Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance
Abstract
:1. Introduction
2. History of Cell-in-Cell Structures
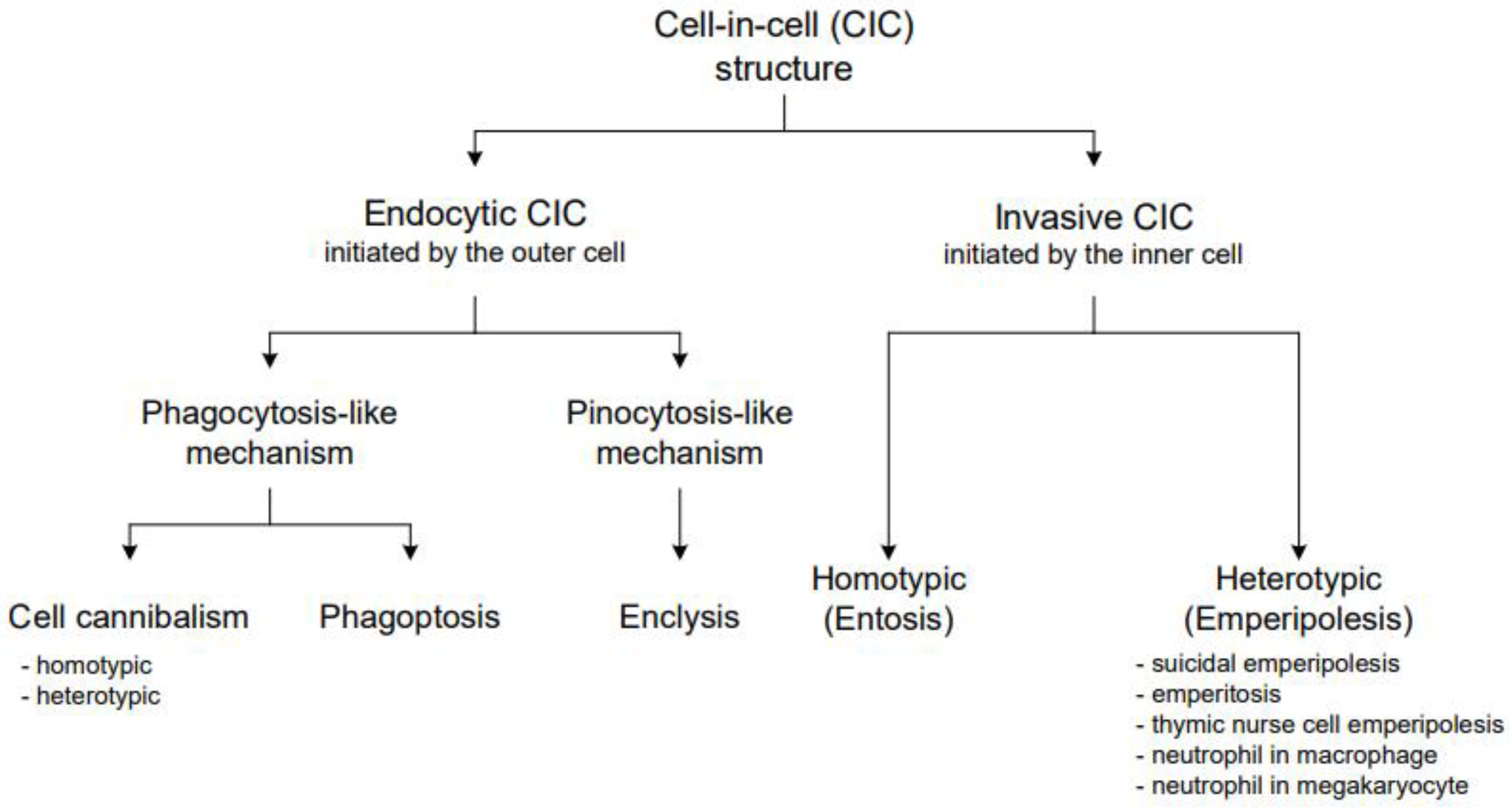
3. General CIC Classification
4. Detailed CIC Characterization
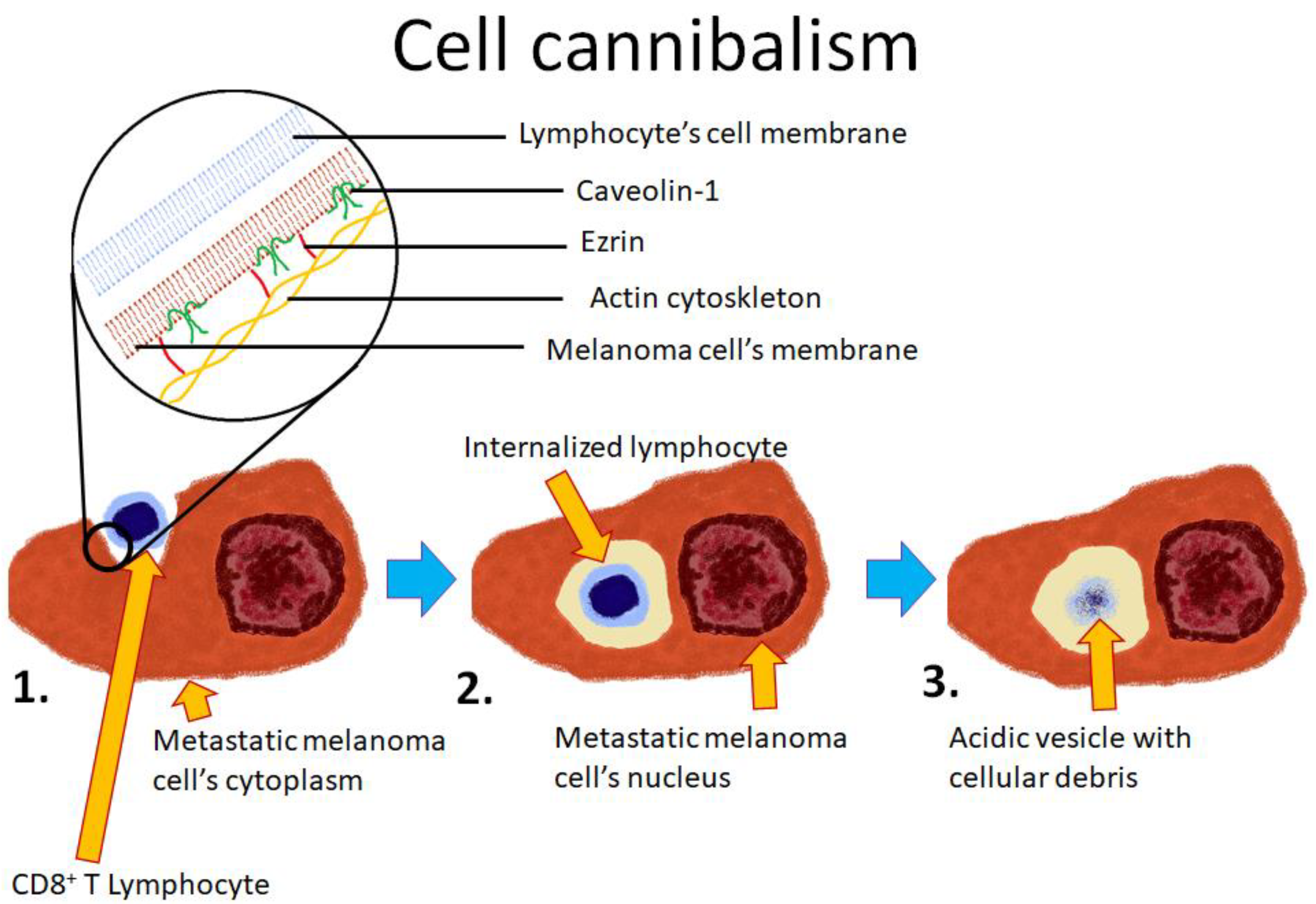
4.1. Cell Cannibalism
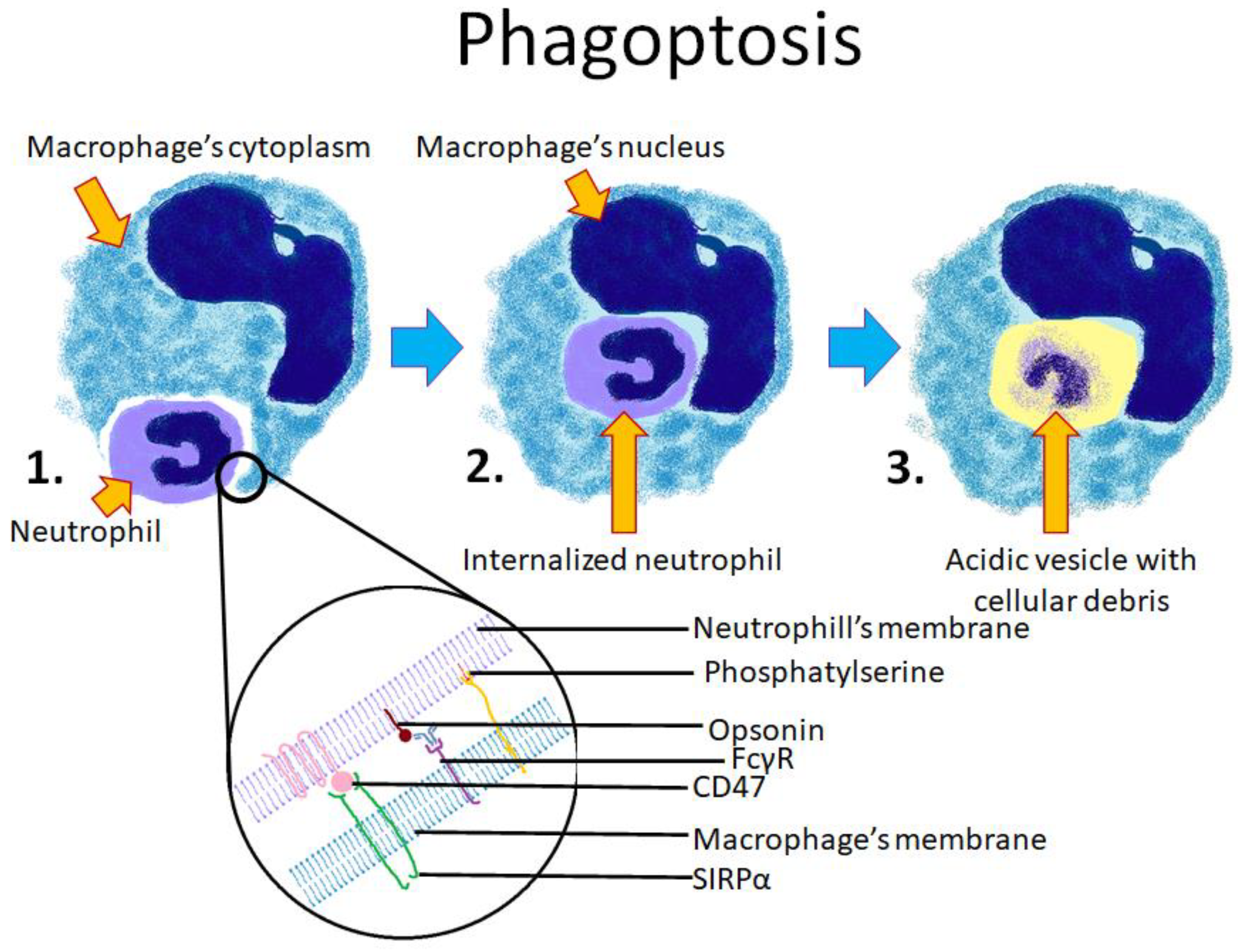
4.2. Phagoptosis
4.3. Enclysis
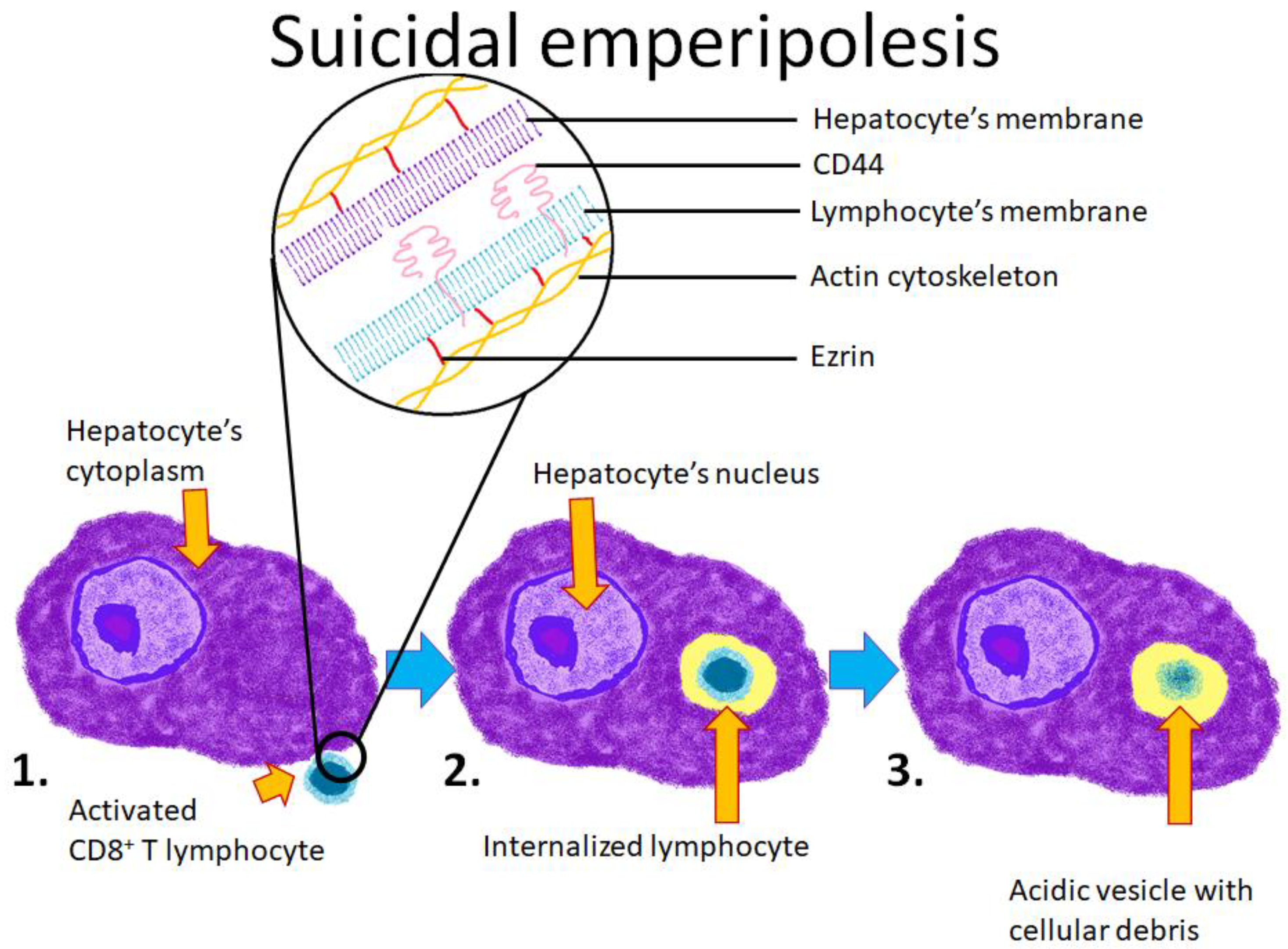
4.4. Emperipolesis
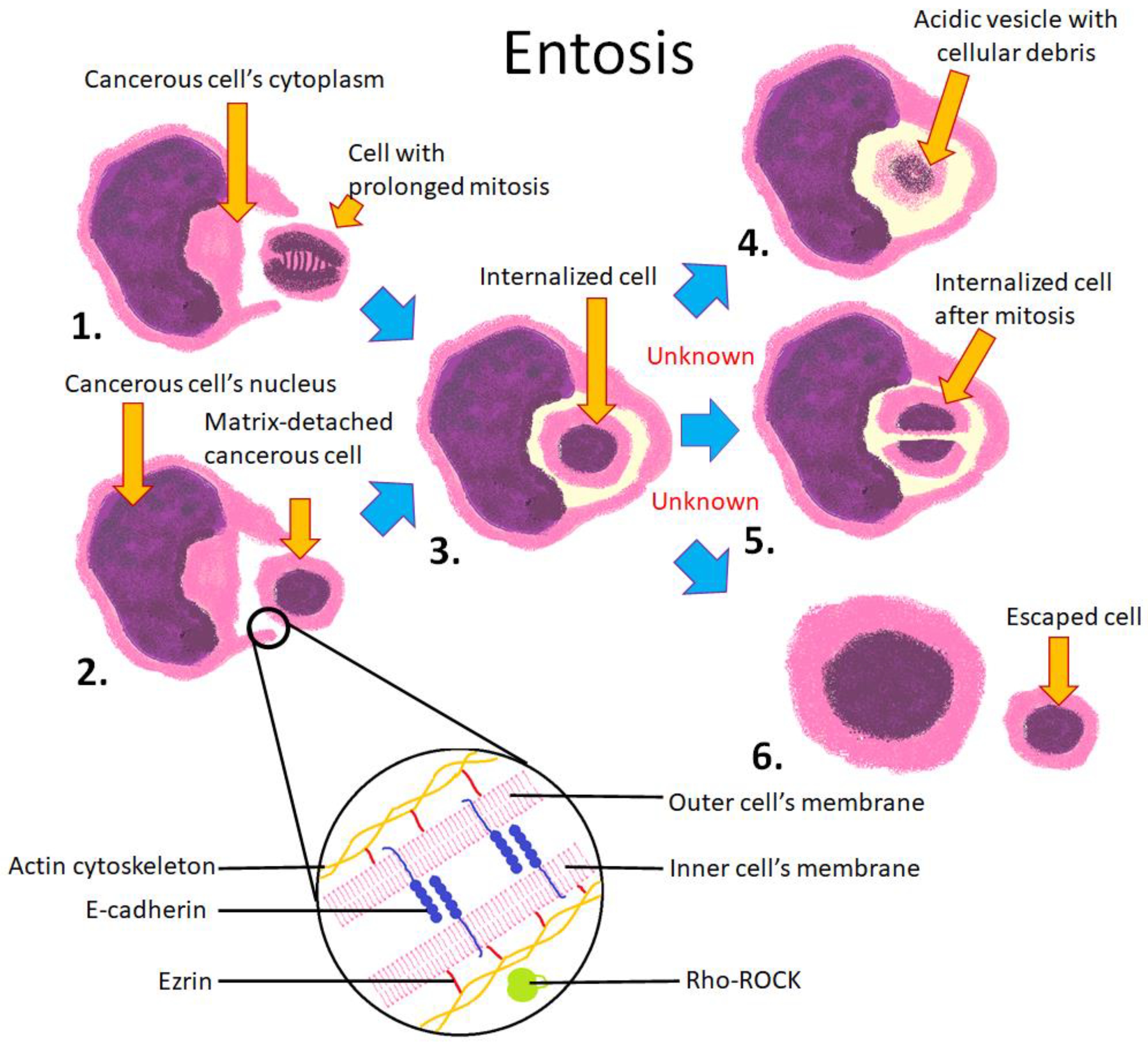
4.5. Entosis
4.6. CIC, Entosis, and Autophagy
4.7. Unclassifiable CIC Structures
5. Concluding Remarks: CICs in Physiology and Pathology
Author Contributions
Funding
Conflicts of Interest
References
- Ichimura, T.; Kakizuka, T.; Horikawa, K.; Seiriki, K.; Kasai, A.; Hashimoto, H.; Fujita, K.; Watanabe, T.M.; Nagai, T. Exploring rare cellular activity in more than one million cells by a transscale scope. Sci. Rep. 2021, 11, 16539. [Google Scholar] [CrossRef]
- Florey, O.; Overholtzer, M. Macropinocytosis and autophagy crosstalk in nutrient scavenging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180154. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, T.; Ren, Y.; Florey, O.; Shirasawa, S.; Sasazuki, T.; Robinson, D.N.; Overholtzer, M. Competition between human cells by entosis. Cell Res. 2014, 24, 1299–1310. [Google Scholar] [CrossRef]
- Krishna, S.; Palm, W.; Lee, Y.; Yang, W.; Bandyopadhyay, U.; Xu, H.; Florey, O.; Thompson, C.B.; Overholtzer, M. PIKfyve Regulates Vacuole Maturation and Nutrient Recovery following Engulfment. Dev. Cell 2016, 38, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena in cancer. Nat. Rev. Cancer 2018, 18, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Krajcovic, M.; Overholtzer, M. Mechanisms of ploidy increase in human cancers: A new role for cell cannibalism. Cancer Res. 2012, 72, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlynarczuk-Bialy, I.; Dziuba, I.; Sarnecka, A.; Platos, E.; Kowalczyk, M.; Pels, K.K.; Wilczynski, G.M.; Wojcik, C.; Bialy, L.P. Entosis: From Cell Biology to Clinical Cancer Pathology. Cancers 2020, 12, 2481. [Google Scholar] [CrossRef]
- Hamann, J.C.; Overholtzer, M. Entosis enables a population response to starvation. Oncotarget 2017, 8, 57934–57935. [Google Scholar] [CrossRef]
- Hamann, J.C.; Surcel, A.; Chen, R.; Teragawa, C.; Albeck, J.G.; Robinson, D.N.; Overholtzer, M. Entosis Is Induced by Glucose Starvation. Cell Rep. 2017, 20, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; He, M.; Li, L.; Liang, Z.; Zou, Z.; Tao, A. Cell-in-Cell Death Is Not Restricted by Caspase-3 Deficiency in MCF-7 Cells. J. Breast Cancer 2016, 19, 231–241. [Google Scholar] [CrossRef]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007, 131, 966–979. [Google Scholar] [CrossRef] [Green Version]
