A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System
Abstract
1. Background
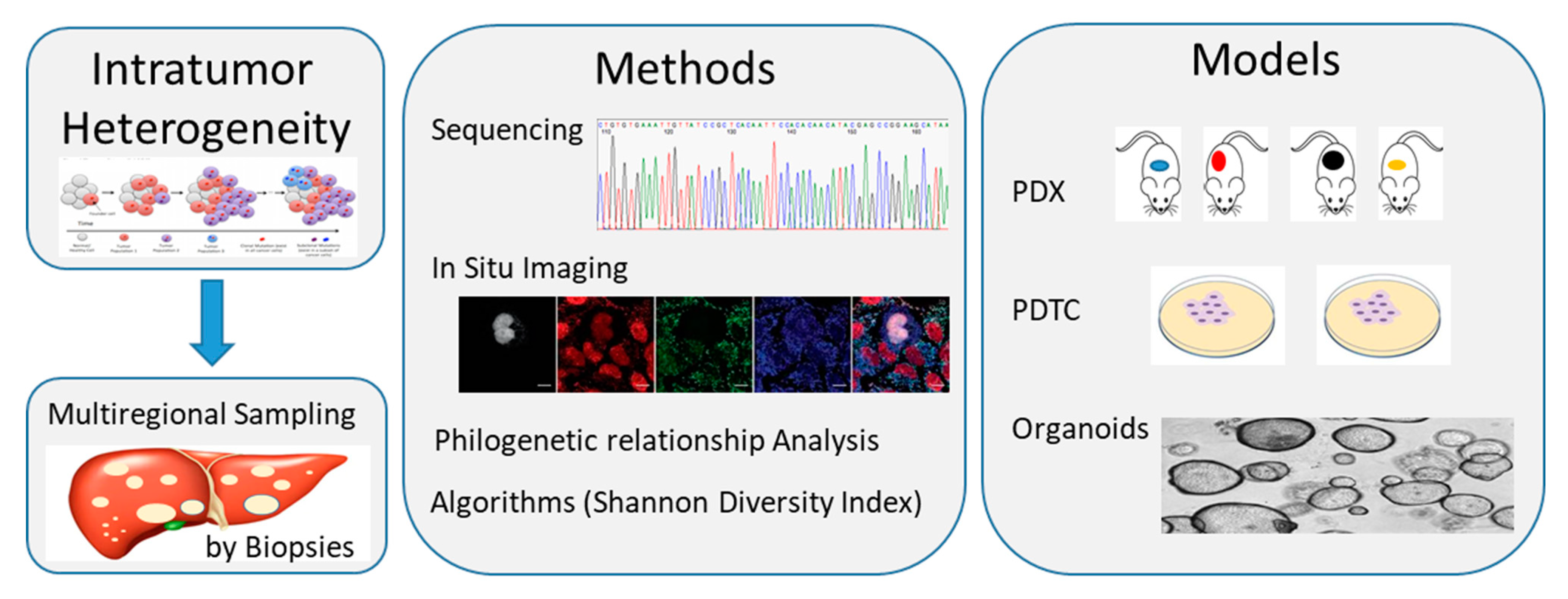
2. CCA Biology, Tumoral Heterogeneity and Molecular Characterization
3. Current Treatment Options for Locally Advanced or Metastatic iCCA
3.1. Standard of Treatment
3.2. Local and Regional Treatment Strategies
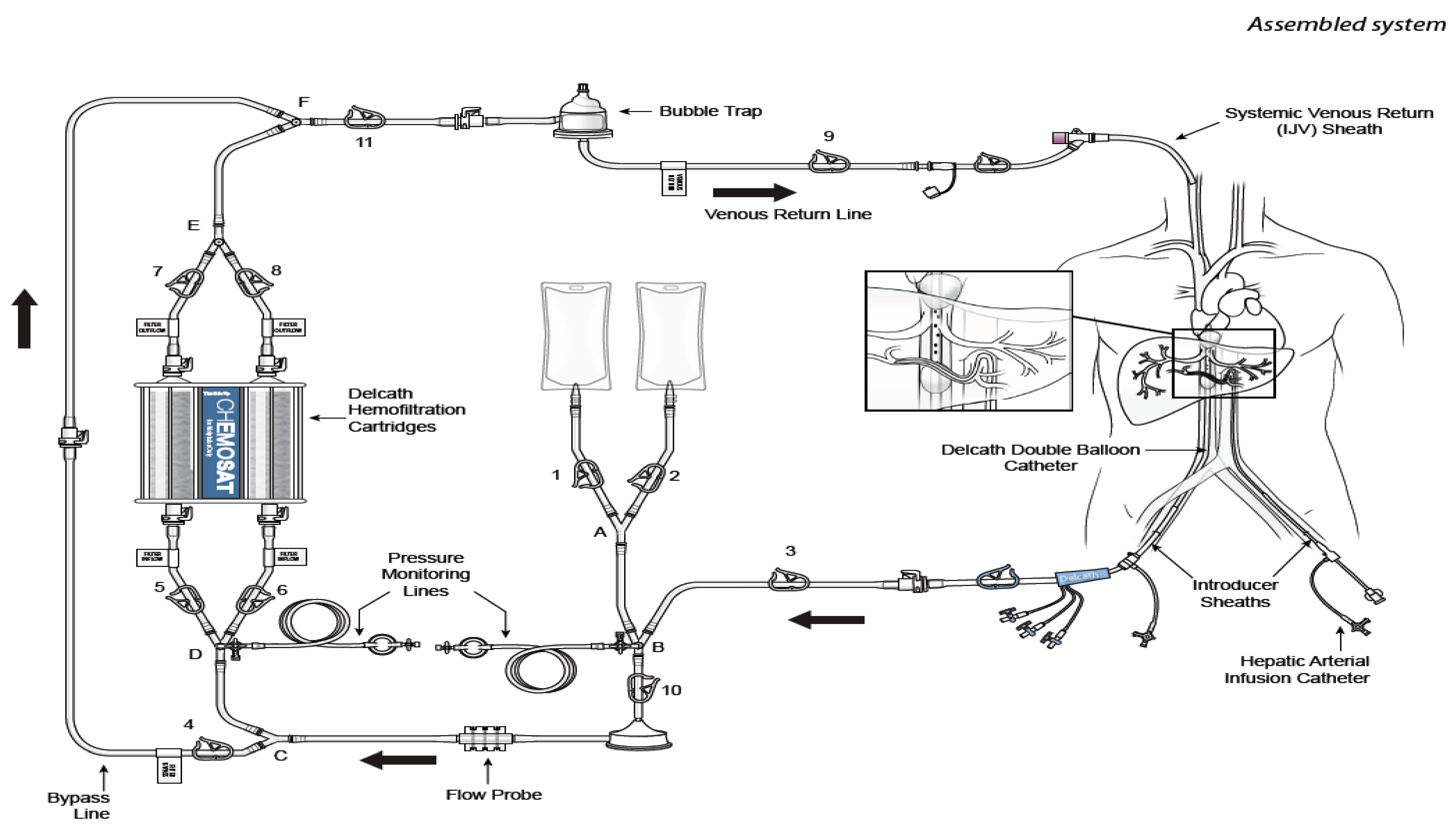
4. CS-PHP Clinical Development
4.1. Overview of Previous Studies in Tumors with Liver Metastases
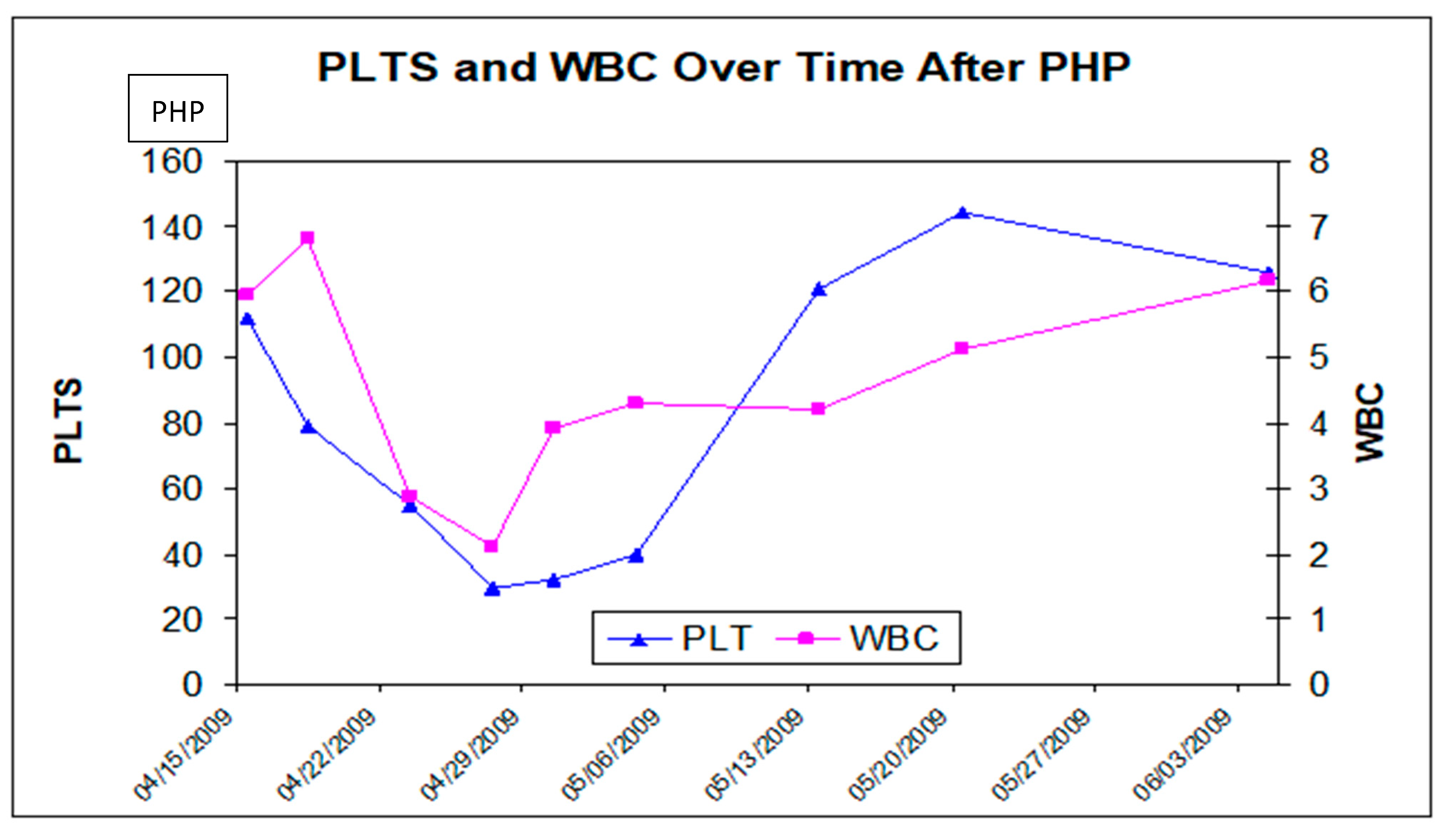
4.2. Safety Experience with CS/HDS Treatment
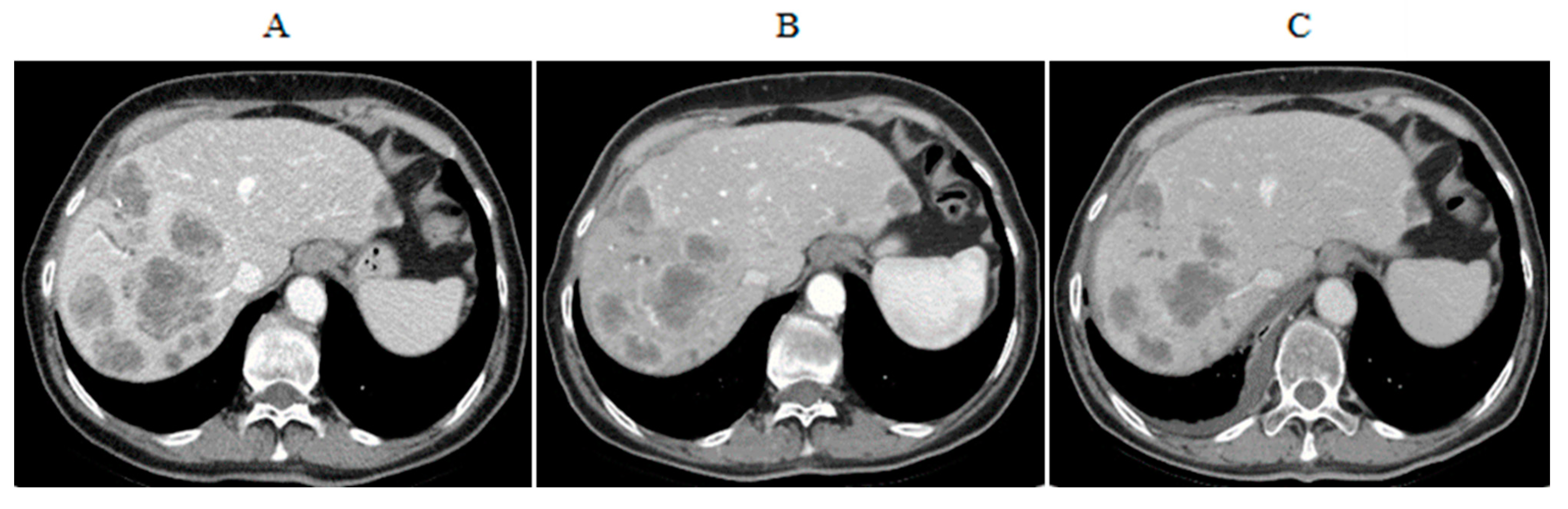
4.3. CS-PHP Efficacy in Cholangiocarcinoma
5. Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’angelica, M.; De Matteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic Cholangiocarcinoma. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef] [PubMed]
- August, D.A.; Nathan, H.; Sotiropoulos, G.C. Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of Prognostic Factors and Lymph Node Assessment. J. Clin. Oncol. 2011, 29, 3140–3145. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology for Hepatobiliary Cancers: Intrahepatic Cholangiocarcinoma. Version 4.2020. 19 June 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 8 July 2020).
