IL-6 Is Not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium tuberculosis H37rv
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bacteria and Infection
2.3. Colony Enumeration Assay
2.4. Histopathology and Immunohistochemistry
2.5. RT-PCR
2.6. Preparation of Single Cell Suspensions from Infected Lungs
2.7. Flow Cytometry
2.8. ESAT61-20-Specific ELISPOT Assays
2.9. Statistical Analysis
3. Results
3.1. Mtb Infection of IL-6−/− Mice
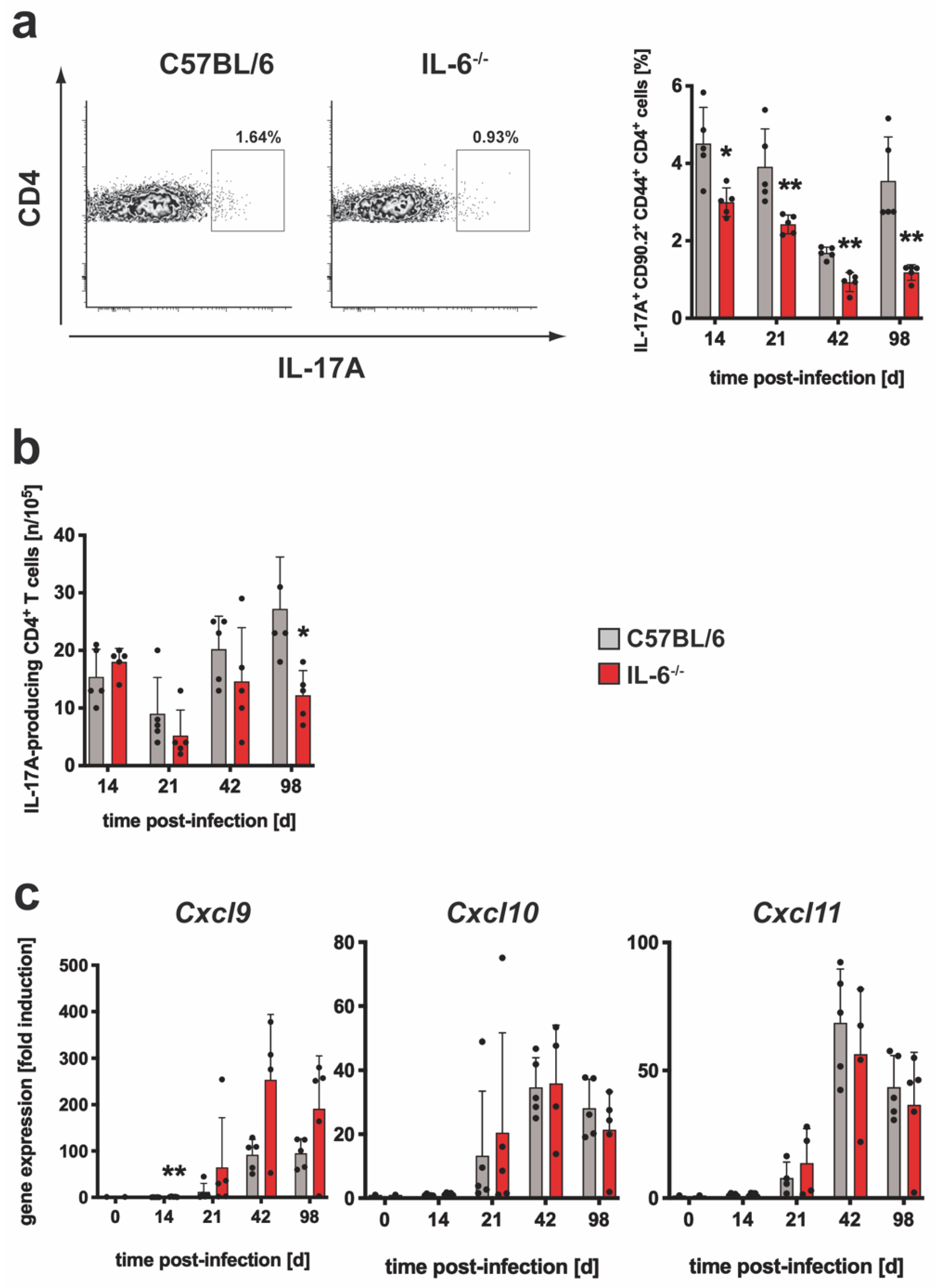
3.1.1. The Absence of IL-6 has a Minor Effect on the Antigen-Specific TH17 Immune Response after Mtb Infection
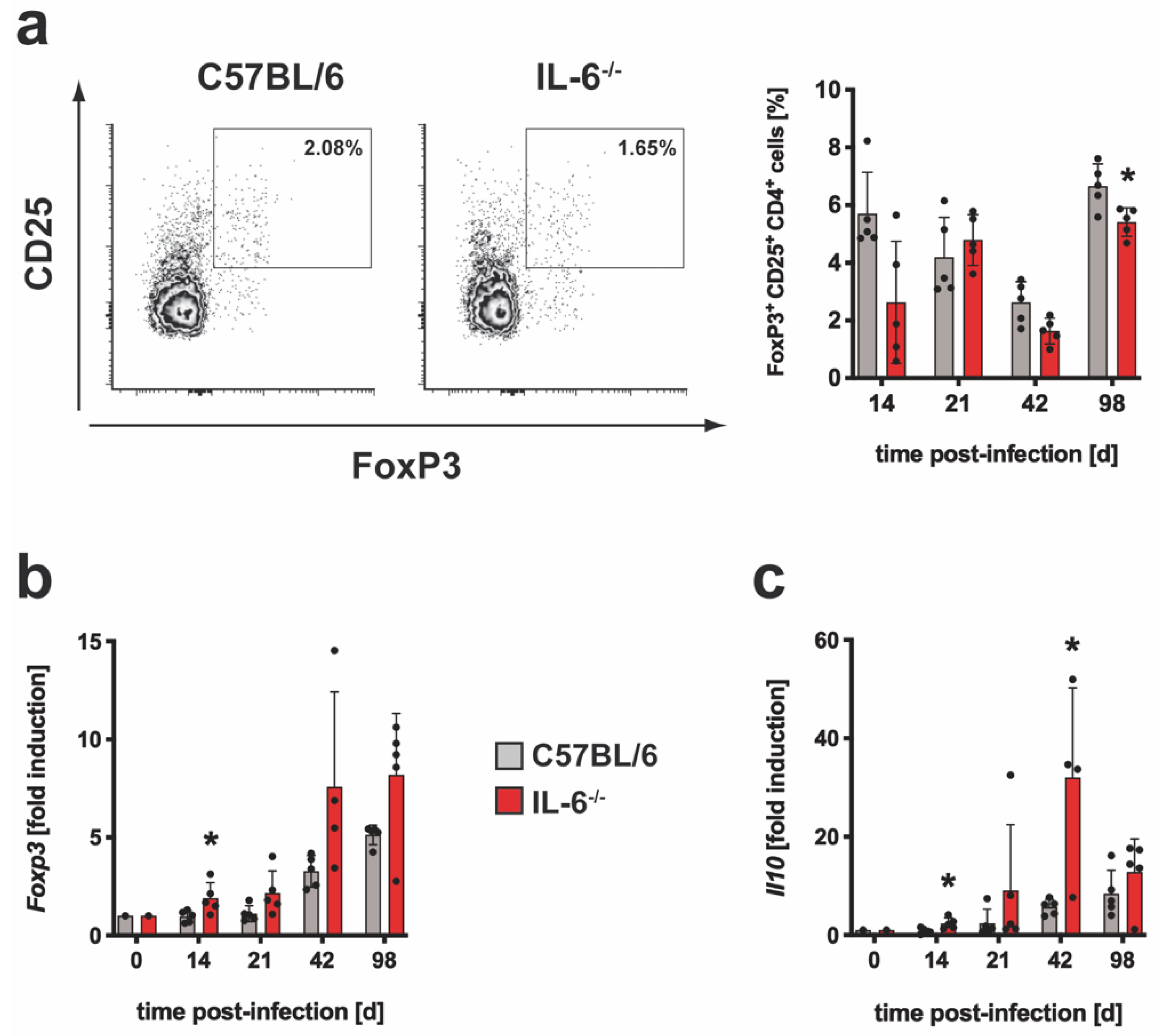
3.1.2. The Regulatory Immune Response in IL-6−/− Mice after Infection with Mtb
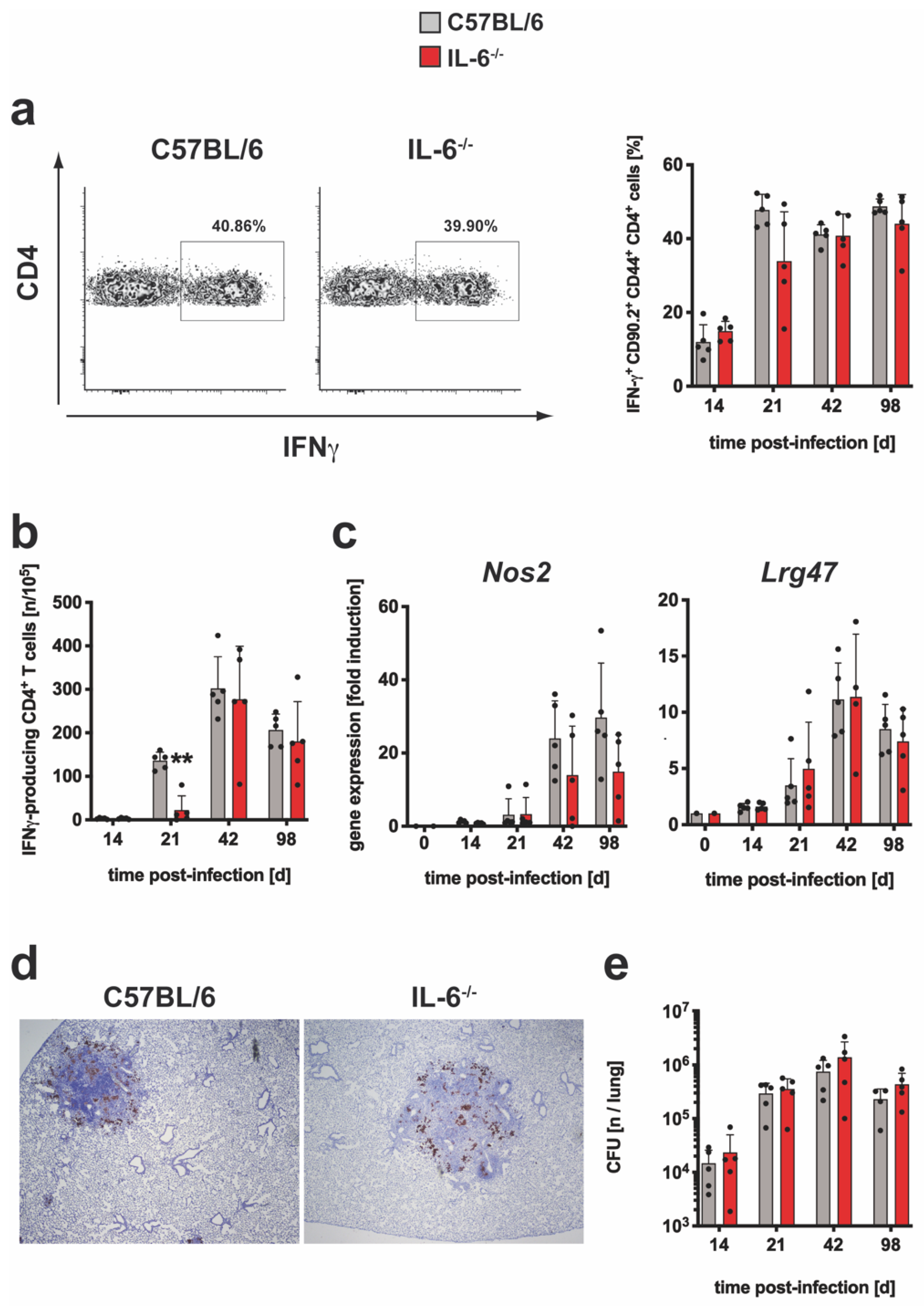
3.1.3. The Overall TH1 Immune Response and Subsequent Macrophage Effector Mechanism Are Not Impaired in Mtb-Infected IL-6−/− Mice
3.2. Experimental TB in CD4cre; gp130loxP/loxP Mice
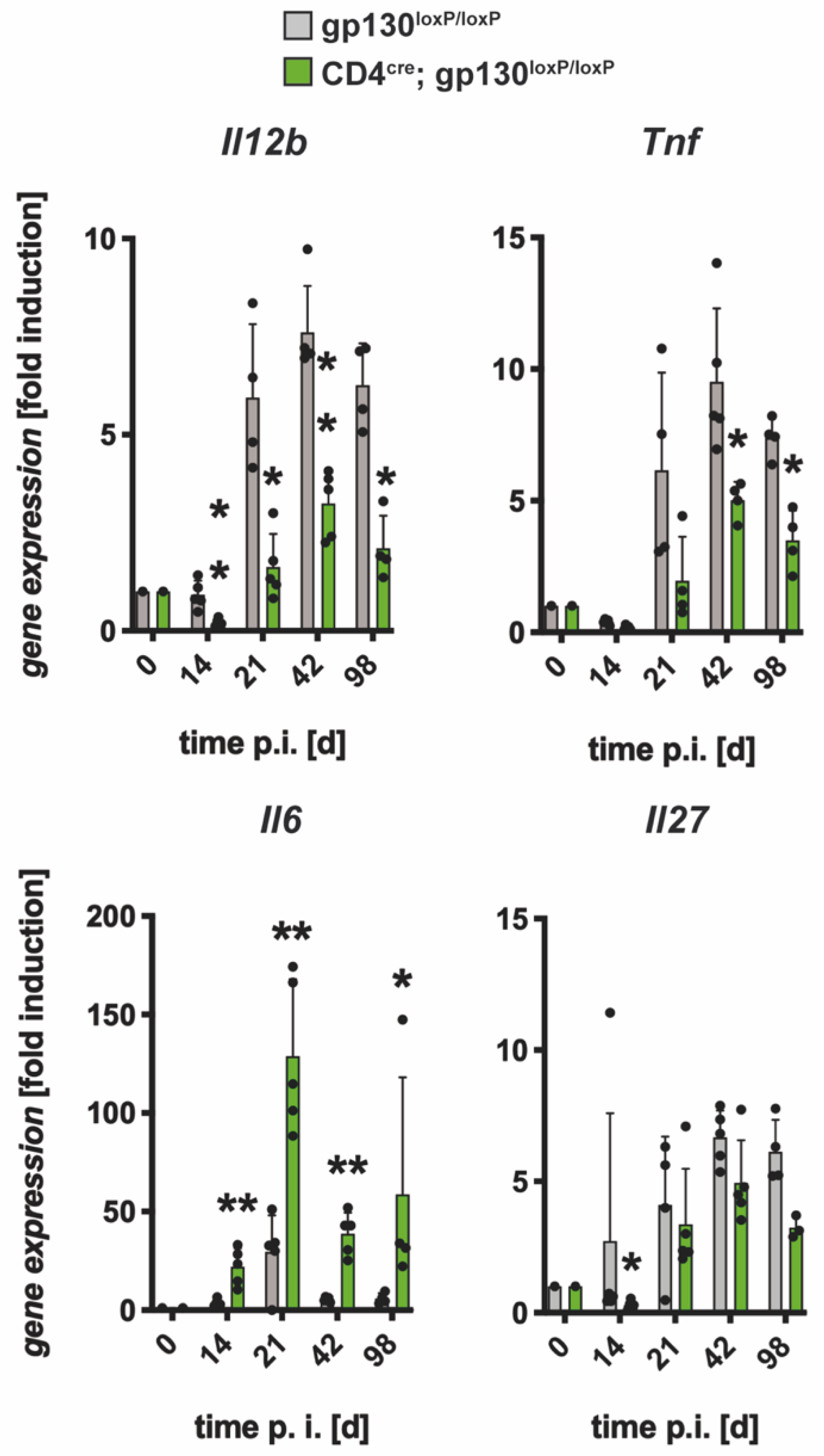
3.2.1. Gene Expression of Il12b, Tnf and Il27 was Decreased Whereas mRNA Levels of Il6 were Elevated in Mtb-Infected CD4cre; gp130loxP/loxP Mice
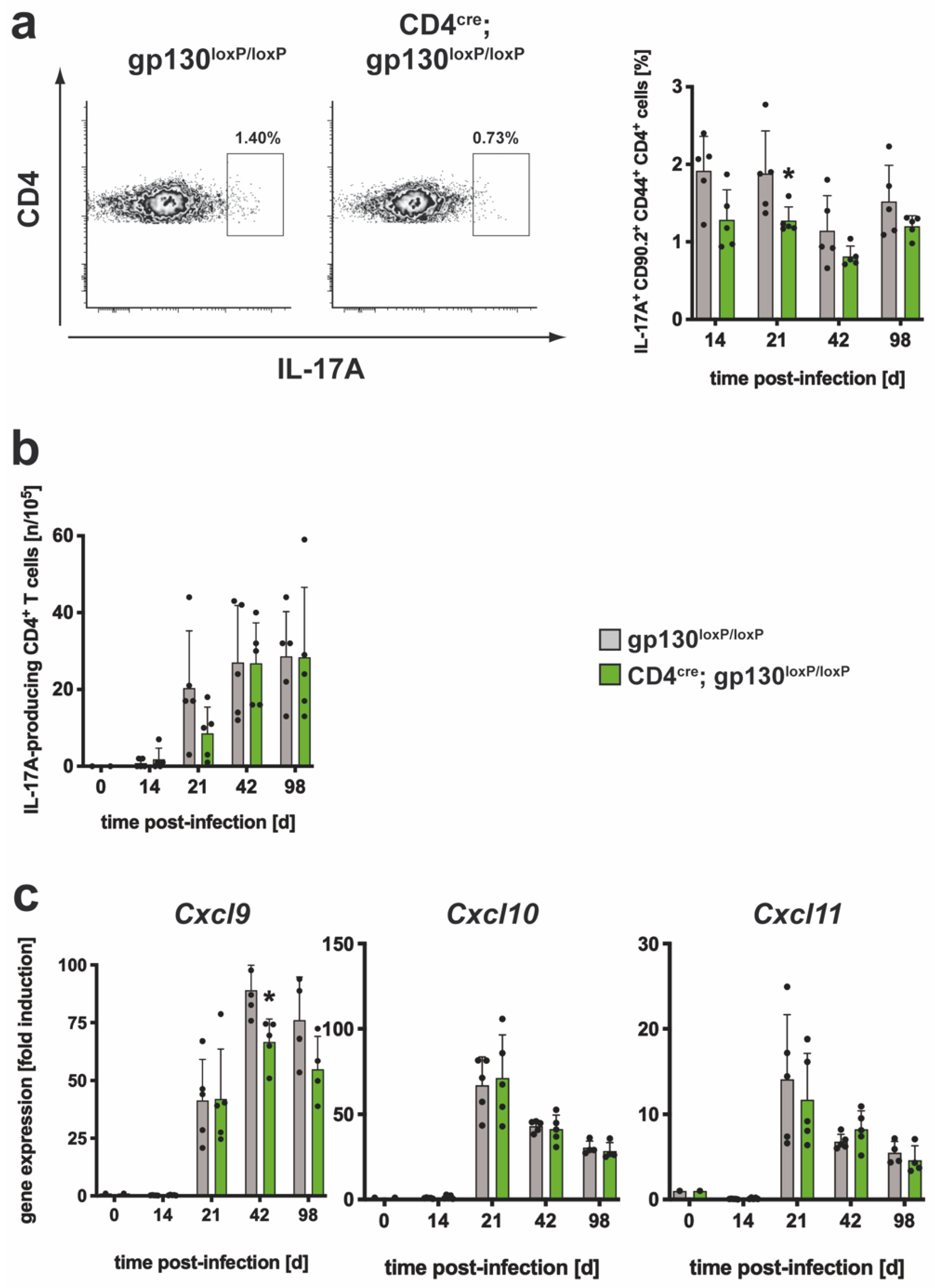
3.2.2. The TH17 Immune Response in Mtb-Infected CD4cre; gp130loxP/loxP Mice Was Only Moderately Affected
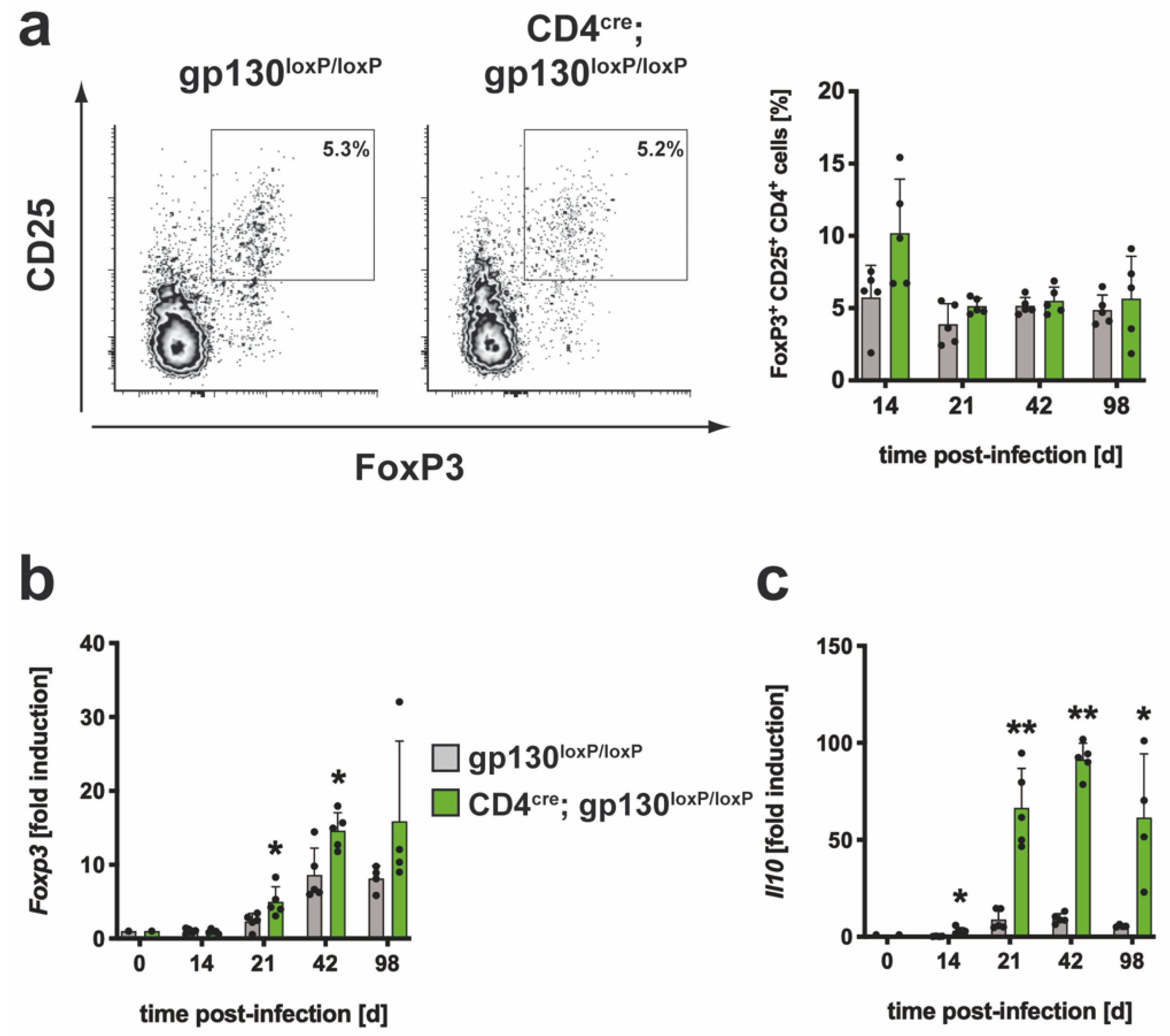
3.2.3. The Regulatory Immune Response in Mtb-Infected CD4cre; gp130loxP/loxP Mice
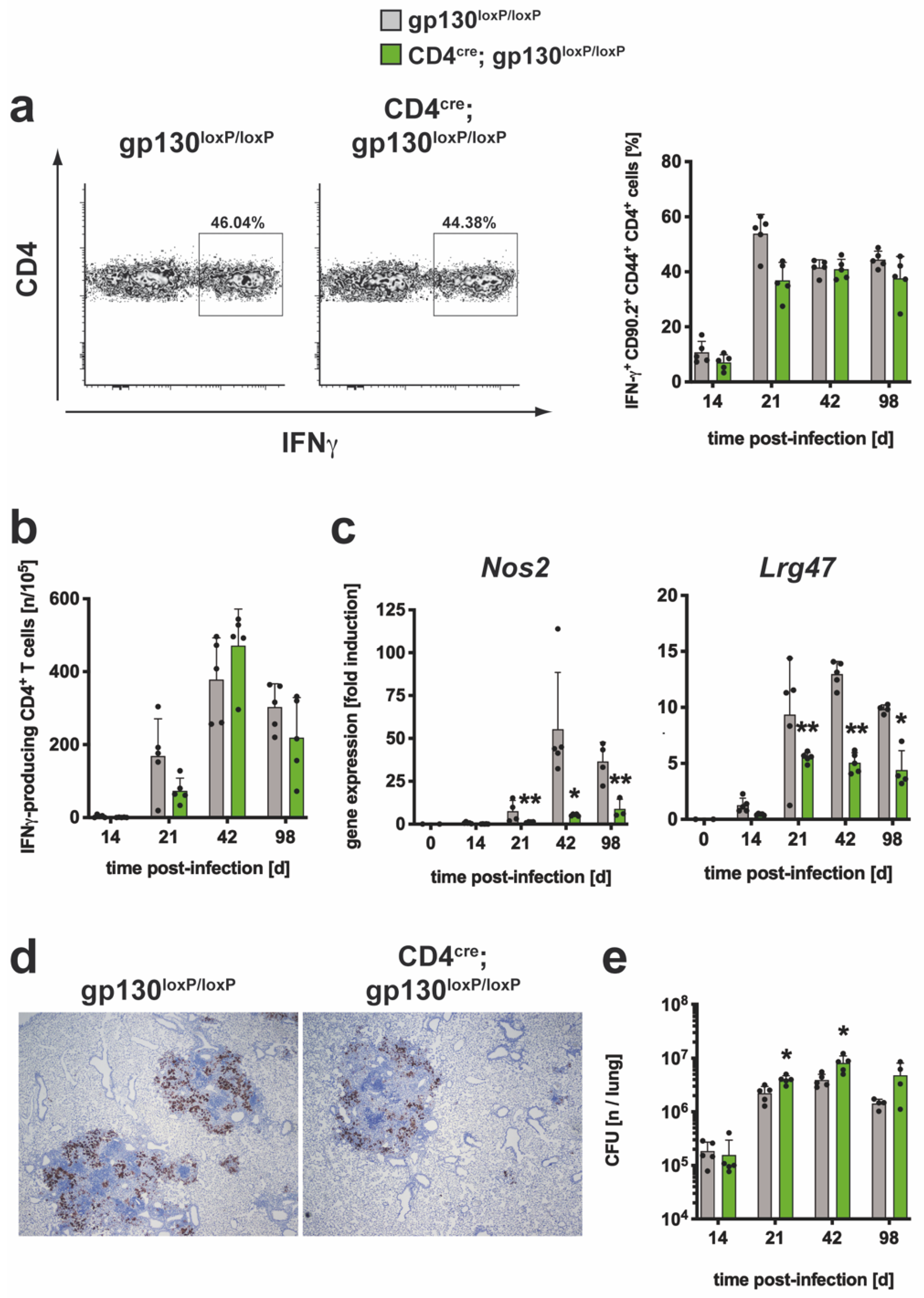
3.2.4. After Mtb Infection, The TH1 Immune Response Was Not Affected by the Absence of gp130 on T Cells yet TH1-Mediated Macrophage Effector Responses Were Severely Impaired
