Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine
Abstract
:1. Introduction
2. Original Diploid Cell Type Establishment, Banking, and Uses
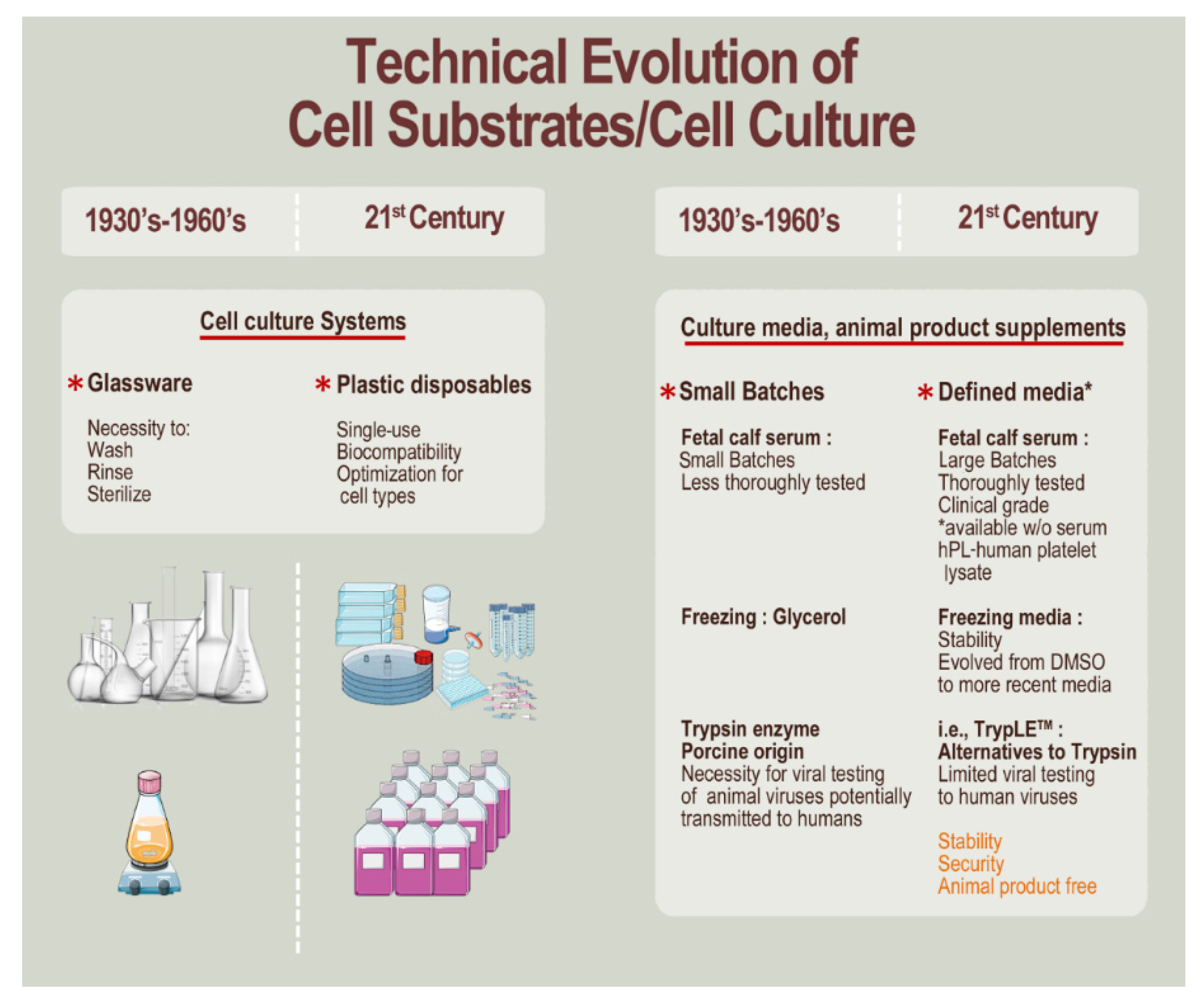
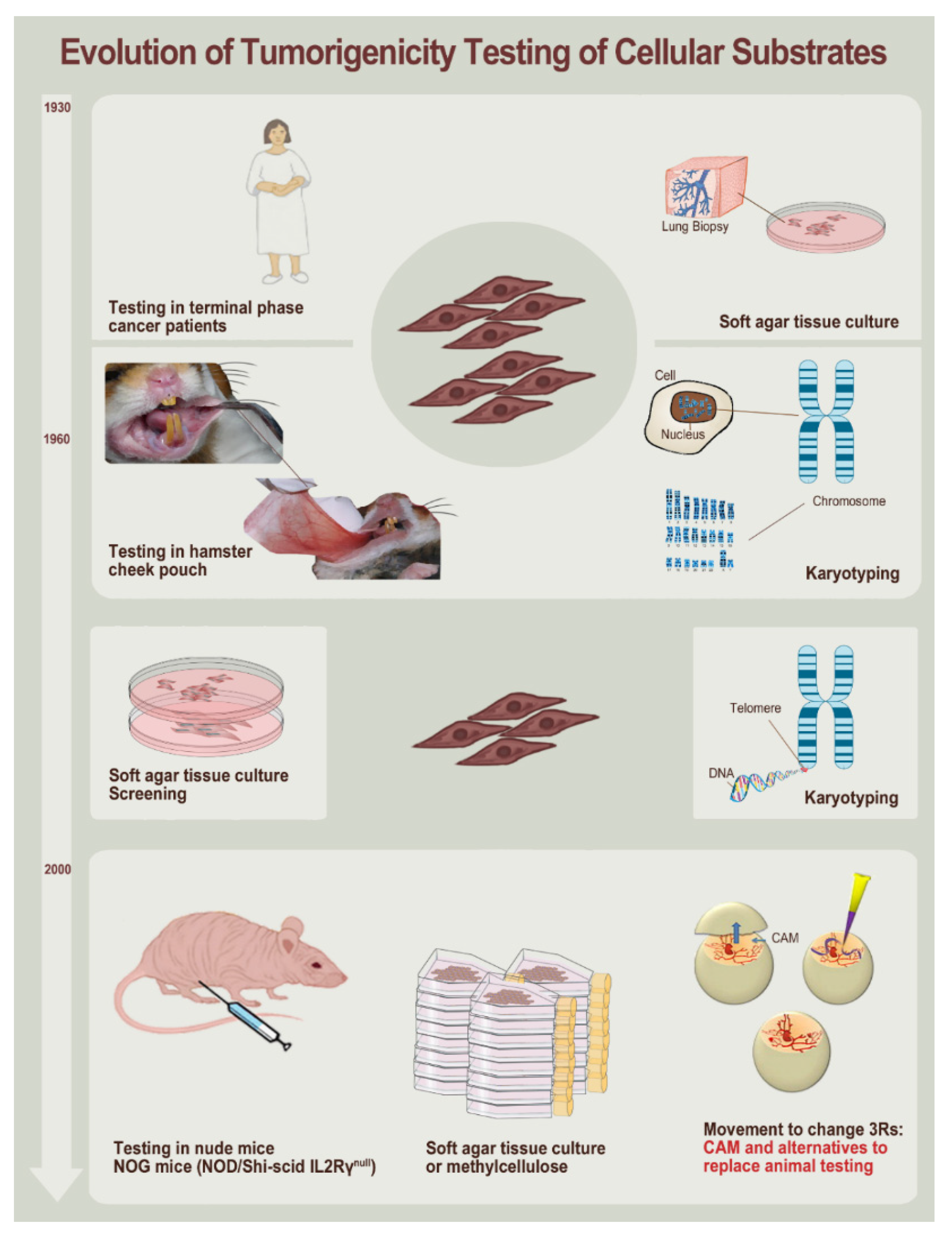
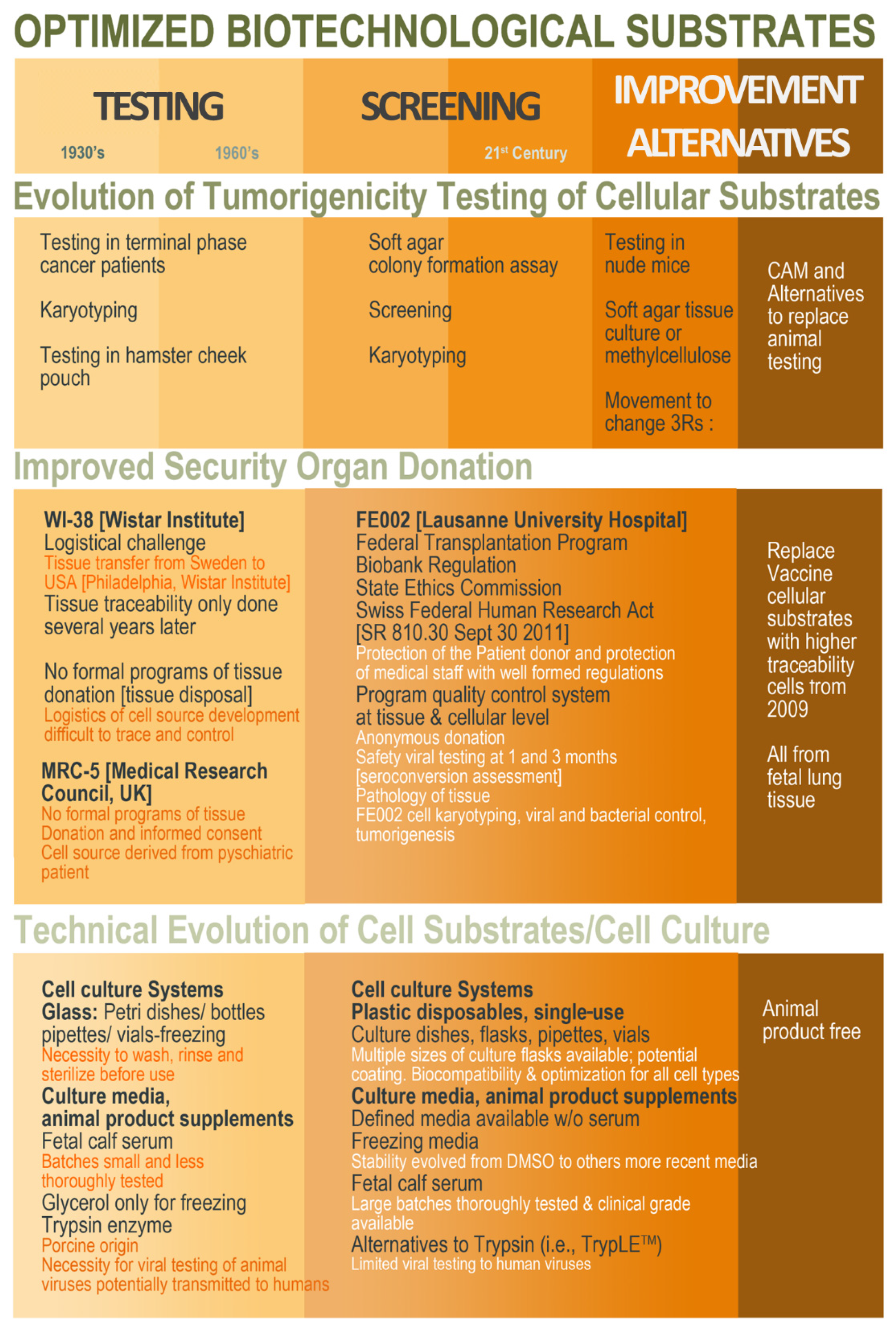
3. Methodological and Technical Evolution since the 1960s toward Modern Cell Isolation, Culture, and Testing
4. Specific Use of Diploid Cells, Derivatives, and Alternatives as Vaccine Substrates
- Development stages: to identify optimal mechanisms and processes to be used in product manufacture.
