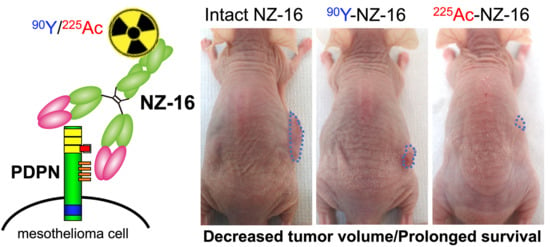
Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibody
2.2. Cell Culture
2.3. Radiolabeling of Antibodies
2.4. Cell Binding and Competitive Inhibition Assays
2.5. Tumor Model
2.6. Biodistribution of Radiolabeled Antibodies
2.7. Dosimetry
2.8. Radioimmunotherapy with 90Y- and 225Ac-Labeled Antibody
2.9. Histologic Analysis
2.10. Statistical Analysis
3. Results
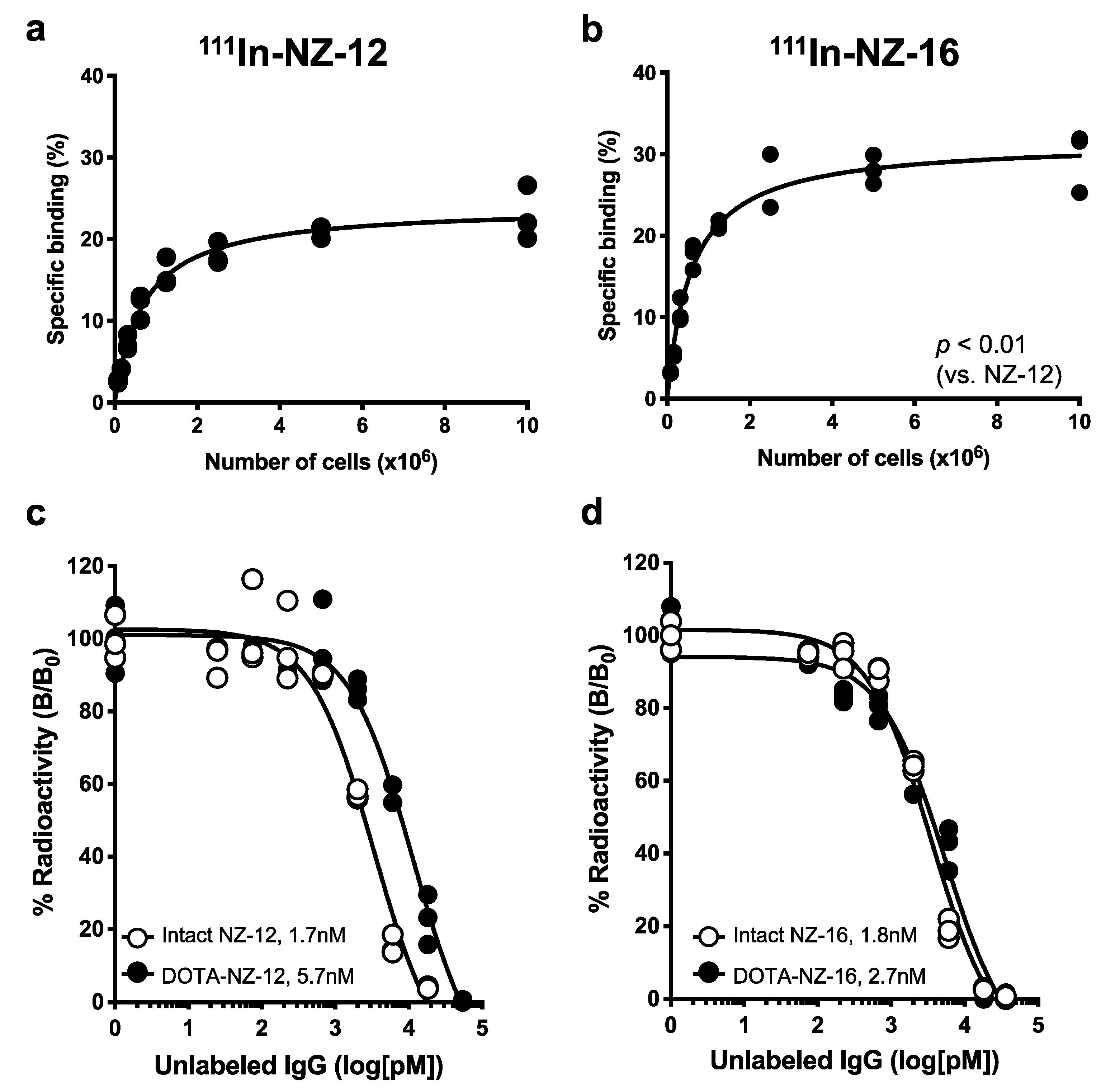
3.1. In Vitro Characterization of the Antibodies
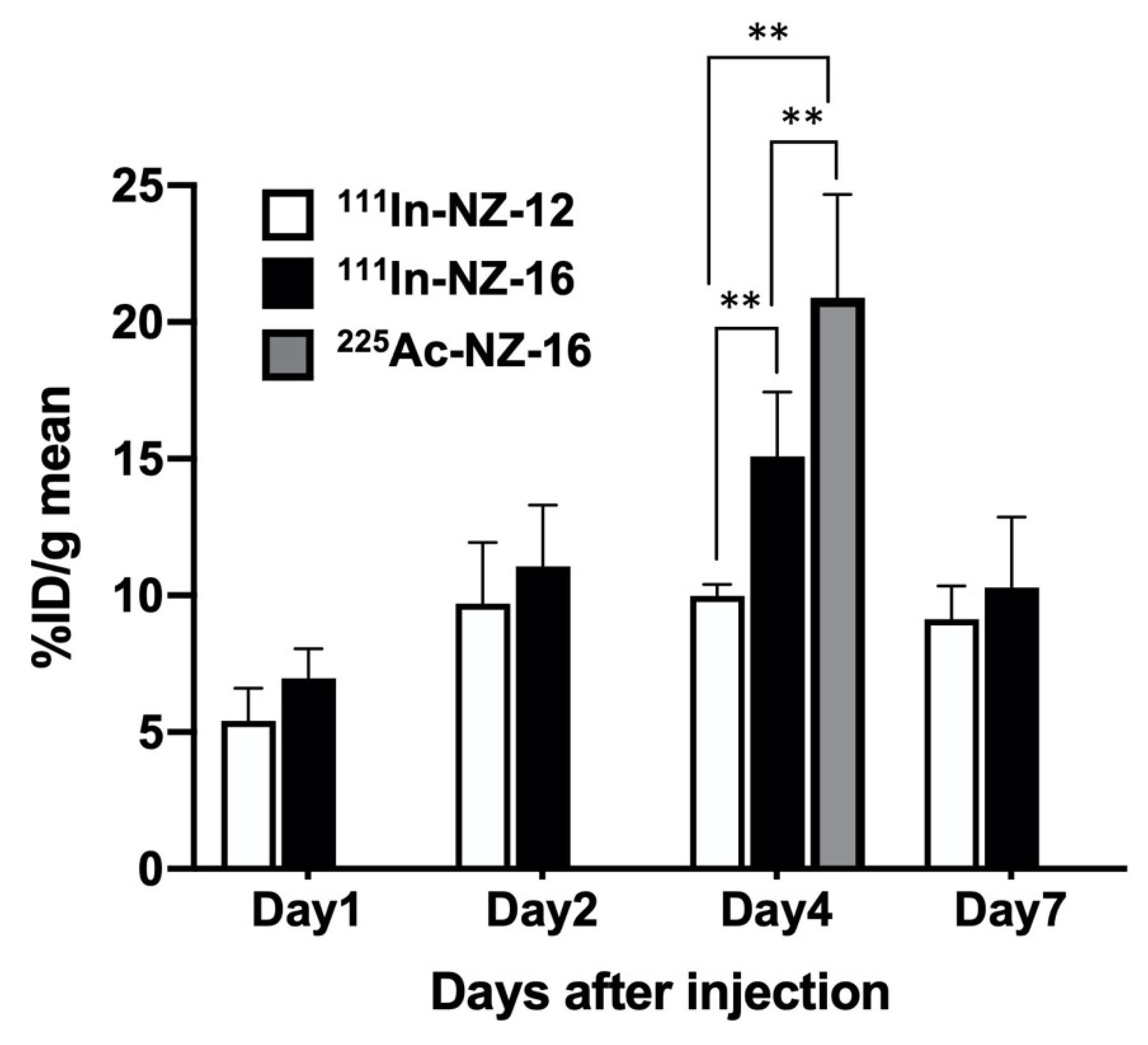
3.2. Biodistribution of 111In-Labeled Antibodies in Nude Mice Bearing H226 Tumors
3.3. Dosimetry
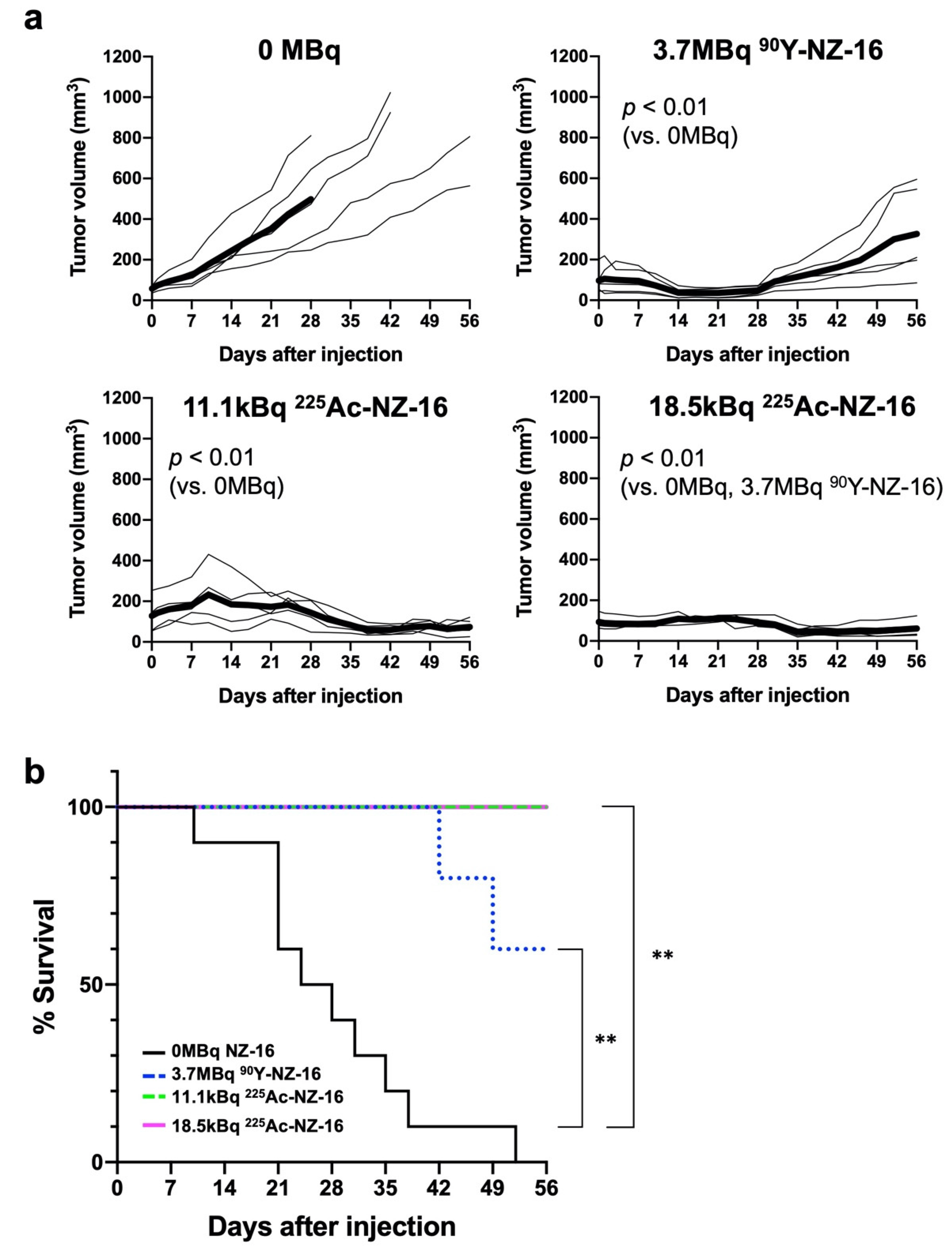
3.4. Treatment Effects of Radiolabeled Antibodies in Nude Mice Bearing H226 Tumors
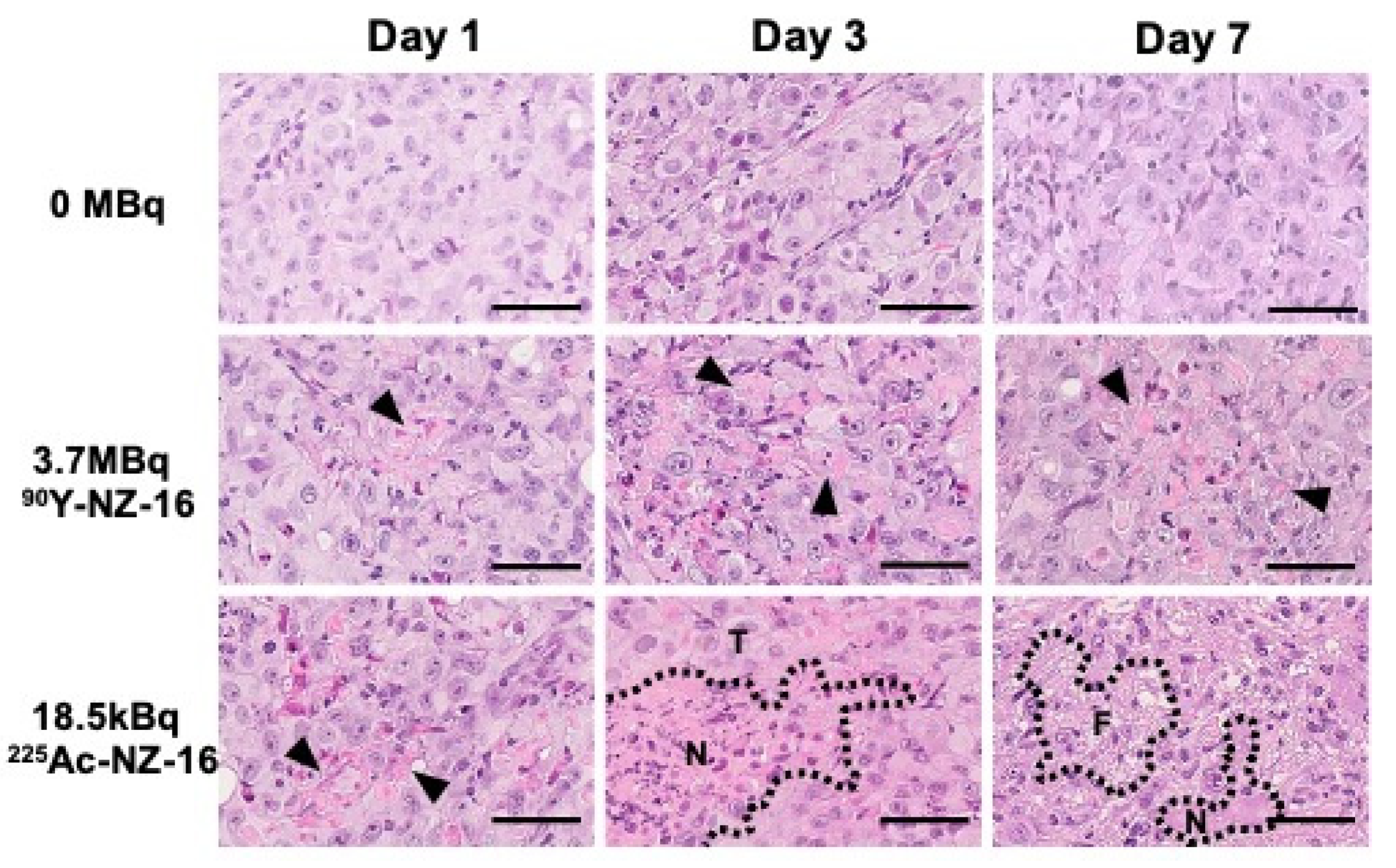
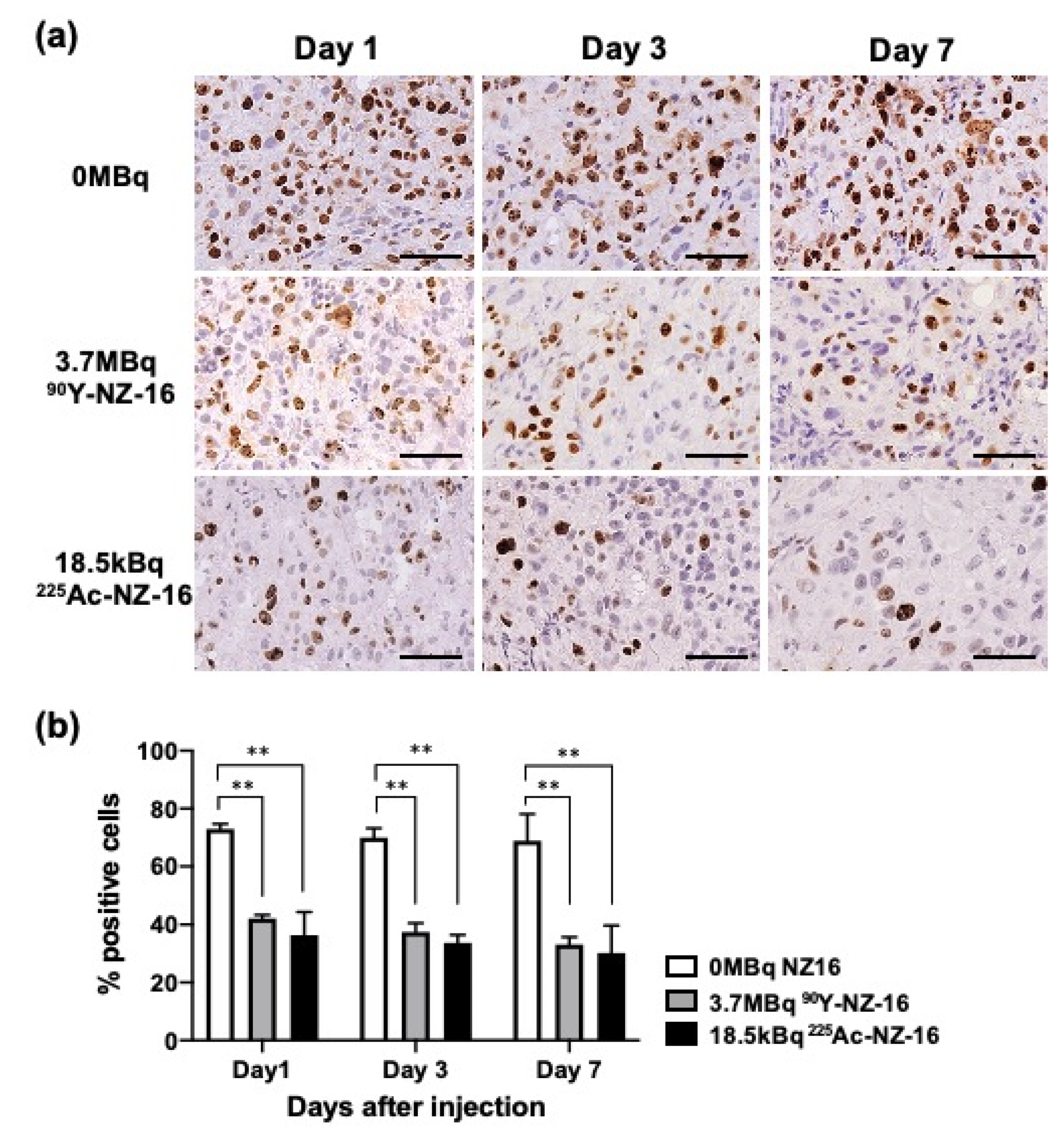
3.5. Histologic Analysis of H226 Tumors Treated with 90Y- and 225Ac-Labeled NZ-16
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, B.W.; Lake, R.A. Advances in malignant mesothelioma. N. Engl. J. Med. 2005, 353, 1591–1603. [Google Scholar] [CrossRef] [Green Version]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., III; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef]
- Ordonez, N.G. Immunohistochemical diagnosis of epithelioid mesothelioma: An update. Arch. Pathol. Lab. Med. 2005, 129, 1407–1414. [Google Scholar] [CrossRef]