- Durgan, J.; Tseng, Y.Y.; Hamann, J.C.; Domart, M.C.; Collinson, L.; Hall, A.; Overholtzer, M.; Florey, O. Mitosis can drive cell cannibalism through entosis. Elife 2017, 6, e27134. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhang, Y.; Li, S.; Sun, F.; Wang, G.; Yang, T.; Wei, D.; Guo, L.; Xiao, H. Induction of entosis in prostate cancer cells by nintedanib and its therapeutic implications. Oncol. Lett. 2019, 17, 3151–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalkar, P.; Diaz-Argelich, N.; Antonio Palop, J.; Sanmartin, C.; Fernandes, A.P. Novel Methylselenoesters Induce Programed Cell Death via Entosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florey, O.; Kim, S.E.; Sandoval, C.P.; Haynes, C.M.; Overholtzer, M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 2011, 13, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, A.; Yavas, A.; McIntyre, C.A.; Ho, Y.J.; Erakky, A.; Wong, W.; Varghese, A.M.; Melchor, J.P.; Overholtzer, M.; O’Reilly, E.M.; et al. Genetic and clinical correlates of entosis in pancreatic ductal adenocarcinoma. Mod. Pathol. 2020, 33, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Olszewska, A.; Klemba, A.; Marciniak, M.; Synowiec, A.; Kieda, C. Polyploidy formation in cancer cells: How a Trojan horse is born. Semin. Cancer Biol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Eberth, J. Über die feineren bau der darmschleithaut. Wurzb. Nat. Zeitschr. 1864, 5, 11. [Google Scholar]
- Steinhaus, J. Ueber Carcinom-Einschlüsse. Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1891, 126, 533–541. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, P. Cell cannibalism: Diagnostic marker of malignancy. Diagn. Cytopathol. 2003, 28, 86–87. [Google Scholar] [CrossRef]
- Lewis, W.H. The engulfment of living blood cells by others of the same type. Anat. Rec. 1925, 31, 43–49. [Google Scholar] [CrossRef]
- Humble, J.G.; Jayne, W.H.W.; Pulvertaft, R.J.V. Biological Interaction Between Lymphocytes and. Other Cells 1956, 2, 283–294. [Google Scholar] [CrossRef]
- Davies, S.P.; Reynolds, G.M.; Wilkinson, A.L.; Li, X.; Rose, R.; Leekha, M.; Liu, Y.S.; Gandhi, R.; Buckroyd, E.; Grove, J.; et al. Hepatocytes Delete Regulatory T Cells by Enclysis, a CD4(+) T Cell Engulfment Process. Cell Rep. 2019, 29, 1610–1620.e4. [Google Scholar] [CrossRef]
- Lozupone, F.; Fais, S. Cancer Cell Cannibalism: A Primeval Option to Survive. Curr. Mol. Med. 2015, 15, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, F.; Perdicchio, M.; Brambilla, D.; Borghi, M.; Meschini, S.; Barca, S.; Marino, M.L.; Logozzi, M.; Federici, C.; Iessi, E.; et al. The human homologue of Dictyostelium discoideum phg1A is expressed by human metastatic melanoma cells. EMBO Rep. 2009, 10, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.P.; Terry, L.V.; Wilkinson, A.L.; Stamataki, Z. Cell-in-Cell Structures in the Liver: A Tale of Four E’s. Front. Immunol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends. Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Lugini, L.; Matarrese, P.; Tinari, A.; Lozupone, F.; Federici, C.; Iessi, E.; Gentile, M.; Luciani, F.; Parmiani, G.; Rivoltini, L.; et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006, 66, 3629–3638. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa, L.S.; Holst, M.; Baarlink, C.; Grosse, R. MRTF transcription and Ezrin-dependent plasma membrane blebbing are required for entotic invasion. J. Cell Biol. 2017, 216, 3087–3095. [Google Scholar] [CrossRef] [Green Version]
- Cunin, P.; Bouslama, R.; Machlus, K.R.; Martinez-Bonet, M.; Lee, P.Y.; Wactor, A.; Nelson-Maney, N.; Morris, A.; Guo, L.; Weyrich, A.; et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. Elife 2019, 8, e44031. [Google Scholar] [CrossRef]