- Confirmation stage: to assure that optimal mechanisms and processes may be tangibly transposed to product manufacture.
- Production stage: to validate the use in the actual manufacturing system of the final product formula.

5. Renewal of Vaccine Substrates Using Original Seed Stocks or Modern Diploid Progenitor Cell Types
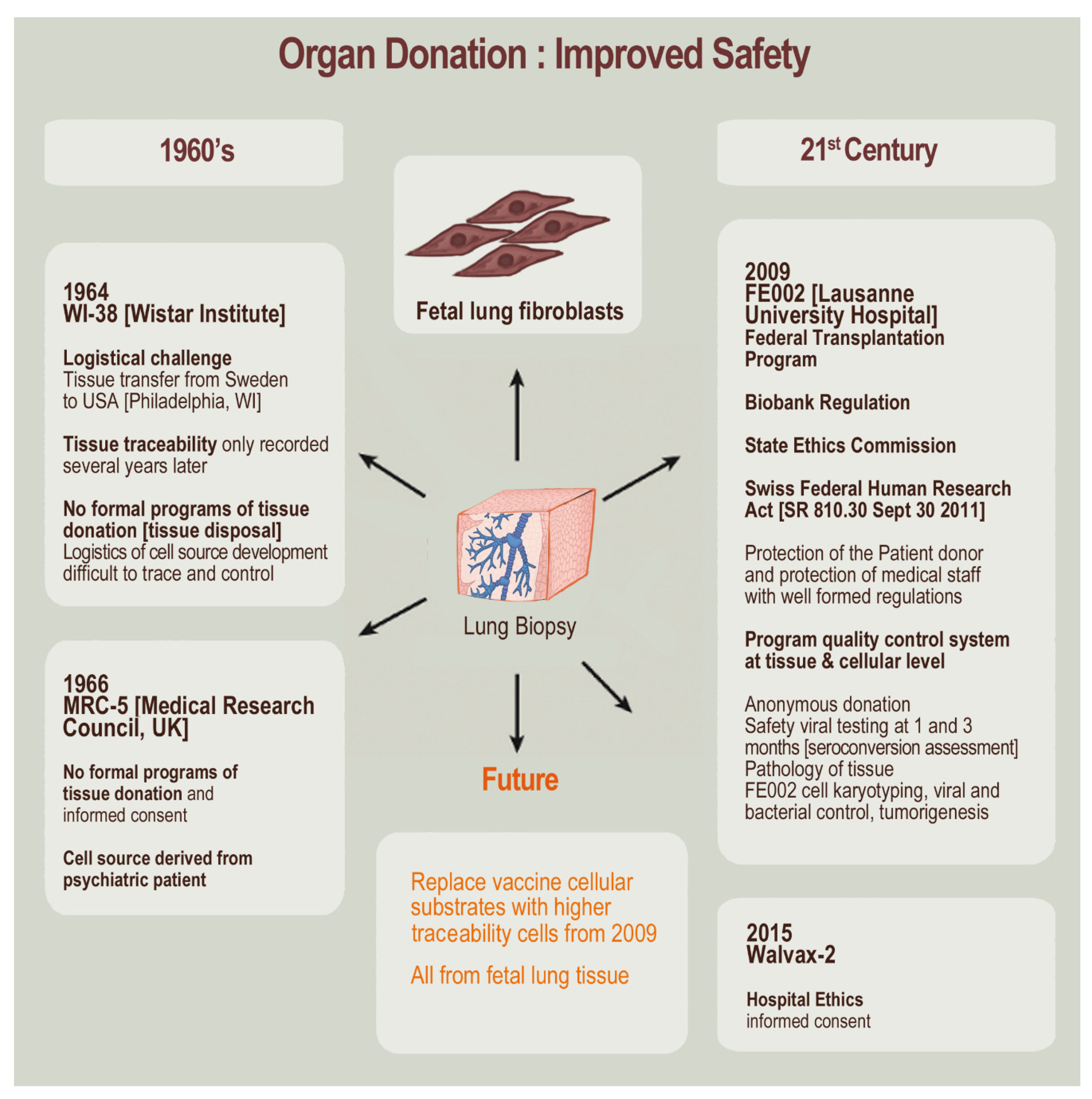
6. Critical Methodological Aspects of Modern Cell Type Sourcing from Organ Donations and Cell Source Establishment
7. Safe Clinical Experience around the Use of Skin Diploid Progenitor Cells as Active Pharmaceutical Ingredients in Cutaneous Regenerative Medicine
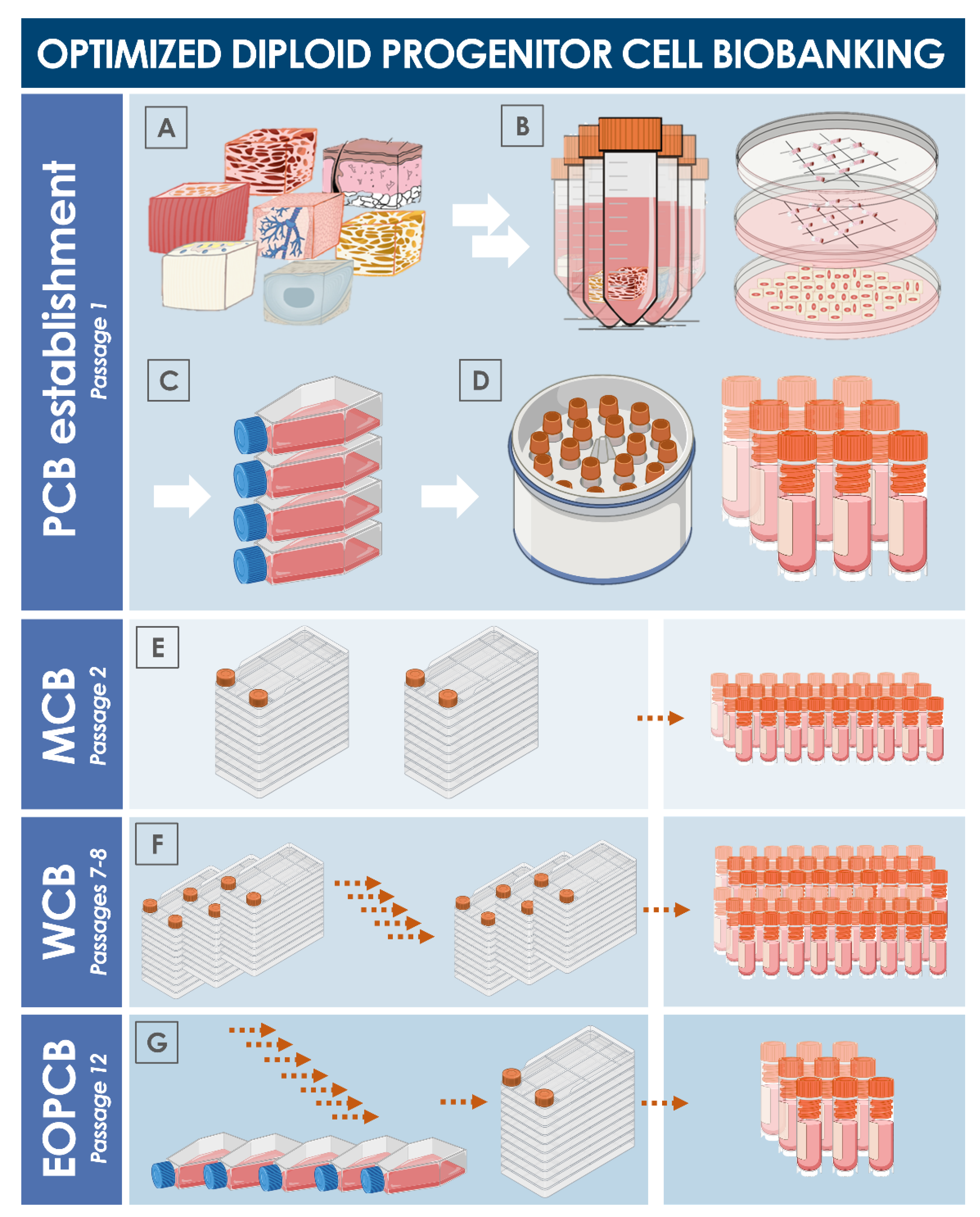
8. Multi-Tiered Cell Banking of Diploid Lung Tissue-Derived Progenitors
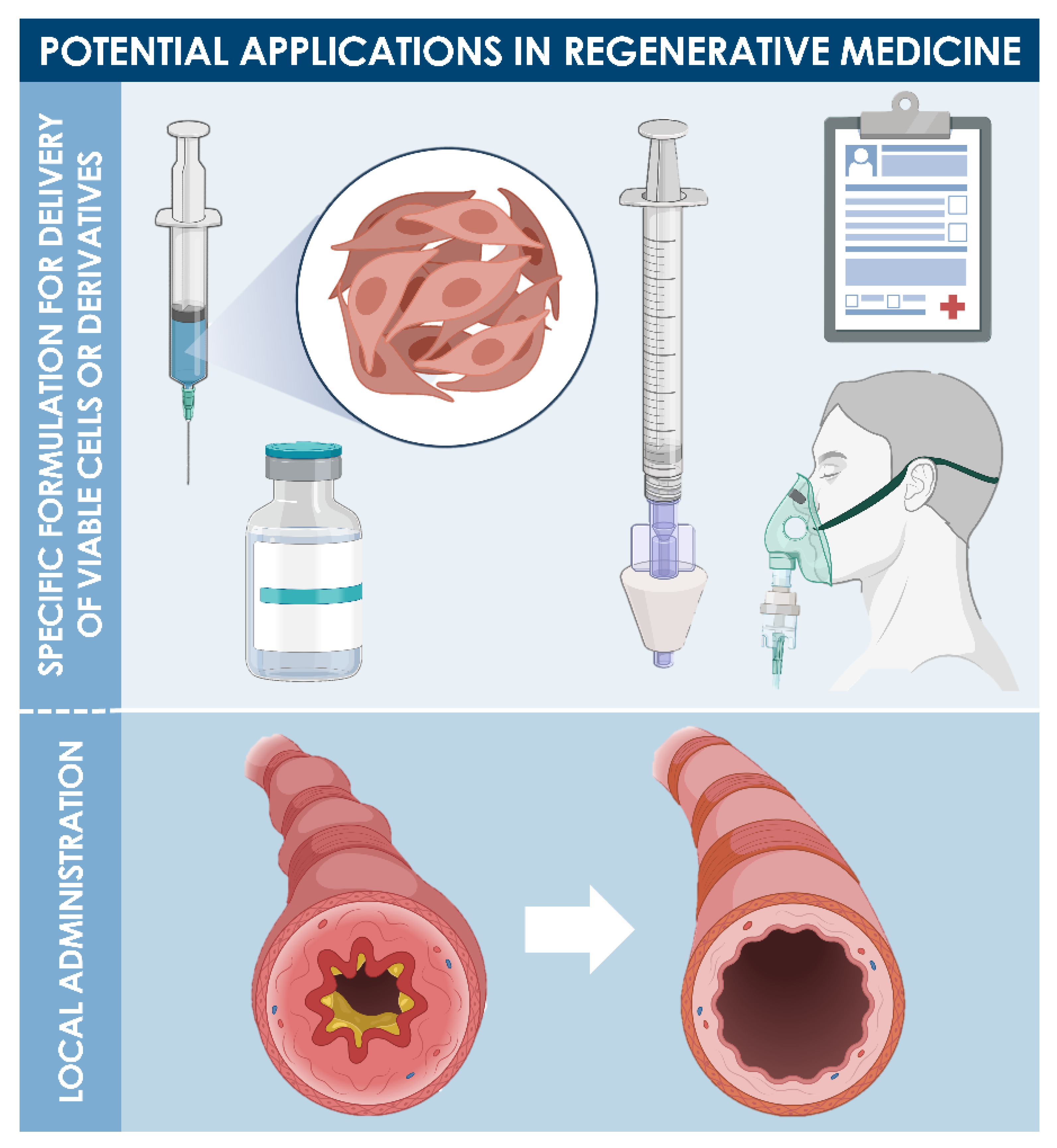
9. Potential Therapeutic Applications of Diploid Lung Progenitors in Respiratory Tract Regenerative Medicine
10. Potential Pathways for Diploid Lung Progenitor Cell Type Homologation as an API
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | active pharmaceutical ingredient |
| ATCC | American type culture collection |
| ATMP | advanced therapy medicinal product |
| bp | base pairs |
| CAM | chorioallantoic membrane |
| CHUV | Centre hospitalier universitaire Vaudois |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| EDQM | European Directorate for the Quality of Medicines & Healthcare |
| EOPCB | end of production cell bank |
| EP | European Pharmacopoeia |
| EU | European Union |
| FBS | fetal bovine serum |
| FDA | US Food and Drug Administration |
| FPC | fibroblast progenitor cell |
| GMP | good manufacturing practices |
| HIV | human immunodeficiency virus |
| hPL | human platelet lysate |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| iPSC | induced pluripotent stem cells |
| MCB | master cell bank |
| MRC | Medical Research Council |
| MSC | mesenchymal stem cells |
| NA | not applicable |
| NIBSC | National Institute for Biological Standards and Control |
| NIH | National Institutes of Health |
| PBB | Progenitor Biological Bandage |
| PCB | parental cell bank |
| PDL | population doubling level |
| PL | passage level |
| SV40 | simian virus 40 |
| US | United States of America |
| WCB | working cell bank |
| WHO | World Health Organization |
| WI | Wistar Institute |
References
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Hayflick, L.; Plotkin, S.A.; Norton, T.W.; Koprowski, H. Preparation of poliovirus vaccines in a human fetal diploid cell strain. Am. J. Hyg. 1962, 75, 240–258. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Tarrant, G.M. Error theory and ageing in human diploid fibroblasts. Nature 1972, 239, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Petes, T.D.; Farber, R.A.; Tarrant, G.M.; Holliday, R. Altered rate of DNA replication in ageing human fibroblast cultures. Nature 1974, 251, 434–436. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Garrett, A.J.; Merton, R. Characteristics of a serially propagated human diploid cell designated MRC-9. J. Biol. Stand. 1979, 7, 113–122. [Google Scholar] [CrossRef]