- Sterman, D.H.; Albelda, S.M. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology 2005, 10, 266–283. [Google Scholar] [CrossRef]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Saga, T.; Kaneko, M.K.; Kato, Y.; Higashi, T. Therapeutic efficacy evaluation of radioimmunotherapy with 90Y-labeled anti-podoplanin antibody NZ-12 for mesothelioma. Cancer Sci. 2019, 110, 1653–1664. [Google Scholar] [CrossRef] [Green Version]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, P.; Temam, S.; El-Naggar, A.; Zhou, X.; Liu, D.D.; Lee, J.J.; Mao, L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 2006, 107, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Kato, Y.; Kaneko, M.K.; Nishikawa, R.; Hirose, T.; Matsutani, M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006, 111, 483–488. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sato, S.; Naito, M.; Kato, Y.; Mishima, K.; Arai, H.; Tsuruo, T.; Fujita, N. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood 2008, 112, 1730–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramohan, V.; Bao, X.; Kato Kaneko, M.; Kato, Y.; Keir, S.T.; Szafranski, S.E.; Kuan, C.T.; Pastan, I.H.; Bigner, D.D. Recombinant anti-podoplanin (NZ-1) immunotoxin for the treatment of malignant brain tumors. Int. J. Cancer 2013, 132, 2339–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Morita, Y.; Kaneko, M.K.; Hanibuchi, M.; Tsujimoto, Y.; Goto, H.; Kakiuchi, S.; Aono, Y.; Huang, J.; Sato, S.; et al. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J. Immunol. 2013, 190, 6239–6249. [Google Scholar] [CrossRef] [Green Version]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int. J. Mol. Sci. 2016, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miederer, M.; Scheinberg, D.A.; McDevitt, M.R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Deliv. Rev. 2008, 60, 1371–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, M.K.; Abe, S.; Ogasawara, S.; Fujii, Y.; Yamada, S.; Murata, T.; Uchida, H.; Tahara, H.; Nishioka, Y.; Kato, Y. Chimeric Anti-Human Podoplanin Antibody NZ-12 of Lambda Light Chain Exerts Higher Antibody-Dependent Cellular Cytotoxicity and Complement-Dependent Cytotoxicity Compared with NZ-8 of Kappa Light Chain. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 25–29. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; Uchiyama, N.; Amano, K.; Chiba, Y.; Hasegawa, Y.; Hirabayashi, J.; Narimatsu, H.; Mishima, K.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem. Biophys. Res. Commun. 2006, 349, 1301–1307. [Google Scholar] [CrossRef]
- Maguire, W.F.; McDevitt, M.R.; Smith-Jones, P.M.; Scheinberg, D.A. Efficient 1-step radiolabeling of monoclonal antibodies to high specific activity with 225Ac for alpha-particle radioimmunotherapy of cancer. J. Nucl. Med. 2014, 55, 1492–1498. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, C.; Tsuji, A.B.; Sudo, H.; Sugyo, A.; Kikuchi, T.; Koizumi, M.; Arano, Y.; Saga, T. Therapeutic efficacy of c-kit-targeted radioimmunotherapy using 90Y-labeled anti-c-kit antibodies in a mouse model of small cell lung cancer. PLoS ONE 2013, 8, e59248. [Google Scholar] [CrossRef]
- Eckerman, K.F.; Endo, A. MIRD Radionuclide Data and Decay Schemes, 2nd ed.; Society of Nuclear Medicine: Reston, VA, USA, 2007; p. 671. [Google Scholar]
- Behr, T.M.; Sgouros, G.; Stabin, M.G.; Behe, M.; Angerstein, C.; Blumenthal, R.D.; Apostolidis, C.; Molinet, R.; Sharkey, R.M.; Koch, L.; et al. Studies on the red marrow dosimetry in radioimmunotherapy: An experimental investigation of factors influencing the radiation-induced myelotoxicity in therapy with beta-, Auger/conversion electron-, or alpha-emitters. Clin. Cancer Res. 1999, 5, 3031s–3043s. [Google Scholar]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of α-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Kaneko, M.; Sata, M.; Fujita, N.; Tsuruo, T.; Osawa, M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour. Biol. 2005, 26, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ogose, A.; Kawashima, H.; Hotta, T.; Ariizumi, T.; Li, G.; Umezu, H.; Endo, N. High-level expression of podoplanin in benign and malignant soft tissue tumors: Immunohistochemical and quantitative