- Bridgewater, J.; Galle, P.R.; Khan, S.A. Guidelines for the diagnosis and management of intrahe-patic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Khan, S.A.; Davidson, B.R.; Goldin, R.D. Guidelines for the diagnosis and treatment of cholangio-carcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kim, J.K.; Kim, M.Y.; Rhim, H.; Han, J.K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2008, 49, 453–459. [Google Scholar] [CrossRef]
- Beheshti, M.V.; Denny, D.F., Jr.; Glickman, M.G. Percutaneous isolated liver perfusion for treatment of hepatic malignancy: Preliminary report. J. Vasc. Interv. Radiol. 1992, 3, 453–458. [Google Scholar] [CrossRef]
- Ravikumar, T.S.; Pizzorno, G.; Bodden, W.; Marsh, J.; Strair, R.; Pollack, J.; Hendler, R.; Hanna, J.; D’Andrea, E. Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J. Clin. Oncol. 1994, 12, 2723–2736. [Google Scholar] [CrossRef]
- Curley, S.A.; Newman, R.A.; Dougherty, T.B.; Fuhrman, G.M.; Stone, D.L.; Mikolajek, J.A.; Guercio, S.; Guercio, A.; Carrasco, C.H.; Kuo, M.T.; et al. Complete hepatic venous isolation and extracorporeal chemofiltration as treatment for human hepatocellular carcinoma: A phase I study. Ann. Surg. Oncol. 1994, 1, 389–399. [Google Scholar] [CrossRef]