- Furton, E.J. Vaccines originating in abortion. Ethics Med. 1999, 24, 3–4. [Google Scholar]
- Maher, D.P.; Panicola, M.R.; Harte, C. Vaccines, abortions and moral coherence. Natl. Cathol. Bioeth. Q. 2002, 2, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Rudd, G. Is vaccination complicit with abortion? Ann. Pharmacother. 2003, 37, 1340–1341. [Google Scholar] [CrossRef]
- Ehreth, J. The global value of vaccination. Vaccine 2003, 21, 596–600. [Google Scholar] [CrossRef]
- Pruss, A.R. Cooperation with past evil and use of cell-lines derived from aborted fetuses. Linacre Q. 2004, 71, 335–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiva, R. Moral reflections on vaccines prepared from cells derived from aborted human fetuses. Natl. Cathol. Bioeth. Q. 2006, 6, 537–541. [Google Scholar]
- Rodriguez Luno, A. Ethical reflections on vaccines using cells from aborted fetuses. Natl. Cathol. Bioeth. Q. 2006, 6, 453–459. [Google Scholar] [CrossRef]
- Wong, A. The ethics of HEK 293. Natl. Cathol. Bioeth. Q. 2006, 6, 473–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norrby, E.; Prusiner, S.B. Polio and Nobel prizes: Looking back 50 years. Ann. Neurol. 2007, 61, 385–395. [Google Scholar] [CrossRef]
- Grabenstein, J.D. What the world’s religions teach, applied to vaccines and immune globulins. Vaccine 2013, 31, 2011–2023. [Google Scholar] [CrossRef]
- Jordan, I.; Sandig, V. Matrix and backstage: Cellular substrates for viral vaccines. Viruses 2014, 6, 1672–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Na, T.; Wang, L.; Gao, Q.; Yin, W.; Wang, J.; Yuan, B.Z. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014, 32, 6820–6827. [Google Scholar] [CrossRef]
- Ma, B.; He, L.F.; Zhang, Y.L.; Chen, M.; Wang, L.L.; Yang, H.W.; Yan, T.; Sun, M.X.; Zheng, C.Y. Characteristics and viral propagation properties of a new human diploid cell line. Hum. Vaccin. Immunother. 2015, 11, 998–1009. [Google Scholar] [CrossRef]
- Wan, X.Y.; Xu, L.Y.; Li, B.; Sun, Q.H.; Ji, Q.L.; Huang, D.D.; Zhao, L.; Xiao, Y.T. Chemical conversion of human lung fibroblasts into neuronal cells. Int. J. Mol. Med. 2018, 41, 1463–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, R.; Stylianou, M.; Expert Committee on Biological Standardization. Second replacement seed stock for MRC-5 cells. In Proposal for WHO Reference Cell Bank Status; WHO/BS/2018.2347; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Quintin, A.; Hirt-Burri, N.; Scaletta, C.; Schizas, C.; Pioletti, D.P.; Applegate, L.A. Consistency and safety of cell banks for research and clinical use: Preliminary analysis of fetal skin banks. Cell Transplant. 2007, 16, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwiche, S.E.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012, 4, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 23, 557758. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking progenitor cells for hippiatric regenerative medicine: Optimized establishment of safe and consistent cell sources for standardized veterinary therapeutic protocols. AJBSR 2020, 8, 252–271. [Google Scholar] [CrossRef]