real-time RT-PCR analysis. Oncol. Rep. 2011, 25, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Sasagawa, I.; Kaneko, M.; Osawa, M.; Fujita, N.; Tsuruo, T. Aggrus: A diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene 2004, 23, 8552–8556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshima, Y.; Sudo, H.; Watanabe, S.; Nagatsu, K.; Tsuji, A.B.; Sakashita, T.; Ito, Y.M.; Yoshinaga, K.; Higashi, T.; Ishioka, N.S. Antitumor effects of radionuclide treatment using alpha-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Frampas, E.; Maurel, C.; Remaud-Le Saec, P.; Mauxion, T.; Faivre-Chauvet, A.; Davodeau, F.; Goldenberg, D.M.; Bardies, M.; Barbet, J. Pretargeted radioimmunotherapy of colorectal cancer metastases: Models and pharmacokinetics predict influence of the physical and radiochemical properties of the radionuclide. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2153–2164. [Google Scholar] [CrossRef]
- Nadal, E.; Bosch-Barrera, J.; Cedres, S.; Coves, J.; Garcia-Campelo, R.; Guirado, M.; Lopez-Castro, R.; Ortega, A.L.; Vicente, D.; de Castro-Carpeno, J. SEOM clinical guidelines for the treatment of malignant pleural mesothelioma (2020). Clin. Transl. Oncol. 2021, 23, 980–987. [Google Scholar] [CrossRef]