- Hwu, W.-J.; Salem, R.R.; Pollak, J.; Rosenblatt, M.; D’Andrea, E.; Leffert, J.J.; Faraone, S.; Marsh, J.C.; Pizzorno, G. A clinical-pharmacological evaluation of percutaneous isolated hepatic infusion of doxorubicin in patients with unresectable liver tumors. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 1999, 11, 529–537. [Google Scholar]
- van Etten, B.; Brunstein, F.; van Ijken, M.G. Isolated hypoxic hepatic perfusion with orthograde or retrograde flow in patients with irresectable liver metastases using percutaneous balloon catheter techniques: A phase I and II study. Ann. Surg. Oncol. 2004, 11, 598–605. [Google Scholar] [CrossRef]
- Verhoef, C.; de Wilt, J.H.; Brunstein, F. Isolated hypoxic hepatic perfusion with retrograde outflow in patients with irresectable liver metastases; a new simplified technique in isolated hepatic per-fusion. Ann. Surg. Oncol. 2008, 15, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Pingpank, J.F.; Libutti, S.K.; Chang, R. Phase I study of hepatic arterial melphalan infusion and he-patic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J. Clin. Oncol. 2005, 23, 3465–3474. [Google Scholar] [CrossRef] [PubMed]
- Pingpank, J.F.; Hughes, M.S.; Faries, M.B. A phase III random assignment trial comparing percuta-neous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutanous melanoma. J. Clin. Oncol. 2010, 28 (Suppl. 18), LBA8512. [Google Scholar] [CrossRef]