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Maniar, J.K.; Kamath, R.R.; Mandalia, S.; Shah, K.; Maniar, A. HIV and tuberculosis: Partners in crime. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, J.M.; Chan, J.; Flynn, J.L. Latent tuberculosis: Mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 2003, 3, 578–590. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.P.; Scanga, C.A.; Yu, K.; Scott, H.M.; Tanaka, K.E.; Tsang, E.; Tsai, M.M.; Flynn, J.L.; Chan, J. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: Possible role for limiting pathology. Infect. Immun. 2001, 69, 1847–1855. [Google Scholar] [CrossRef]
- North, R.J.; Jung, Y.-J. Immunity to tuberculosis. Annu. Rev. Immunol. 2004, 22, 599–623. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Barber, D.L. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Korbel, D.S.; Schneider, B.E.; Schaible, U.E. Innate immunity in tuberculosis: Myths and truth. Microbes Infect./Inst. Pasteur 2008, 10, 995–1004. [Google Scholar] [CrossRef]
- Gopal, R.; Monin, L.; Slight, S.; Uche, U.; Blanchard, E.; Junecko, B.A.F.; Ramos-Payan, R.; Stallings, C.L.; Reinhart, T.A.; Kolls, J.K.; et al. Unexpected Role for IL-17 in Protective Immunity against Hypervirulent Mycobacterium tuberculosis HN878 Infection. PLoS Pathog. 2014, 10, e1004099. [Google Scholar] [CrossRef]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4(+) T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lindenstrom, T.; Woodworth, J.; Dietrich, J.; Aagaard, C.; Andersen, P.; Agger, E.M. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 2012, 80, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Desel, C.; Dorhoi, A.; Bandermann, S.; Grode, L.; Eisele, B.; Kaufmann, S.H. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 2011, 204, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, H.; Behrends, J.; Ritter, K.; Holscher, A.; Volz, J.; Rosenkrands, I.; Holscher, C. The increased protection and pathology in Mycobacterium tuberculosis-infected IL-27R-alpha-deficient mice is supported by IL-17A and is associated with the IL-17A-induced expansion of multifunctional T cells. Mucosal Immunol. 2018, 11, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Fouser, L.A.; Wright, J.F.; Dunussi-Joannopoulos, K.; Collins, M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol. Rev. 2008, 226, 87–102. [Google Scholar] [CrossRef]
- Singh, R.P.; Hasan, S.; Sharma, S.; Nagra, S.; Yamaguchi, D.T.; Wong, D.T.; Hahn, B.H.; Hossain, A. Th17 cells in inflammation and autoimmunity. Autoimmun. Rev. 2014, 13, 1174–1181. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Yen, D.; Cheung, J.; Scheerens, H.; Poulet, F.; McClanahan, T.; McKenzie, B.; Kleinschek, M.A.; Owyang, A.; Mattson, J.; Blumenschein, W.; et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Investig. 2006, 116, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Eugster, H.P.; Frei, K.; Kopf, M.; Lassmann, H.; Fontana, A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 1998, 28, 2178–2187. [Google Scholar] [CrossRef]
- Okuda, Y.; Sakoda, S.; Bernard, C.C.; Fujimura, H.; Saeki, Y.; Kishimoto, T.; Yanagihara, T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998, 10, 703–708. [Google Scholar] [CrossRef]
- Samoilova, E.B.; Horton, J.L.; Hilliard, B.; Liu, T.S.; Chen, Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: Roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 1998, 161, 6480–6486. [Google Scholar]