- Wang, S.; He, M.F.; Chen, Y.H.; Wang, M.Y.; Yu, X.M.; Bai, J.; Zhu, H.Y.; Wang, Y.Y.; Zhao, H.; Mei, Q.; et al. Rapid reuptake of granzyme B leads to emperitosis: An apoptotic cell-in-cell death of immune killer cells inside tumor cells. Cell Death Dis. 2013, 4, e856. [Google Scholar] [CrossRef]
- Bauer, M.F.; Hader, M.; Hecht, M.; Buttner-Herold, M.; Fietkau, R.; Distel, L.V.R. Cell-in-cell phenomenon: Leukocyte engulfment by non-tumorigenic cells and cancer cell lines. BMC Mol. Cell Biol. 2021, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Benseler, V.; Warren, A.; Vo, M.; Holz, L.E.; Tay, S.S.; Le Couteur, D.G.; Breen, E.; Allison, A.C.; van Rooijen, N.; McGuffog, C.; et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16735–16740. [Google Scholar] [CrossRef] [Green Version]
- Guyden, J.C.; Martinez, M.; Chilukuri, R.V.; Reid, V.; Kelly, F.; Samms, M.O. Thymic Nurse Cells Participate in Heterotypic Internalization and Repertoire Selection of Immature Thymocytes; Their Removal from the Thymus of Autoimmune Animals May be Important to Disease Etiology. Curr. Mol. Med. 2015, 15, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Yener, Y.; Dikmenli, M. The effects of acrylamide on the frequency of megakaryocytic emperipolesis and the mitotic activity of rat bone marrow cells. J. Sci. Food Agric. 2011, 91, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Tonnessen-Murray, C.A.; Frey, W.D.; Rao, S.G.; Shahbandi, A.; Ungerleider, N.A.; Olayiwola, J.O.; Murray, L.B.; Vinson, B.T.; Chrisey, D.B.; Lord, C.J.; et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 2019, 218, 3827–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fais, S. Cannibalism: A way to feed on metastatic tumors. Cancer Lett. 2007, 258, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, F.; Borghi, M.; Marzoli, F.; Azzarito, T.; Matarrese, P.; Iessi, E.; Venturi, G.; Meschini, S.; Canitano, A.; Bona, R.; et al. TM9SF4 is a novel V-ATPase-interacting protein that modulates tumor pH alterations associated with drug resistance and invasiveness of colon cancer cells. Oncogene 2015, 34, 5163–5174. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Finocchiaro, G.; Arena, G.; Venuti, A. Neutrophil-tumor cell phagocytosis (cannibalism) in human tumors: An update and literature review. Exp. Oncol. 2012, 34, 306–311. [Google Scholar]
- Wang, X.; Li, Y.; Li, J.; Li, L.; Zhu, H.; Chen, H.; Kong, R.; Wang, G.; Wang, Y.; Hu, J.; et al. Cell-in-Cell Phenomenon and Its Relationship With Tumor Microenvironment and Tumor Progression: A Review. Front. Cell Dev. Biol. 2019, 7, 311. [Google Scholar] [CrossRef] [Green Version]
- Sikora, E.; Bielak-Żmijewska, A.; Mosieniak, G. What is and what is not cell senescence. Postepy Biochem. 2018, 64, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef] [Green Version]