- Hughes, M.S.; Zager, J.S.; Faries, M.B.; Alexander, H.R.; Royal, R.E.; Wood, B.J.; Choi, J.; McCluskey, K.; Whitman, E.; Agarwala, S.; et al. Results of a Randomized Controlled Multicenter Phase III Trial of Percutaneous Hepatic Perfusion Compared with Best Available Care for Patients with Melanoma Liver Metastases. Ann. Surg. Oncol. 2015, 23, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.R.; Libutti, S.K.; Bartlett, D.L.; Puhlmann, M.; Fraker, D.L.; Bachenheimer, L.C. A phase I–II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin. Cancer Res. 2000, 6, 3062–3070. [Google Scholar]
- Alexander, H.R., Jr.; Libutti, S.K.; Pingpank, J.F. Hyperthermic isolated hepatic perfusion using mel-phalan for patients with ocular melanoma metastatic to liver. Clin. Cancer Res. 2003, 9, 6343–6349. [Google Scholar]
- Noter, S.L.; Rothbarth, J.; Pijl, M.E.; Keunen, J.E.; Hartgrink, H.H.; Tijl, F.G.; Kuppen, P.J.; Van De Velde, C.J.; Tollenaar, R.A. Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res. 2004, 14, 67–72. [Google Scholar] [CrossRef]
- Rizell, M.; Mattson, J.; Cahlin, C.; Hafström, L.; Lindner, P.; Olausson, M. Isolated hepatic perfusion for liver metastases of malignant melanoma. Melanoma Res. 2008, 18, 120–126. [Google Scholar] [CrossRef]