- Hirt-Burri, N.; Scaletta, C.; Gerber, S.; Pioletti, D.; Applegate, L.A. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Artif. Organs 2008, 32, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt-Burri, N.; de Buys Roessingh, A.S.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A.; Hohlfeld, J. Human muscular fetal cells: A potential cell source for muscular therapies. Pediatr. Surg. Int. 2008, 24, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Quintin, A.; Schizas, C.; Scaletta, C.; Jaccoud, S.; Gerber, S.; Osterheld, M.C.; Juillerat, L.; Applegate, L.A.; Pioletti, D.P. Isolation and in vitro chondrogenic potential of human foetal spine cells. J. Cell Mol. Med. 2009, 13, 2559–2569. [Google Scholar] [CrossRef]
- Applegate, L.A.; Weber, D.; Simon, J.P.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W. Organ donation and whole-cell bioprocessing in the Swiss fetal progenitor cell transplantation platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. [Google Scholar]
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Swiss fetal transplantation program and non-enzymatically isolated primary progenitor cell types for regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. GMP tiered cell banking of non-enzymatically isolated dermal progenitor fibroblasts for allogenic regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; Hohlfeld, J.; Scaletta, C.; Hirt-Burri, N.; Gerber, S.; Hohlfeld, P.; Gebbers, J.O.; Applegate, L.A. Development, characterization and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006, 15, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Applegate, L.A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; Pioletti, D. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol. Physiol. 2009, 22, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramelet, A.A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2009, 44, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt-Burri, N.; Ramelet, A.A.; Raffoul, W.; de Buys Roessingh, A.S.; Scaletta, C.; Pioletti, D.P.; Applegate, L.A. Biologicals and fetal cell therapy for wound and scar management. ISRN Dermatol. 2011, 2011, 549870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Buys Roessingh, A.S.; Guerid, S.; Que, Y.; Berger, M.; Hirt-Burri, N.; Scaletta, C.; Raffoul, W.; Alatossava, T.; Applegate, L.A. Cell therapy assistance in reconstructive surgery for musculoskeletal tissues following burn and trauma: Swiss cellular Transplantation Platform. Def. Manag. 2013, S3, 003. [Google Scholar] [CrossRef] [Green Version]
