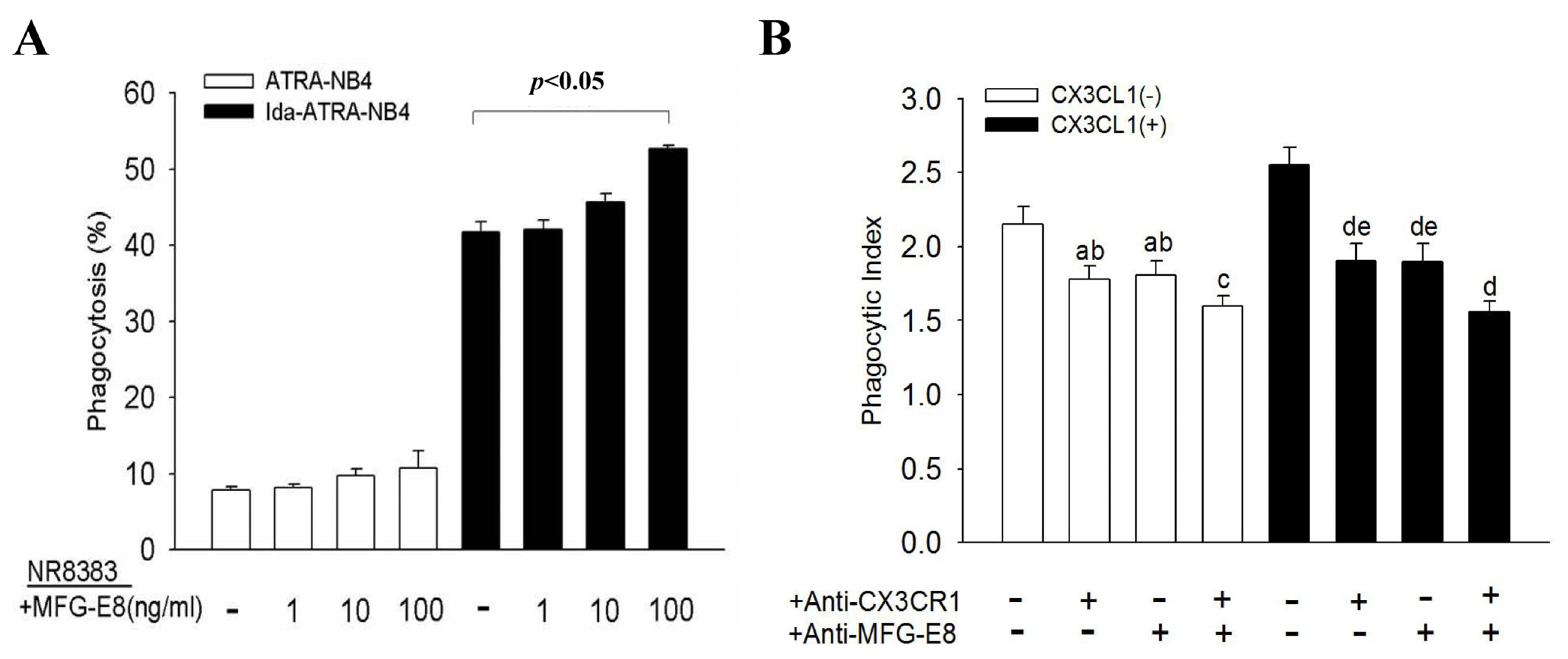
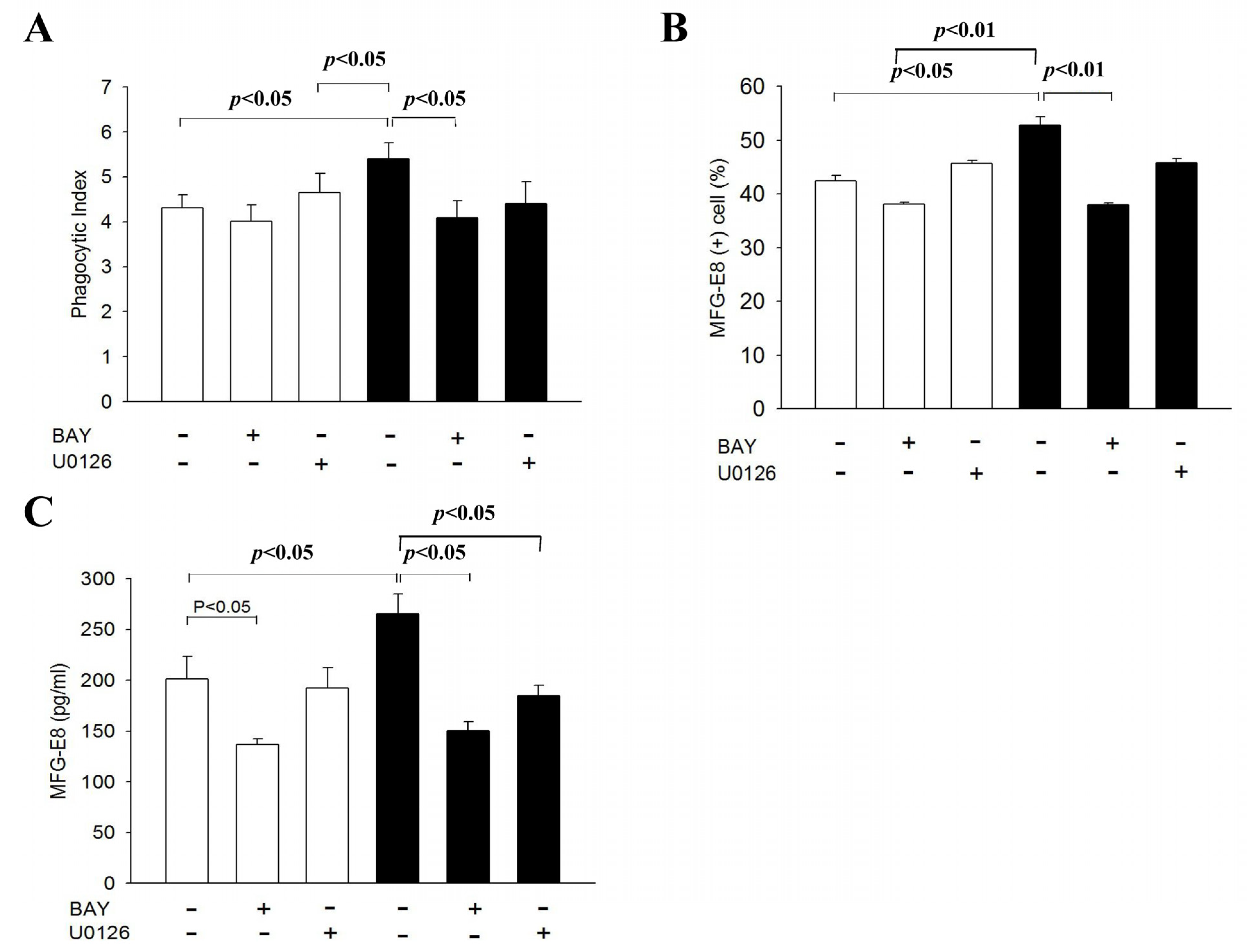
3.5. MFG-E8 Contributes to the CX3CL1-Enhanced Phagocytic Activity of NR8383 Cells
3.6. CX3CL1 Promotes Phagocytic Activity of NR8383 Cells via the NF-Κb Signal Transduction Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankel, S.R.; Eardley, A.; Lauwers, G.; Weiss, M.; Warrell, R.P., Jr. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann. Intern. Med. 1992, 117, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, P.; Bergua, J.M.; Vellenga, E.; Rayón, C.; Parody, R.; de la Serna, J.; León, A.; Esteve, J.; Milone, G.; Debén, G.; et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: Characteristics, outcome, and prognostic factors. Blood 2009, 113, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.H.; Soignet, S.L.; Chanel, S.; Ho, R.; Heller, G.; Scheinberg, D.A.; Ellison, R.; Warrell, R.P., Jr. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J. Clin. Oncol. 2000, 18, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Chale, R.S.; Abad, A.C.; Schally, A.V. Acute promyelocytic leukemia (APL): A review of the literature. Oncotarget 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Luesink, M.; Jansen, J.H. Advances in understanding the pulmonary infiltration in acute promyelocytic leukaemia. Br. J. Haematol. 2010, 151, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Schlageter, M.H.; de Gentile, A.; Guidez, F.; Balitrand, N.; Toubert, M.E.; Krawice, I.; Fenaux, P.; Castaigne, S.; Najean, Y.; et al. Hematopoietic growth factor expression and ATRA sensitivity in acute promyelocytic blast cells. Blood 1994, 83, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Montesinos, P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood 2014, 123, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Seale, J.; Delva, L.; Renesto, P.; Balitrand, N.; Dombret, H.; Scrobohaci, M.L.; Degos, L.; Paul, P.; Chomienne, C. All-trans retinoic acid rapidly decreases cathepsin G synthesis and mRNA expression in acute promyelocytic leukemia. Leukemia 1996, 10, 95–101. [Google Scholar] [PubMed]
- Marchetti, M.; Falanga, A.; Giovanelli, S.; Oldani, E.; Barbui, T. All-trans-retinoic acid increases adhesion to endothelium of the human promyelocytic leukaemia cell line NB4. Br. J. Haematol. 1996, 93, 360–366. [Google Scholar] [CrossRef]