- Van Iersel, L.B.; Gelderblom, H.; Vahrmeijer, A.L. Isolated hepatic melphalan perfusion of colorectal liver metastases: Outcome and prognostic factors in 154 patients. Ann. Oncol. 2008, 19, 1127–1134. [Google Scholar] [CrossRef]
- Alexander, H.R., Jr.; Bartlett, D.L.; Libutti, S.K. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann. Surg. Oncol. 2009, 16, 1852–1859. [Google Scholar] [CrossRef]
- Rothbarth, J.; Pijl, M.E.J.; Vahrmeijer, A.L.; Hartgrink, H.H.; Tijl, F.G.J.; Kuppen, P.J.; Tollenaar, R.A.E.M.; Van De Velde, C. Isolated hepatic perfusion with high-dose melphalan for the treatment of colorectal metastasis confined to the liver. BJS 2003, 90, 1391–1397. [Google Scholar] [CrossRef]
- Feldman, E.D.; Wu, P.C.; Beresneva, T. Treatment of patients with unresectable primary hepatic malignancies using hyperthermic isolated hepatic perfusion. J. Gastrointest. Surg. 2004, 8, 200–207. [Google Scholar] [CrossRef]
- Grover, A.C.; Libutti, S.K.; Pingpank, J.F.; Helsabeck, C.; Beresnev, T.; Alexander, H.R., Jr. Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery 2004, 136, 1176–1182. [Google Scholar] [CrossRef]
- CHEMOSAT® Hepatic Delivery System. Instructions for Use; Delcath Systems Inc.: New York, NY, USA; Available online: http://www.chemosat.com/home/ (accessed on 1 March 2020).