- Emami, B. Tolerance of Normal Tissue to Therapeutic Radiation. Rep. Radiother. Oncol. 2013, 1, 123–127. [Google Scholar]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Back, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMMI Res. 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurcic, J.G. Targeted Alpha-Particle Therapy for Hematologic Malignancies. J. Med. Imaging Radiat. Sci. 2019, 50, S53–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleinick, N.L.; Biswas, T.; Patel, R.; Tao, M.; Patel, R.; Weeks, L.; Sharma, N.; Dowlati, A.; Gerson, S.L.; Fu, P.; et al. Radiosensitization of non-small-cell lung cancer cells and xenografts by the interactive effects of pemetrexed and methoxyamine. Radiother. Oncol. 2016, 121, 335–341. [Google Scholar] [CrossRef] [PubMed]
| Day 1 | Day 2 | Day 4 | Day 7 | |
|---|---|---|---|---|
| NZ-12 | ||||
| Blood | 18.0 ± 1.5 | 13.8 ± 1.5 | 8.6 ± 2.3 | 5.1 ± 0.5 |
| Brain | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Lung | 6.2 ± 0.4 | 5.0 ± 1.0 | 3.6 ± 1.0 | 2.1 ± 0.3 |
| Liver | 15.2 ± 2.1 | 14.1 ± 1.1 | 10.8 ± 1.0 | 7.3 ± 0.9 |
| Spleen | 9.9 ± 0.7 | 9.0 ± 1.4 | 8.6 ± 0.9 | 5.4 ± 1.4 |
| Intestine | 2.0 ± 0.3 | 1.6 ± 0.2 | 1.0 ± 0.2 | 0.6 ± 0.1 |
| Kidney | 12.1 ± 0.3 | 9.5 ± 0.6 | 6.4 ± 1.0 | 3.7 ± 0.7 |
| Muscle | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.3 ± 0.1 |
| NZ-16 | ||||
| Blood | 21.8 ± 2.9 | 19.7 ± 2.6 ** | 13.6 ± 1.4 * | 8.2 ± 4.0 |
| Brain | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.2 |
| Lung | 7.7 ± 1.3 | 6.9 ± 0.7 * | 7.0 ± 2.7 | 3.4 ± 1.4 |