- Khader, S.A.; Pearl, J.E.; Sakamoto, K.; Gilmartin, L.; Bell, G.K.; Jelley-Gibbs, D.M.; Ghilardi, N.; Desauvage, F.; Cooper, A.M. IL-23 Compensates for the Absence of IL-12p70 and Is Essential for the IL-17 Response during Tuberculosis but Is Dispensable for Protection and Antigen-Specific IFN-{gamma} Responses if IL-12p70 Is Available. J. Immunol. 2005, 175, 788–795. [Google Scholar] [CrossRef]
- Hölscher, C.; Atkinson, R.A.; Arendse, B.; Brown, N.; Myburgh, E.; Alber, G.; Brombacher, F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 2001, 167, 6957–6966. [Google Scholar] [CrossRef]
- Ladel, C.H.; Blum, C.; Dreher, A.; Reifenberg, K.; Kopf, M.; Kaufmann, S.H. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 1997, 65, 4843–4849. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Betz, U.A.; Bloch, W.; van den Broek, M.; Yoshida, K.; Taga, T.; Kishimoto, T.; Addicks, K.; Rajewsky, K.; Muller, W. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J. Exp. Med. 1998, 188, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Brombacher, F.; Kohler, G.; Kienzle, G.; Widmann, K.H.; Lefrang, K.; Humborg, C.; Ledermann, B.; Solbach, W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J. Exp. Med. 1996, 184, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Behrends, J.; Renauld, J.-C.; Ehlers, S.; Hölscher, C. IL-22 Is Mainly Produced by IFNγ-Secreting Cells but Is Dispensable for Host Protection against Mycobacterium tuberculosis Infection. PLoS ONE 2013, 8, e57379. [Google Scholar] [CrossRef]