- Lim, J.H. Cholangiocarcinoma:Morphologic Classification according to Growth Pattern and Imaging Findings. Am. J. Roentgenol. 2003, 181, 819–827. [Google Scholar] [CrossRef]
- Shaib, Y.; El–Serag, H.B. The Epidemiology of Cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Aaltonen, L.A. World Health Organization Classification of Tumours: Pathology and Genetics: Tumours of the Digestive System; IARC Press: Lyon, France, 2000. [Google Scholar]
- Parkin, D.M.; Ohshima, H.; Srivatanakul, P.; Vatanasapt, V. Cholangiocarcinoma: Epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol. Biomark. Prev. 1993, 2, 537–544. [Google Scholar]
- Bragazzi, M.C.; Cardinale, V.; Carpino, G. Cholangiocarcinoma: Epidemiology and risk factors. Transl. Gastrointest. Cancer 2012, 1, 21–32. [Google Scholar]
- Anderson, C.D.; Pinson, C.W.; Berlin, J.; Chari, R.S. Diagnosis and Treatment of Cholangiocarcinoma. Oncologist 2004, 9, 43–57. [Google Scholar] [CrossRef]
- Cardinale, V.; Carpino, G.; Reid, L.; Gaudio, E.; Alvaro, D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J. Gastrointest. Oncol. 2012, 4, 94–102. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, Z.-C.; Duan, M.; Lin, Y.-H.; Zhou, X.-Y.; Worthley, D.L.; Wang, X.-Y.; Niu, G.; Xia, Y.; Deng, M.; et al. Cell Culture System for Analysis of Genetic Heterogeneity Within Hepatocellular Carcinomas and Response to Pharmacologic Agents. Gastroenterology 2017, 152, 232–242.e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Wan, L.; Pei, X.; Liu, B.; Yan, X. Single-cell transcriptomic architecture and inter-cellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals two classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Deshpande, V.; Beyter, D.; Koga, T.; Rusert, J.; Lee, C.; Li, B.; Arden, K.; Ren, B.; Nathanson, D.A.; et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nat. Cell Biol. 2017, 543, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kumar, M.R.; Roessler, S.; Ji, J.; Forgues, M.; Budhu, A. Transcriptomic profiling reveals he-patic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology 2012, 56, 1792–1803. [Google Scholar] [CrossRef]
- Lee, J.-S.; Chu, I.-S.; Mikaelyan, A.; Calvisi, D.F.; Heo, J.; Reddy, J.K.; Thorgeirsson, S.S. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat. Genet. 2004, 36, 1306–1311. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Rimassa, L.; Personeni, N.; Aghemo, A.; Lleo, A. The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine. J. Autoimmun. 2019, 100, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Rizvi, S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, Resectability, and Outcome in 225 Patients with Hilar Cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef]
- Weber, S.M.; Jarnagin, W.R.; Klimstra, D.S.; De Matteo, R.P.; Fong, Y.; Blumgart, L.H. Intrahepatic Cholangiocarcinoma: Resectability, recurrence pattern, and outcomes. J. Am. Coll. Surg. 2001, 193, 384–391. [Google Scholar] [CrossRef]
- Primrose, J.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; D’Angelica, M.I.; Abrams, T.A. Hepatobiliary cancers. version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 1152–1182. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Eckmann, K.R.; Patel, D.K.; Landgraf, A.; Slade, J.H.; Lin, E.; Kaur, H.; Loyer, E.; Weatherly, J.M.; Javle, M. Chemotherapy Outcomes for the Treatment of Unresectable Intrahepatic and Hilar Cholangiocarcinoma: A Retrospective Analysis. Gastrointest. Cancer Res. 2011, 4, 155–160. [Google Scholar] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Gemcitabine Hydrochloride and Cisplatin with or without Nab-Paclitaxel in Treating Patients with Newly Diagnosed Advanced Biliary Tract Cancers. Updated 7 November 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03768414 (accessed on 8 April 2020).