| Liver | 11.3 ± 3.5 * | 9.3 ± 0.9 ** | 7.9 ± 2.3 | 7.1 ± 1.6 |
| Spleen | 7.5 ± 0.8 | 9.7 ± 0.7 | 8.0 ± 0.9 | 8.1 ± 3.1 * |
| Intestine | 2.6 ± 0.6 | 2.2 ± 0.2 | 1.9 ± 0.4 | 1.0 ± 0.4 |
| Kidney | 7.9 ± 1.2 ** | 7.8 ± 0.7 | 7.0 ± 0.7 | 4.9 ± 1.6 |
| Muscle | 1.2 ± 0.2 | 1.2 ± 0.3 * | 1.1 ± 0.1 | 0.7 ± 0.4 |
| Day 4 | |
|---|---|
| Blood | 12.4 ± 0.8 |
| Brain | 2.0 ± 0.4 ** |
| Lung | 9.9 ± 1.1 ** |
| Liver | 10.5 ± 1.5 |
| Spleen | 11.9 ± 1.0 ** |
| Intestine | 2.7 ± 0.6 * |
| Kidney | 7.9 ± 1.0 |
| Muscle | 3.6 ± 1.1 ** |
| 90Y | 225Ac | |||
| NZ-12 | NZ-16 | NZ-12 | NZ-16 | |
| Brain | 0.1 ± 0.0 | 0.2 ± 0.0 | 6.4 ± 0.5 | 9.7 ± 0.8 |
| Lung | 1.7 ± 0.1 | 2.4 ± 0.2 | 84.6 ± 4.7 | 124.2 ± 11.4 |
| Liver | 4.6 ± 0.1 | 3.5 ± 0.2 | 239.0 ± 7.3 | 185.5 ± 13.5 |
| Spleen | 3.2 ± 0.1 | 3.1 ± 0.1 | 168.7 ± 9.4 | 172.1 ± 36.4 |
| Intestine | 0.5 ± 0.0 | 0.7 ± 0.0 | 25.8 ± 1.1 | 37.1 ± 2.0 |
| Kidney | 3.1 ± 0.1 | 2.6 ± 0.1 | 156.9 ± 4.7 | 140.0 ± 9.5 |
| Muscle | 0.3 ± 0.0 | 0.4 ± 0.0 | 15.5 ± 0.9 | 21.1 ± 1.7 |
| Bone marrow a | 1.8 ± 0.1 | 2.5 ± 0.1 | 89.4 ± 4.0 | 126.1 ± 6.1 |
| Tumor | 3.0 ± 0.2 | 3.8 ± 0.2 | 174.6 ± 158.0 | 236.0 ± 108.6 |
| 3.7 MBq 90Y | 11.1 kBq 225Ac | 18.5 kBq 225Ac | |
|---|---|---|---|
| Brain | 0.7 | 0.1 | 0.2 |
| Lung | 8.8 | 1.4 | 2.3 |
| Liver | 12.8 | 2.1 | 3.4 |
| Spleen | 11.4 | 1.9 | 3.2 |
| Intestine | 2.7 | 0.4 | 0.7 |
| Kidney | 9.7 | 1.6 | 2.6 |