- Robb, C.; Regan, K.; Dorward, D.; Rossi, A. Key Mechanisms Governing Resolution of Lung Inflammation, Seminars in Immunopathology. Semin Immunopathol. 2016, 38, 425–448. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153, S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, I.-S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp. Mol. Med. 2017, 49, e331. [Google Scholar] [CrossRef]
- McCracken, J.M.; Allen, L.-A.H. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; D’acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of in flammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Bezema, T.; Calder, P.C.; Charloux, A.; Frossard, N.; Garssen, J.; Haworth, O.; Dilevskaya, K.; Levi-Schaffer, F.; Lonsdorfer, E. Activation of resolution pathways to prevent and fight chronic inflammation: Lessons from asthma and inflammatory bowel disease. Front. Immunol. 2019, 10, 1699. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef]
- Doran, A.C.; Yurdagul, A.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Mitroulis, I.; von Renesse, J.; Hajishengallis, G.; Chavakis, T. From leukocyte recruitment to resolution of inflammation: The cardinal role of integrins. J. Leukoc. Biol. 2017, 102, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX 3 C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-H.; Shih, C.-H.; Feng, S.-Y.; Li, I.-T.; Chang, S.-C.; Lin, Y.-C.; Hsu, H.-C. CX3CL1 (+) microparticles mediate the chemoattraction of alveolar macrophages toward apoptotic acute promyelocytic leukemic cells. Cell. Physiol. Biochem. 2014, 33, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-H.; Shih, C.-H.; Feng, S.-Y.; Chang, S.-C.; Lin, Y.-C.; Hsu, H.-C. Role of CX3CL1 in the chemotactic migration of all-trans retinoic acid-treated acute promyelocytic leukemic cells toward apoptotic cells. J. Chin. Med. Assoc. 2014, 77, 367–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanotte, M.; Martin-Thouvenin, V.; Najman, S.; Balerini, P.; Valensi, F.; Berger, R. NB4, a maturation inducible cell line with t (15; 17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77, 1080–1086. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Wang, S.; Guo, X.; Shi, P.; Wang, W.; Xu, B. Low-dose triptolide in combination with idarubicin induces apoptosis in AML leukemic stem-like KG1 a cell line by modulation of the intrinsic and extrinsic factors. Cell Death Dis. 2013, 4, e948. [Google Scholar] [CrossRef] [PubMed]