- De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A decade after foetal skin progenitor cell therapy in pediatric burn treatment. J. Regen. Med. 2015, 4, 1. [Google Scholar] [CrossRef]
- Dimitropoulos, G.; Jafari, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn patient care lost in good manufacturing practices? Ann. Burn. Fire Disasters 2016, 29, 111–115. [Google Scholar]
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef]
- Laurent, A.; Simon, J.P.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A.; de Buys Roessingh, A.S. GMP-grade allogeneic musculoskeletal primary progenitor cell types: Standardized candidates for general or pharmacopeial monograph elaboration. J. Transl. Sci. 2020, 7, 1–3. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Progenitor Biological Bandages: An authentic Swiss tool for safe therapeutic management of burns, ulcers, and donor site grafts. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.P.; Ezzi, O.E.; et al. Retrospective evaluation of Progenitor Biological Bandage use: A Complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 5 D. Quality of Biotechnological Products: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products; CPMP/ICH/294/95; European Medicines Agency: Amsterdam, The Netherlands, 1998. [Google Scholar]
- European Parliament and Council. Directive 2004/23/EC on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2004, 102, 48–58. [Google Scholar]
- European Commission. Commission Directive (EC) No 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Off. J. Eur. Union 2006, L 38. [Google Scholar]
- European Commission. Commission Directive (EC) No 2006/86/EC of 24 October 2006 implementing Directive 2004/23/EC of the European parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2006, L 294. [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Directorate for the Quality of Medicines & Healthcare; European Committee (Partial Agreement) on organ transplantation (CD-P-TO). Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; ISBN 978-92-871-8945-5. [Google Scholar]
- Wadman, M. The Vaccine Race: Science, Politics, and the Human Costs of Defeating Disease; Penguin Books: New York, NY, USA, 2017; ISBN 978-0143111313. [Google Scholar]
- Rivers, T.M.; Haagen, E.; Muckenfuss, R.S. Observations concerning the persistence of living cells in Maitland’s medium for the cultivation of vaccine virus. J. Exp. Med. 1929, 50, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Expert Committee on Biological Standardization. Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks. WHO Tech. Rep. Ser. 2013, 978. [Google Scholar]
- Center for Biologics Evaluation and Reasearch, US Food and Drug Administration. Guidance for Industry on Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications; FDA: Silver Spring, MD, USA, 2010.