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019, 37, 4003. [Google Scholar] [CrossRef]
- Naboush, A.; Roman, C.A.J.; Shapira, I. Immune checkpoint inhibitors in malignancies with mismatch repair deficiency: A review of the state of the current knowledge. J. Investig. Med. 2017, 65, 754–758. [Google Scholar] [CrossRef]
- Keytruda [Prescribing Information]; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2020; Available online: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (accessed on 8 April 2020).
- Ott, P.A.; Bang, Y.-J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell–Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated with Pembrolizumab Across 20 Cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Abou-Alfa, G.; Mercade, T.M.; Javle, M.; Kelley, R.; Lubner, S.; Adeva, J.; Cleary, J.; Catenacci, D.; Borad, M.; Bridgewater, J.; et al. ClarIDHy: A global, phase III, randomized, double-blind study of ivosidenib (IVO) vs. placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann. Oncol. 2019, 30, v872–v873. [Google Scholar] [CrossRef]
- Ghedini, G.C.; Ronca, R.; Presta, M.; Giacomini, A. Future applications of FGF/FGFR inhibitors in cancer. Expert Rev. Anticancer Ther. 2018, 18, 861–872. [Google Scholar] [CrossRef]
- Pemazyre [Prescribing Information]; Incyte Corporation: Wilmington, DE, USA, 2020; Available online: https://www.pemazyre.com/pdf/prescribing-information.pdf (accessed on 20 April 2020).
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.; Borad, M.; Gallinson, D.; Murphy, A.; et al. FIGHT-202: A phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Ann. Oncol. 2019, 30, v876. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.A.; Wasan, H.S.; et al. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J. Clin. Oncol. 2020, 38, TPS592. [Google Scholar] [CrossRef]
- Javle, M.; Kelley, R.; Roychowdhury, S.; Weiss, K.; Abou-Alfa, G.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Ng, M.C.H.; Goyal, L.; Bang, Y.-J.; Oh, -Y.; Chao, T.-Y.; Cleary, J.M.; Voss, M.H.; Meric-Bernstam, F.; Iyer, G.; Heist, R.S.; et al. AB065. P-36. Debio 1347 in patients with cholangiocarcinoma harboring an FGFR gene alteration: Preliminary results. HepatoBiliary Surg. Nutr. 2019, 8, AB065. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Busset, M.D.D.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Park, J.; Su, W.-C.; Oh, D.-Y.; Kim, K.-P.; Feng, Y.-H.; Shen, L.; Liao, H.; Nie, J.; Qing, M.; et al. Preliminary results of a ph2a study to evaluate the clinical efficacy and safety of erdafitinib in Asian patients with biomarker-selected advanced cholangiocarcinoma (CCA). Ann. Oncol. 2018, 29, viii209. [Google Scholar] [CrossRef]
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Dudeck, O.; Ricke, J. Advances in regional chemotherapy of the liver. Expert Opin. Drug Deliv. 2011, 8, 1057–1069. [Google Scholar] [CrossRef]
- Cao, J.; Hu, J.; Liu, S. Intrahepatic Cholangiocarcinoma: Genomic Heterogeneity between Eastern and Western Patients. JCO Precis. Oncol. 2020, 4, 557–569. [Google Scholar] [CrossRef]
- Breedis, C.; Young, G. The Blood Supply of Neoplasms in the Liver. Am. J. Pathol. 1954, 30, 969–985. [Google Scholar]
- Hinshaw, J.L.; Lubner, M.G.; Ziemlewicz, T.J.; Lee, F.T.; Brace, C.L. Percutaneous Tumor Ablation Tools: Microwave, Radiofrequency, or Cryoablation—What Should You Use and Why? RadioGraphics 2014, 34, 1344–1362. [Google Scholar] [CrossRef]
- Meloni, M.F.; Chiang, J.; Laeseke, P.F.; Dietrich, C.F.; Sannino, A.; Solbiati, M.; Nocerino, E.; Brace, C.; Lee, F.T. Microwave ablation in primary and secondary liver tumours: Technical and clinical approaches. Int. J. Hyperth. 2016, 33, 15–24. [Google Scholar] [CrossRef]