- Hasper, H.; Weghorst, R.; Richel, D.; Meerwaldt, J.; Olthuis, F.; Schenkeveld, C. A new four-color flow cytometric assay to detect apoptosis in lymphocyte subsets of cultured peripheral blood cells. Cytom. J. Int. Soc. Anal. Cytol. 2000, 40, 167–171. [Google Scholar] [CrossRef]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.-C. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chien, H.Y.; Shih, C.H.; Lai, S.L.; Li, I.T.; Hsu, S.C.; Kou, Y.R.; Hsu, H.C. Annexin A1 mediates the anti-inflammatory effects during the granulocytic differentiation process in all-trans retinoic acid-treated acute promyelocytic leukemic cells. J. Cell. Physiol. 2012, 227, 3661–3669. [Google Scholar] [CrossRef]
- Tsai, W.H.; Shih, C.H.; Feng, S.Y.; Li, I.T.; Chang, S.C.; Lin, Y.C.; Hsu, H.C. CX3CL1(+) Microparticles Mediate the Chemoattraction of Alveolar Macrophages toward Apoptotic Acute Promyelocytic Leukemic Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 594–604. [Google Scholar] [CrossRef]
- Elliott, M.R.; Ravichandran, K.S. The dynamics of apoptotic cell clearance. Dev. Cell 2016, 38, 147–160. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Akakura, S.; Singh, S.; Spataro, M.; Akakura, R.; Kim, J.-I.; Albert, M.L.; Birge, R.B. The opsonin MFG-E8 is a ligand for the αvβ5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. cell Res. 2004, 292, 403–416. [Google Scholar] [CrossRef]
- Leonardi-Essmann, F.; Emig, M.; Kitamura, Y.; Spanagel, R.; Gebicke-Haerter, P.J. Fractalkine-upregulated milk-fat globule EGF factor−8 protein in cultured rat microglia. J. Neuroimmunol. 2005, 160, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.D.; Van Eldik, L.J. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 2008, 3, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Amin, D.; Wu, R.; Dong, W.; Ravikumar, T.S.; Wang, P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol. Med. 2007, 13, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miyasaka, K.; Aozasa, K.; Koike, M.; Uchiyama, Y.; Nagata, S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Miwa, M.; Miwa, K.; Hanayama, R.; Nagase, H.; Nagata, S.; Tanaka, M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 2004, 200, 459–467. [Google Scholar] [CrossRef]