- Cano, C.E.; Sandi, M.J.; Hamidi, T.; Calvo, E.L.; Turrini, O.; Bartholin, L.; Loncle, C.; Secq, V.; Garcia, S.; Lomberk, G.; et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 2012, 4, 964–979, Erratum in 2021, 13, e14243. [Google Scholar] [CrossRef]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Dias-Baruffi, M.; Zhu, H.; Cho, M.; Karmakar, S.; McEver, R.P.; Cummings, R.D. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 2003, 278, 41282–41293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neher, J.J.; Neniskyte, U.; Zhao, J.W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 2011, 186, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Neniskyte, U.; Neher, J.J.; Brown, G.C. Neuronal death induced by nanomolar amyloid beta is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 2011, 286, 39904–39913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyurina, Y.Y.; Basova, L.V.; Konduru, N.V.; Tyurin, V.A.; Potapovich, A.I.; Cai, P.; Bayir, H.; Stoyanovsky, D.; Pitt, B.R.; Shvedova, A.A.; et al. Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: Implications for the resolution of inflammation. J. Biol. Chem. 2007, 282, 8498–8509. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, H.; Li, X.; Wu, J.; Xue, T.; Wu, J.; Shen, H.; Li, X.; Shen, M.; Chen, G. TMEM16F Aggravates Neuronal Loss by Mediating Microglial Phagocytosis of Neurons in a Rat Experimental Cerebral Ischemia and Reperfusion Model. Front. Immunol. 2020, 11, 1144. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.P.; Liu, J.; Lim, J.S.; et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; van Rooijen, N.; Saxena, R.K. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 2007, 47, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.; Tan, M.; Webster, D. Histology, Kupffer Cell; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; George, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018, 19, 76–84. [Google Scholar] [CrossRef]
- Gul, N.; van Egmond, M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015, 75, 5008–5013. [Google Scholar] [CrossRef] [Green Version]
- Overdijk, M.B.; Verploegen, S.; Bogels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K.; et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015, 7, 311–321. [Google Scholar] [CrossRef]
- Golay, J.; Da Roit, F.; Bologna, L.; Ferrara, C.; Leusen, J.H.; Rambaldi, A.; Klein, C.; Introna, M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013, 122, 3482–3491. [Google Scholar] [CrossRef] [Green Version]
- Metayer, L.E.; Vilalta, A.; Burke, G.A.A.; Brown, G.C. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by phagoptosis. Oncotarget 2017, 8, 60892–60903. [Google Scholar] [CrossRef] [Green Version]
- Ishidome, T.; Yoshida, T.; Hanayama, R. Induction of Live Cell Phagocytosis by a Specific Combination of Inflammatory Stimuli. EBioMedicine 2017, 22, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Ye, J.; Zhou, C.; Qi, L.; Fu, Z.; Yan, B.; Liang, Z.; Li, R.; Zhai, W. Calreticulin down-regulation inhibits the cell growth, invasion and cell cycle progression of human hepatocellular carcinoma cells. Diagn. Pathol. 2015, 10, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Chen, J.Y.; Weissman-Tsukamoto, R.; Volkmer, J.P.; Ho, P.Y.; McKenna, K.M.; Cheshier, S.; Zhang, M.; Guo, N.; Gip, P.; et al. Macrophages eat cancer cells using their own calreticulin as a guide: Roles of TLR and Btk. Proc. Natl. Acad. Sci. USA 2015, 112, 2145–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018, 285, 3566–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuo, Q.Z.; Zhang, S.T.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Neniskyte, U.; Brown, G.C. Lactadherin/MFG-E8 is essential for microglia-mediated neuronal loss and phagoptosis induced by amyloid beta. J. Neurochem. 2013, 126, 312–317. [Google Scholar] [CrossRef]