- McLoughlin, R.M.; Jenkins, B.J.; Grail, D.; Williams, A.S.; Fielding, C.A.; Parker, C.R.; Ernst, M.; Topley, N.; Jones, S.A. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 9589–9594. [Google Scholar] [CrossRef]
- Schreiber, T.; Ehlers, S.; Heitmann, L.; Rausch, A.; Mages, J.; Murray, P.J.; Lang, R.; Holscher, C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J. Immunol. 2009, 183, 1301–1312. [Google Scholar] [CrossRef]
- Murray, P.J.; Wang, L.; Onufryk, C.; Tepper, R.I.; Young, R.A. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 1997, 158, 315–321. [Google Scholar]
- MacMicking, J.D.; Nathan, C.; Hom, G.; Chartrain, N.; Fletcher, D.S.; Trumbauer, M.; Stevens, K.; Xie, Q.W.; Sokol, K.; Hutchinson, N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1995, 81, 641–650. [Google Scholar] [CrossRef]
- MacMicking, J.D.; Taylor, G.A.; McKinney, J.D. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 2003, 302, 654–659. [Google Scholar] [CrossRef]
- Korn, T.; Mitsdoerffer, M.; Croxford, A.L.; Awasthi, A.; Dardalhon, V.A.; Galileos, G.; Vollmar, P.; Stritesky, G.L.; Kaplan, M.H.; Waisman, A.; et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18460–18465. [Google Scholar] [CrossRef]
- Yamashita, T.; Iwakura, T.; Matsui, K.; Kawaguchi, H.; Obana, M.; Hayama, A.; Maeda, M.; Izumi, Y.; Komuro, I.; Ohsugi, Y.; et al. IL-6-mediated Th17 differentiation through RORγt is essential for the initiation of experimental autoimmune myocarditis. Cardiovasc. Res. 2011, 91, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, T.; Linehan, J.L.; Moon, J.J.; Pepper, M.; Jenkins, M.K.; Cleary, P.P. Robust antigen specific th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 2011, 7, e1002252. [Google Scholar] [CrossRef] [PubMed]