- Arur, S.; Uche, U.E.; Rezaul, K.; Fong, M.; Scranton, V.; Cowan, A.E.; Mohler, W.; Han, D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 2003, 4, 587–598. [Google Scholar] [CrossRef]
- Sheridan, G.K.; Murphy, K.J. Neuron–glia crosstalk in health and disease: Fractalkine and CX3CR1 take centre stage. Open Biol. 2013, 3, 130181. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-M.; Pan, L.; Wang, Y.; Xu, Q.-Z. Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-κB pathway in CX3CL1-knockout mice. Int. J. Mol. Med. 2016, 37, 703–715. [Google Scholar] [CrossRef]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Miller, R.J. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. USA 2000, 97, 8075–8080. [Google Scholar] [CrossRef]
- Moon, S.O.; Kim, W.; Sung, M.J.; Lee, S.; Kang, K.P.; Kim, D.H.; Lee, S.Y.; So, J.N.; Park, S.K. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol. Pharmacol. 2006, 70, 112–119. [Google Scholar] [CrossRef]
- Garcia, G.E.; Xia, Y.; Chen, S.; Wang, Y.; Ye, R.D.; Harrison, J.K.; Bacon, K.B.; Zerwes, H.G.; Feng, L. NF-κB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL−1β, TNF-α, and LPS. J. Leukoc. Biol. 2000, 67, 577–584. [Google Scholar] [CrossRef]
- Aziz, M.M.; Ishihara, S.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kadowaki, Y.; Rumi, M.A.K.; Amano, Y.; Kinoshita, Y. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent αvβ3 integrin signaling. J. Immunol. 2009, 182, 7222–7232. [Google Scholar] [CrossRef]
- Yi, Y.-S. Functional role of milk fat globule-epidermal growth factor VIII in macrophage-mediated inflammatory responses and inflammatory/autoimmune diseases. Mediat. Inflamm. 2016, 2016, 5628486. [Google Scholar] [CrossRef]
- Miksa, M.; Amin, D.; Wu, R.; Jacob, A.; Zhou, M.; Dong, W.; Yang, W.-L.; Ravikumar, T.S.; Wang, P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int. J. Mol. Med. 2008, 22, 743–748. [Google Scholar]