- Neher, J.J.; Neniskyte, U.; Hornik, T.; Brown, G.C. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia 2014, 62, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Aghabi, Y.O.; Yasin, A.; Kennedy, J.I.; Davies, S.P.; Butler, A.E.; Stamataki, Z. Targeting Enclysis in Liver Autoimmunity, Transplantation, Viral Infection and Cancer. Front. Immunol. 2021, 12, 662134. [Google Scholar] [CrossRef]
- Palm, W. Metabolic functions of macropinocytosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180285. [Google Scholar] [CrossRef]
- Miao, Q.; Bian, Z.; Tang, R.; Zhang, H.; Wang, Q.; Huang, S.; Xiao, X.; Shen, L.; Qiu, D.; Krawitt, E.L.; et al. Emperipolesis mediated by CD8 T cells is a characteristic histopathologic feature of autoimmune hepatitis. Clin. Rev. Allergy Immunol. 2015, 48, 226–235. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, S.; He, M.F.; Wang, Y.; Zhao, H.; Zhu, H.Y.; Yu, X.M.; Ma, J.; Che, X.J.; Wang, J.F.; et al. Prevalence of heterotypic tumor/immune cell-in-cell structure in vitro and in vivo leading to formation of aneuploidy. PLoS ONE 2013, 8, e59418. [Google Scholar] [CrossRef] [Green Version]
- Xiaonin, W. The mechanism of natural killer cell-mediated tumor cell cytolysis at a single cell level. J. First Mil. Med. Univ. 1986, 4. [Google Scholar]
- Liang, J.B.; Chen, Y.; Chen, R.L.; Li, Y.K.; Li, B.; You, Z.R.; Li, Y.; Zhang, J.; Huang, B.Y.; Wei, Y.R.; et al. CD8(+) T cells actively penetrate hepatocytes via the CD44/p-ERM/F-actin pathway in autoimmune hepatitis. J. Dig. Dis. 2021, 22, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, S.; Guo, Z.; Yao, X. Emperipolesis, entosis and beyond: Dance with fate. Cell Res. 2008, 18, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Rousalova, I.; Krepela, E. Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int. J. Oncol. 2010, 37, 1361–1378. [Google Scholar] [CrossRef] [Green Version]
- Dalia, S.; Sagatys, E.; Sokol, L.; Kubal, T. Rosai-Dorfman disease: Tumor biology, clinical features, pathology, and treatment. Cancer Control 2014, 21, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Chen, Y.; Zeng, M.; Pei, R.; Du, Y.; Tang, L.; Wang, M.; Hu, Y.; Zhu, H.; He, M.; et al. In-cell infection: A novel pathway for Epstein-Barr virus infection mediated by cell-in-cell structures. Cell Res. 2015, 25, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.; Liu, J.; Zheng, Z.; Zhu, H.; Chu, X.; Dong, Z.; Huang, S.; Du, Q. Regulation of myosin activation during cell-cell contact formation by Par3-Lgl antagonism: Entosis without matrix detachment. Mol. Biol. Cell 2012, 23, 2076–2091. [Google Scholar] [CrossRef] [PubMed]
- Krajcovic, M.; Johnson, N.B.; Sun, Q.; Normand, G.; Hoover, N.; Yao, E.; Richardson, A.L.; King, R.W.; Cibas, E.S.; Schnitt, S.J.; et al. A non-genetic route to aneuploidy in human cancers. Nat. Cell Biol. 2011, 13, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Wang, M.; Niu, Z.; Qin, H.; Ruan, B.; Zheng, Y.; Ning, X.; Gu, S.; Gao, L.; Chen, Z.; Wang, X.; et al. Mechanical Ring Interfaces between Adherens Junction and Contractile Actomyosin to Coordinate Entotic Cell-in-Cell Formation. Cell Rep. 2020, 32, 108071. [Google Scholar] [CrossRef]