- Okamoto Yoshida, Y.; Umemura, M.; Yahagi, A.; O’Brien, R.L.; Ikuta, K.; Kishihara, K.; Hara, H.; Nakae, S.; Iwakura, Y.; Matsuzaki, G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 2010, 184, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Yahagi, A.; Hamada, S.; Begum, M.D.; Watanabe, H.; Kawakami, K.; Suda, T.; Sudo, K.; Nakae, S.; Iwakura, Y.; et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007, 178, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef]
- Wei, L.; Laurence, A.; Elias, K.M.; O’Shea, J.J. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007, 282, 34605–34610. [Google Scholar] [CrossRef]
- Lei, L.; Zhong, X.N.; He, Z.Y.; Zhao, C.; Sun, X.J. IL-21 induction of CD4+ T cell differentiation into Th17 cells contributes to bleomycin-induced fibrosis in mice. Cell Biol. Int. 2015, 39, 388–399. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jäger, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Zhao, Z.; Yu, Y.; Fan, H.; Xu, X.; Bu, X.; Gu, J. IL-21 Induces an Imbalance of Th17/Treg Cells in Moderate-to-Severe Plaque Psoriasis Patients. Front. Immunol. 2019, 10, 1865. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, W.; Liu, C.; Zheng, X.; Guo, A.; Long, J. Effect of IL-21 on the Balance of Th17 Cells/Treg Cells in the Pathogenesis of Graves’ Disease. Endocr. Res. 2019, 44, 138–147. [Google Scholar] [CrossRef]
- Cheekatla, S.S.; Tripathi, D.; Venkatasubramanian, S.; Paidipally, P.; Welch, E.; Tvinnereim, A.R.; Nurieva, R.; Vankayalapati, R. IL-21 Receptor Signaling Is Essential for Optimal CD4(+) T Cell Function and Control of Mycobacterium tuberculosis Infection in Mice. J. Immunol. 