- European Medicines Agency. ICH Topic Q 5 A (R1). Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin; European Medicines Agency: Amsterdam, The Netherlands, 1997; CPMP/ICH/295/95. [Google Scholar]
- US Food and Drug Administration. Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals; FDA: Silver Springs, MD, USA, 1993.
- European Pharmacopoeia. Cell Substrates for the Production of Vaccines for Human Use; EDQM: Strasbourg, France, 2008. [Google Scholar]
- Xie, L.; Metallo, C.; Warren, J.; Pilbrough, W.; Peltier, J.; Zhong, T.; Pikus, L.; Yancy, A.; Leung, J.; Auniņs, J.G.; et al. Large-scale propagation of a replication-defective adenovirus vector in stirred-tank bioreactor PER.C6 cell culture under sparging conditions. Biotechnol. Bioeng. 2003, 83, 45–52. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, N.; Sambhara, S.; Mittal, S.K. Egg-independent vaccine strategies for highly pathogenic H5N1 influenza viruses. Hum. Vaccin. 2010, 6, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Schiedner, G.; Hertel, S.; Kochanek, S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: Generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 2000, 11, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Trent, D.; Shin, J.; Hombach, J.; Knezevic, I.; Minor, P.; WHO Working Group. WHO Working Group on technical specifications for manufacture and evaluation of dengue vaccines, Geneva, Switzerland, 11–12 May 2009. Vaccine 2010, 28, 8246–8255. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Regulation (EC) No 726/2004 laying down Community procedures for the authorization and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Off. J. Eur. Union 2004, L 136. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off. J. Eur. Union 2007, L 324. [Google Scholar]
- Federal Assembly of the Swiss Confederation. Federal Act on Medicinal Products and Medical Devices (Therapeutic Products Act, TPA); 2000; SR 812: 21.
- European Parliament and Council. Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. Eur. Union 2001, L 311. [Google Scholar]
- Committee for medicinal product for human use. Guideline on Human Cell-Based Medicinal Products; EMEA/CHMP/410869/2006; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- European Commission. Commission Directive (EC) No 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use. Off. J. Eur. Union 2003, L 262. [Google Scholar]
- European Commission. EudraLex, The Rules Governing Medicinal Products in the European Union, Volume 4, Good Manufacturing Practice, Guidelines of 22.11.2017, Good Manufacturing Practice for Advanced Therapy Medicinal Products; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Medicines Agency. ICH Topic Q 7. Good Manufacturing Practice for Active Pharmaceutical Ingredients; CPMP/ICH/4106/00; European Medicines Agency: Amsterdam, The Netherlands, 2000. [Google Scholar]
- European Medicines Agency. Re-Establishment of Working Seeds and Working Cell Banks Using TSE Compliant Materials; EMEA/22314/02; European Medicines Agency: Amsterdam, The Netherlands, 2002. [Google Scholar]
- European Medicines Agency. Guideline on the Use of Porcine Trypsin Used in the Manufacture of Human Biological Medicinal Products; EMA/CHMP/BWP/814397/2011; European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- European Medicines Agency. Creutzfeldt-Jakob Disease and Advanced Therapy Medicinal Products; EMA/CHMP/BWP/353632/2010; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- European Medicines Agency. Note for Guidance on the Use of Bovine Serum in the Manufacture of Human Biological Medicinal Products; CPMP/BWP/1793/02; European Medicines Agency: Amsterdam, The Netherlands, 2003. [Google Scholar]
- European Medicines Agency. ICH Topic Q 5 E. Note for Guidance on Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process; CPMP/ICH/5721/03; European Medicines Agency: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Kusakawa, S.; Machida, K.; Yasuda, S.; Takada, N.; Kuroda, T.; Sawada, R.; Okura, H.; Tsutsumi, H.; Kawamata, S.; Sato, Y. Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rγnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products. Regen. Ther. 2015, 1, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlotte Lozier Institute. Update: COVID-19 Vaccine Candidates and Abortion-Derived Cell Lines; Charlotte Lozier Institute: Arlington, TX, USA, 2021. [Google Scholar]
- European Medicines Agency. ICH Topic Q 9. Guideline on Quality Risk Management; EMA/CHMP/ICH/24235/2006; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- European Medicines Agency. ICH Topic Q 10. Guideline on Pharmaceutical Quality System; EMA/CHMP/ICH/214732/2007; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- European Medicines Agency. ICH Topic Q 6 B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products; CPMP/ICH/365/96; European Medicines Agency: Amsterdam, The Netherlands, 1999. [Google Scholar]
- European Medicines Agency. ICH Topic Q 5 C. Stability Testing of Biotechnological/Biological Products; CPMP/ICH/138/95; European Medicines Agency: Amsterdam, The Netherlands, 1996. [Google Scholar]