- Garanina, A.S.; Kisurina-Evgenieva, O.P.; Erokhina, M.V.; Smirnova, E.A.; Factor, V.M.; Onishchenko, G.E. Consecutive entosis stages in human substrate-dependent cultured cells. Sci. Rep. 2017, 7, 12555. [Google Scholar] [CrossRef] [Green Version]
- Purvanov, V.; Holst, M.; Khan, J.; Baarlink, C.; Grosse, R. G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. Elife 2014, 3, e02786. [Google Scholar] [CrossRef]
- Su, Y.; Ren, H.; Tang, M.; Zheng, Y.; Zhang, B.; Wang, C.; Hou, X.; Niu, Z.; Wang, Z.; Gao, X.; et al. Role and dynamics of vacuolar pH during cell-in-cell mediated death. Cell Death Dis. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ram, A.; Albeck, J.G.; Overholtzer, M. Entosis is induced by ultraviolet radiation. iScience 2021, 24, 102902. [Google Scholar] [CrossRef]
- Liang, J.; Niu, Z.; Zhang, B.; Yu, X.; Zheng, Y.; Wang, C.; Ren, H.; Wang, M.; Ruan, B.; Qin, H.; et al. p53-dependent elimination of aneuploid mitotic offspring by entosis. Cell Death Differ. 2021, 28, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Mackay, H.L.; Moore, D.; Hall, C.; Birkbak, N.J.; Jamal-Hanjani, M.; Karim, S.A.; Phatak, V.M.; Pinon, L.; Morton, J.P.; Swanton, C.; et al. Genomic instability in mutant p53 cancer cells upon entotic engulfment. Nat. Commun. 2018, 9, 3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenker, H.; Buttner-Herold, M.; Fietkau, R.; Distel, L.V. Cell-in-cell structures are more potent predictors of outcome than senescence or apoptosis in head and neck squamous cell carcinomas. Radiat. Oncol. 2017, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Schwegler, M.; Wirsing, A.M.; Schenker, H.M.; Ott, L.; Ries, J.M.; Buttner-Herold, M.; Fietkau, R.; Putz, F.; Distel, L.V. Prognostic Value of Homotypic Cell Internalization by Nonprofessional Phagocytic Cancer Cells. Biomed. Res. Int. 2015, 2015, 359392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Niu, Z.; Qin, H.; Fan, J.; Wang, M.; Zhang, B.; Zheng, Y.; Gao, L.; Chen, Z.; Tai, Y.; et al. Subtype-Based Prognostic Analysis of Cell-in-Cell Structures in Early Breast Cancer. Front. Oncol. 2019, 9, 895. [Google Scholar] [CrossRef]
- Ruan, B.; Niu, Z.; Jiang, X.; Li, Z.; Tai, Y.; Huang, H.; Sun, Q. High Frequency of Cell-in-Cell Formation in Heterogeneous Human Breast Cancer Tissue in a Patient With Poor Prognosis: A Case Report and Literature Review. Front. Oncol. 2019, 9, 1444. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Niu, Z.; Zhou, L.; Zhou, Y.; Ma, Q.; Zhu, Y.; Liu, M.; Shi, Y.; Tai, Y.; Shao, Q.; et al. Subtype-Based Analysis of Cell-in-Cell Structures in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 670051. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab. Investig. 2003, 83, 1555–1567. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, D.; Fais, S. The Janus-faced role of ezrin in "linking" cells to either normal or metastatic phenotype. Int. J. Cancer 2009, 125, 2239–2245. [Google Scholar] [CrossRef]