2017, 199, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Booty, M.G.; Barreira-Silva, P.; Carpenter, S.M.; Nunes-Alves, C.; Jacques, M.K.; Stowell, B.L.; Jayaraman, P.; Beamer, G.; Behar, S.M. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci. Rep. 2016, 6, 36720. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Griffiths, K.L.; Slight, S.; Lin, Y.; Rangel-Moreno, J.; Khader, S.A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015, 8, 1099–1109. [Google Scholar] [CrossRef]
- Cho, M.L.; Kang, J.W.; Moon, Y.M.; Nam, H.J.; Jhun, J.Y.; Heo, S.B.; Jin, H.T.; Min, S.Y.; Ju, J.H.; Park, K.S.; et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 2006, 176, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun. 2000, 68, 3322–3326. [Google Scholar] [CrossRef] [PubMed]
- Sodenkamp, J.; Behrends, J.; Forster, I.; Muller, W.; Ehlers, S.; Holscher, C. gp130 on macrophages/granulocytes modulates inflammation during experimental tuberculosis. Eur. J. Cell Biol. 2011, 90, 505–514. [Google Scholar] [CrossRef]
- Sodenkamp, J.; Waetzig, G.H.; Scheller, J.; Seegert, D.; Grötzinger, J.; Rose-John, S.; Ehlers, S.; Hölscher, C. Therapeutic targeting of interleukin-6 trans-signaling does not affect the outcome of experimental tuberculosis. Immunobiology 2012, 217, 996–1004. [Google Scholar] [CrossRef]
- Fasnacht, N.; Greweling, M.C.; Bollati-Fogolín, M.; Schippers, A.; Müller, W. T-cell-specific deletion of gp130 renders the highly susceptible IL-10-deficient mouse resistant to intestinal nematode infection. Eur. J. Immunol. 2009, 39, 2173–2183. [Google Scholar] [CrossRef]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef]