- Mohning, M.P.; Thomas, S.M.; Barthel, L.; Mould, K.J.; McCubbrey, A.L.; Frasch, S.C.; Bratton, D.L.; Henson, P.M.; Janssen, W.J. Phagocytosis of microparticles by alveolar macrophages during acute lung injury requires MerTK. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 314, L69–L82. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Wilson, M.R.; O’Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Reutershan, J.; Basit, A.; Galkina, E.V.; Ley, K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L807–L815. [Google Scholar] [CrossRef]
- Han, C.Z.; Juncadella, I.J.; Kinchen, J.M.; Buckley, M.W.; Klibanov, A.L.; Dryden, K.; Onengut-Gumuscu, S.; Erdbrügger, U.; Turner, S.D.; Shim, Y.M. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 2016, 539, 570–574. [Google Scholar] [CrossRef]
- Freeman, S.A.; Grinstein, S. Phagocytosis: How macrophages tune their non-professional counterparts. Curr. Biol. 2016, 26, R1279–R1282. [Google Scholar] [CrossRef]
- Stokes, C.A.; Kaur, R.; Edwards, M.R.; Mondhe, M.; Robinson, D.; Prestwich, E.C.; Hume, R.D.; Marshall, C.A.; Perrie, Y.; O’Donnell, V.B. Human rhinovirus-induced inflammatory responses are inhibited by phosphatidylserine containing liposomes. Mucosal Immunol. 2016, 9, 1303–1316. [Google Scholar] [CrossRef]
- Miksa, M.; Wu, R.; Dong, W.; Das, P.; Yang, D.; Wang, P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock 2006, 25, 586–593. [Google Scholar] [CrossRef]

| Idarubicin (nM) | * Early Apoptosis (%) | * Late Apoptosis (%) |
|---|---|---|
| 0 | 11.0 ± 0.6 | 9.1 ± 0.3 |
| 5 | 21.0 ± 1.0 | 7.6 ± 1.5 |
| 50 | 45.8 ± 0.4 | 6.3 ± 0.4 |
| p value ** | p < 0.001 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-H.; Chang, S.-C.; Lin, Y.-C.; Hsu, H.-C. CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages. Cells 2021, 10, 2583. https://doi.org/10.3390/cells10102583
Tsai W-H, Chang S-C, Lin Y-C, Hsu H-C. CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages. Cells. 2021; 10(10):2583. https://doi.org/10.3390/cells10102583
Chicago/Turabian StyleTsai, Wen-Hui, Shao-Chi Chang, Yu-Chieh Lin, and Hui-Chi Hsu. 2021. "CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages" Cells 10, no. 10: 2583. https://doi.org/10.3390/cells10102583
APA StyleTsai, W.-H., Chang, S.-C., Lin, Y.-C., & Hsu, H.-C. (2021). CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages. Cells, 10(10), 2583. https://doi.org/10.3390/cells10102583