- Hamann, J.C.; Kim, S.E.; Overholtzer, M. Methods for the Study of Entotic Cell Death. Methods Mol. Biol. 2019, 1880, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Garanina, A.S.; Khashba, L.A.; Onishchenko, G.E. Stages of Cell Cannibalism—Entosis—In Normal Human Keratinocyte Culture. Biochemistry 2015, 80, 1469–1477. [Google Scholar] [CrossRef]
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena, cannibalism, and autophagy: Is there a relationship? Cell Death Dis. 2018, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Strus, P.; Borensztejn, K.; Szczepankiewicz, A.A.; Lisiecki, K.; Czarnocki, Z.; Nieznanska, H.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Novel podophyllotoxin and benzothiazole derivative induces transitional morphological and functional changes in HaCaT cells. Toxicol. Vitr. 2021, 73, 105144. [Google Scholar] [CrossRef]
- Marino, M.L.; Fais, S.; Djavaheri-Mergny, M.; Villa, A.; Meschini, S.; Lozupone, F.; Venturi, G.; Della Mina, P.; Pattingre, S.; Rivoltini, L.; et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010, 1, e87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Meng, Z.; Zhu, Y.; Lu, J.; Li, Z.; Zhao, Q.; Huang, Y.; Jiang, L.; Yao, X. TM9SF4 is a novel factor promoting autophagic flux under amino acid starvation. Cell Death Differ. 2018, 25, 368–379. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2020, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.A.; Milasta, S.; Green, D.R. Toll-like receptor signaling in the lysosomal pathways. Immunol. Rev. 2009, 227, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Hou, J.; Li, W.; Wang, X.; Xiang, L.; Tan, D.; Wang, W.; Jiang, L.; Claret, F.X.; et al. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS ONE 2020, 15, e0231003. [Google Scholar] [CrossRef]


| Analysis of Cell-in-Cell Literature | |||||||
|---|---|---|---|---|---|---|---|
| Keyword in Abstract or in Any Field | Cell-in-Cell (in Abstract) | Entosis | Cell Cannibalism (in Abstract) | Phagoptosis | Enclysis | Emperipolesis | Total |
| Number of publications including the selected phrase | 115 | 172 | 147 | 34 | 4 | 540 | 1012 |
| Total number of citations | 2721 | 6036 | 3790 | 1655 | 16 | 6047 | 20,265 |
| Average citation per item | 23.66 | 35.09 | 25.78 | 48.68 | 4.00 | 11.20 | 20.02 |
| Macropinocytosis | Enclysis | |
|---|---|---|
| Endocytosed material | Extracellular fluid and proteins dissolved in it | CD4+ T Lymphocyte |
| Effector cells | Various (best studied in cancers) | Hepatocytes |
| Involved proteins | Ras pathway, PI3-K pathway, β-catenin dependent WNT pathway | ICAM-1, β-catenin |
| Possible roles | Nutrient uptake | Modulating lymphocyte population |
| Cancer Type | CIC Distribution | Ref |
|---|---|---|
| Head and neck squamous cell carcinoma | Lymph nodes metastases, the average CIC numbers were significantly lower than in the corresponding primary tumors | [89] |
| Head and neck squamous cell carcinoma | In the central tumor area, the average value of CIC structures was higher than in the invasive front | [88,89] |
| Pancreatic ductal adenocarcinoma | CIC positivity was significantly more prevalent in liver metastases | [16] |
| Cell-in-Cell Structures | |||||||
|---|---|---|---|---|---|---|---|