- Martinez, A.N.; Mehra, S.; Kaushal, D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J. Infect. Dis. 2013, 207, 1253–1261. [Google Scholar] [CrossRef]
- Taylor, G.A.; Jeffers, M.; Largaespada, D.A.; Jenkins, N.A.; Copeland, N.G.; Vande Woude, G.F. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J. Biol. Chem. 1996, 271, 20399–20405. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J.; Padalko, E. iNOS (NOS2) at a glance. J. Cell Sci. 2004, 117, 2865–2867. [Google Scholar] [CrossRef] [PubMed]
- Redford, P.S.; Murray, P.J.; O’Garra, A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011, 4, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, M.E.; Carow, B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin. Immunol. 2014, 26, 518–532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, K.; Sodenkamp, J.C.; Hölscher, A.; Behrends, J.; Hölscher, C. IL-6 Is Not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium tuberculosis H37rv. Cells 2021, 10, 9. https://doi.org/10.3390/cells10010009
Ritter K, Sodenkamp JC, Hölscher A, Behrends J, Hölscher C. IL-6 Is Not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium tuberculosis H37rv. Cells. 2021; 10(1):9. https://doi.org/10.3390/cells10010009
Chicago/Turabian StyleRitter, Kristina, Jan Christian Sodenkamp, Alexandra Hölscher, Jochen Behrends, and Christoph Hölscher. 2021. "IL-6 Is Not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium tuberculosis H37rv" Cells 10, no. 1: 9. https://doi.org/10.3390/cells10010009
APA StyleRitter, K., Sodenkamp, J. C., Hölscher, A., Behrends, J., & Hölscher, C. (2021). IL-6 Is Not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium tuberculosis H37rv. Cells, 10(1), 9. https://doi.org/10.3390/cells10010009