- European Medicines Agency. Guideline on Risk Management Systems for Medicinal Products for Human Use; EMEA/CHMP/96268/2005; European Medicines Agency: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Zhao, R.C. Stem cell-based therapy for coronavirus disease. Stem Cells Dev. 2020, 29, 679–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Kardia, E.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B. Aerosol-based delivery of fibroblast cells for treatment of lung diseases. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halim, N.S.; Ch’ng, E.S.; Kardia, E.; Ali, S.A.; Radzi, R.; Yahaya, B.H. Aerosolised mesenchymal stem cells expressing angiopoietin-1 enhances airway repair. Stem Cell Rev. Rep. 2019, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, E.G.; Koch, J.M.; Centanni, J.M.; Hacker, T.A.; Braun, R.K.; Eldridge, M.; Hei, D.J.; Hematti, P.; Raval, A.N. Biodistribution and clearance of human mesenchymal stem cells by quantitative three-dimensional cryo-imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl. Med. 2016, 5, 1668–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khawaga, S.; Abdelalim, E.M. Potential application of mesenchymal stem cells and their exosomes in lung injury: An emerging therapeutic option for COVID-19 patients. Stem Cell Res. Ther. 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Hoffmann, A.; Lee-Thedieck, C. Limited potential or unfavorable manipulations? Strategies toward efficient mesenchymal stem/stromal cell applications. Front. Cell Dev. Biol. 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, S.; Guo, B.; Gao, T.; Wang, L.; Wang, Y.; Wang, L.; Tan, Y.; Wu, J.; Hao, J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020, 53, e12939. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Therapeutic potential of mesenchymal stem cells and their products in lung diseases-intravenous administration versus inhalation. Pharmaceutics 2021, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. 1), S32–S34. [Google Scholar] [CrossRef]
- Monti, M.; Perotti, C.; Del Fante, C.; Cervio, M.; Redi, C.A. Fondazione IRCCS Policlinico San Matteo, Pavia. Stem cells: Sources and therapies. Biol. Res. 2012, 45, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Gottlieb, S. Balancing safety and innovation for cell-based regenerative medicine. N. Engl. J. Med. 2018, 378, 954–959. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchellaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic Advanced Therapy Medicinal Product: A national evaluation. Cythotherapy 2016, 18, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Aspal, M.; Zemans, R.L. Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration. Int. J. Mol. Sci. 2020, 21, 3188. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Song, H. Regulation of alveolar type 2 stem/progenitor cells in lung injury and regeneration. Acta Biochim. Biophys. Sin. 2020, 52, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Ionescu, L.I.; Vadivel, A.; Collins, J.; Xu, L.; Zhong, S.; Kang, M.; de Caen, G.; Daneshmand, M.; Shi, J.; et al. Human induced pluripotent stem cell-derived lung progenitor and alveolar epithelial cells attenuate hyperoxia-induced lung injury. Cytotherapy 2018, 20, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Palomo, B.; Sanchez-Lopez, L.I.; Moodley, Y.; Edel, M.J.; Serrano-Mollar, A. Induced pluripotent stem cell-derived lung alveolar epithelial type II cells reduce damage in bleomycin-induced lung fibrosis. Stem Cell Res. Ther. 2020, 11, 213. [Google Scholar] [CrossRef]

|
|
|
|
| Framework Document | Jurisdictional Level and Application |
|---|---|
| Human research and consent | Regulated in federal laws and applicable EU texts |
| Federal Transplantation Program 1 | Registered with the Federal Office of Public Health or the Swiss Institute for Therapeutic Products (Swissmedic) |
| Ethics Commission | Regulated at a state level by the Ethics Committees |
| Biobank Regulations 2 | Regulated at an institutional level in University Hospitals or within the framework of a local/regional/national biobank system |
| • Full traceability is available to the origin of the cell source, around derivation of the cell line/type, and materials used in the preparation of the cell seed stocks. |
| • The research is subject to open international scientific scrutiny and collaborative technical investigations into the characteristics of the cells and the possible presence of adventitious agents. |
| • The cell characterization results are peer-reviewed and published. |
| • Investigations are evaluated under the auspices of WHO expert review and cells are qualified as suitable for industrial use. |
| • The supply of cells is free of any constraint related to intellectual property rights on final products. |
| • A single source of cells exists with a growing, scientifically, and technically updated body of safety-testing data and safe history of use, giving increased confidence to manufacturers, regulators, and public policy makers. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine. Cells 2021, 10, 2526. https://doi.org/10.3390/cells10102526
Laurent A, Abdel-Sayed P, Hirt-Burri N, Scaletta C, Michetti M, de Buys Roessingh A, Raffoul W, Applegate LA. Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine. Cells. 2021; 10(10):2526. https://doi.org/10.3390/cells10102526
Chicago/Turabian StyleLaurent, Alexis, Philippe Abdel-Sayed, Nathalie Hirt-Burri, Corinne Scaletta, Murielle Michetti, Anthony de Buys Roessingh, Wassim Raffoul, and Lee Ann Applegate. 2021. "Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine" Cells 10, no. 10: 2526. https://doi.org/10.3390/cells10102526
APA StyleLaurent, A., Abdel-Sayed, P., Hirt-Burri, N., Scaletta, C., Michetti, M., de Buys Roessingh, A., Raffoul, W., & Applegate, L. A. (2021). Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine. Cells, 10(10), 2526. https://doi.org/10.3390/cells10102526