| Structure | Cell Cannibalism | Phagoptosis | Enclysis | Emperipolesis | Entosis | ||
| In General | Suicidal | Emperitosis | |||||
| First description | 1904 | 2012 (name proposed) | 2019 | 1956 | 2011 | 2013 | 2007 |
| Mechanism | Endocytic (phagocytosis-like) | Endocytic (phagocytosis-like) | Endocytic (pinocytosis-like) | Invasive | Invasive | Invasive | Invasive |
| Type | Homotypic or heterotypic | Heterotypic | Heterotypic | Heterotypic | Heterotypic | Heterotypic | Homotypic |
| Outer cell | Cancerous cell, e.g., melanoma | Macrophage, microglia | Hepatocyte | Cancerous cell or megakaryocyte | Hepatocyte | Cancerous cell | Cancerous cell |
| Inner cell | Cancerous cell, leukocyte, mesenchymal stem cell | Various, e.g., leukocyte or neuron | CD4+ T lymphocyte | Leukocyte or erythrocyte | CD8+ T lymphocyte | NK cell | Cancerous cell |
| Fate of the engulfed cell | Lysosome-mediated cell death | Lysosome-mediated cell death | Lysosome-mediated cell death (usually Tregs) or escape (usually non-Tregs) | Cell death, mitosis, or escape | Cell death | Apoptotic cell death | Cell death, mitosis, or escape |
| Triggering factors | Starvation, acidic environment | Presence of “eat me” or lack of “do not eat me” signals (PS, lack of CD47) on an inner cell’s surface | N/D * | N/D | N/D | N/D | Matrix detachment, starvation, mitosis |
| Involved molecules | Ezrin, caveolin-1, TM9SF4 | PS, antibody, and CD47 receptors | ICAM-1, β-catenin | (Refer to suicidal emperipolesis and emperitosis) | Ezrin, F-actin, CD44 | Ezrin, E-cadherin, ICAM-2 | E-cadherin, ezrin, Rho-ROCK-actin/myosin pathway |
| Possible biological functions | Enhancing survival of tumor cells by acquiring nutrients, immune escape, or entering senescence | Removal of aging erythrocytes and cancerous cells | Modulation of lymphocyte subpopulations (strengthening of the immune response) | (Refer to suicidal emperipolesis and emperitosis); destruction of cancerous cells, viral transmission, platelet membrane circulation | Autoreactive T lymphocyte deletion | Immune escape | Removal of aneuploid cells or enhancing cancer survival |
| Clinical occurrence | Metastatic melanoma | Cell turnover, Alzheimer’s disease | The process was reported in healthy individuals | Rosai–Dorfman disease | Autoimmune hepatitis | N/D | Nasopharyngeal, breast, lung, pancreatic cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borensztejn, K.; Tyrna, P.; Gaweł, A.M.; Dziuba, I.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells 2021, 10, 2569. https://doi.org/10.3390/cells10102569
Borensztejn K, Tyrna P, Gaweł AM, Dziuba I, Wojcik C, Bialy LP, Mlynarczuk-Bialy I. Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells. 2021; 10(10):2569. https://doi.org/10.3390/cells10102569
Chicago/Turabian StyleBorensztejn, Karol, Paweł Tyrna, Agata M. Gaweł, Ireneusz Dziuba, Cezary Wojcik, Lukasz P. Bialy, and Izabela Mlynarczuk-Bialy. 2021. "Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance" Cells 10, no. 10: 2569. https://doi.org/10.3390/cells10102569
APA StyleBorensztejn, K., Tyrna, P., Gaweł, A. M., Dziuba, I., Wojcik, C., Bialy, L. P., & Mlynarczuk-Bialy, I. (2021). Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells, 10(10), 2569. https://doi.org/10.3390/cells10102569