| Muscle | 1.5 | 0.2 | 0.4 |
| Bone marrow a | 8.1 | 1.4 | 2.3 |
| Tumor | 14.9 | 2.6 | 4.4 |
| 3.7 MBq 90Y | 11.1 kBq 225Ac | 18.5 kBq 225Ac | |
|---|---|---|---|
| Brain | 0.7 | 0.5 | 0.9 |
| Lung | 8.8 | 6.9 | 11.5 |
| Liver | 12.8 | 10.3 | 17.2 |
| Spleen | 11.4 | 9.6 | 15.9 |
| Intestine | 2.7 | 2.1 | 3.4 |
| Kidney | 9.7 | 7.8 | 13.0 |
| Muscle | 1.5 | 1.2 | 2.0 |
| Bone marrow b | 8.1 | 7.0 | 11.7 |
| Tumor | 14.9 | 13.1 | 21.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudo, H.; Tsuji, A.B.; Sugyo, A.; Kaneko, M.K.; Kato, Y.; Nagatsu, K.; Suzuki, H.; Higashi, T. Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells 2021, 10, 2503. https://doi.org/10.3390/cells10102503
Sudo H, Tsuji AB, Sugyo A, Kaneko MK, Kato Y, Nagatsu K, Suzuki H, Higashi T. Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells. 2021; 10(10):2503. https://doi.org/10.3390/cells10102503
Chicago/Turabian StyleSudo, Hitomi, Atsushi B. Tsuji, Aya Sugyo, Mika K. Kaneko, Yukinari Kato, Kotaro Nagatsu, Hisashi Suzuki, and Tatsuya Higashi. 2021. "Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma" Cells 10, no. 10: 2503. https://doi.org/10.3390/cells10102503
APA StyleSudo, H., Tsuji, A. B., Sugyo, A., Kaneko, M. K., Kato, Y., Nagatsu, K., Suzuki, H., & Higashi, T. (2021). Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells, 10(10), 2503. https://doi.org/10.3390/cells10102503