- Yamasaki, T.; Hamabe, S.; Saeki, I.; Harima, Y.; Yamaguchi, Y.; Uchida, K.; Terai, S.; Sakaida, I. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: A prospective randomized trial. J. Gastroenterol. 2010, 46, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, A.; Guglielmi, A.; Pacetti, P. Capecitabine plus hepatic intra-arterial epirubicin and cisplatin in unresectable biliary cancer: A phase II study. Anticancer Res. 2007, 27, 3009–3013. [Google Scholar] [PubMed]
- Shitara, K.; Ikami, I.; Munakata, M. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin. Oncol. 2008, 20, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Arai, Y.; Yamaura, H. Phase I/II study of hepatic arterial infusion chemotherapy with gemcitabine in patients with unresectable intrahepatic cholangiocarcinoma (JIVROSG0301). Am. J. Clin. Oncol. 2011, 34, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, N.E.; Schwartz, L.; Gönen, M. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: Does the addition of systemic bevacizumab improve results? Oncology 2011, 80, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Riaz, R.; Awais, R.; Salem, R. Side effects of yttrium-90 radioembolization. Front. Oncol. 2014, 4, 198–207. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef]
- Delcath Systems Inc. Study 04-C-0273: A Phase 2 Study of Hepatic Arterial Infusin of Melphalan via Peripheral Hepatic Perfosuion for Unresectable Primary and Metastatic Cancers of the Liver. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT00096083 (accessed on 8 April 2020).
- Karydis, I.; Gangi, A.; Wheater, M.J. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J. Surg. Oncol. 2018, 117, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.S.; Burgmans, M.C.; Fiocco, M. Safety of Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Liver Metastases from Ocular Melanoma Using the Delcath Systems’ Second-Generation Hemofiltration System: A Prospective Non-randomized Phase II Trial. Cardiovasc. Interv. Radiol. 2019, 42, 841–852. [Google Scholar] [CrossRef]
- Kirstein, M.M.; Marquardt, S.; Jedicke, N. Safety and efficacy of chemosaturation in patients with primary and secondary liver tumors. J. Cancer Res. Clin. Oncol. 2017, 143, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Zangos, S.; Scholtz, J.E. Chemosaturation with percutaneous hepatic perfusions of melphalan for hepatic metastases: Experience from two European centers. Rofo 2014, 186, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, S.; Kirstein, M.M.; Bruning, R. Percutaneous hepatic perfusion (chemosaturation) with melphalan in patients with intrahepatic cholangiocarcinoma: European multicentre study on safety, short-term effects and survival. Eur. Radiol. 2019, 29, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Delcath Systems Inc. Clinical Study Protocol number PHP-HCC-202. EudraCT No. 2014-001585-98. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001585-98/IT (accessed on 8 April 2020).
| Target | Prevalence | Trial/Drug |
|---|---|---|
| TP53 mutation | 27% iCCA; 40% pCCA/dCCA | |
| KRAS mutation | 22% iCCA; 42% pCCA/dCCA | |
| ROS1 rearrangement | 8% to 9% | |
| MSI-H | 14% to 18% iCCA | KEYNOTE-028/Pembrolizumab [59,60,61] |
| TMB-H | 6% to 12% iCCA | [73] |
| CDKN2A mutation | 47% iCCA | |
| IDH1/IDH2 mutation | 25% iCCA | ClarIDHy/Ivosidenib [63] |
| FGFR2 | 10% to 16% iCCA | FIGHT-202 and -203/Pemigatinib [64,65,66,67] Infigratinib [68] Debio1347, Derazantinib, Erdafitinib, Futibatinib [69,70,71,72] |
| EGFR overexpression | 16% iCCA | |
| MET amplification | 2% iCCA |
| Liver Tumor Type | No. of Patients | No. of Treatments |
|---|---|---|
| Ocular Melanoma | 221 | 489 |
| Cholangiocarcinoma | 42 | 76 |
| Colorectal | 15 | 24 |
| Hepatocellular carcinoma | 13 | 20 |
| Pancreatic | 7 | 14 |
| Neuroendocrine | 7 | 12 |
| Cutaneous Melanoma | 6 | 9 |
| Breast | 5 | 11 |
| Others | 5 | 5 |
| TOTAL | 321 | 660 |
| Hematological Toxicity on 160 PHPs | Grade 3/4, n (%) | Grade 5, (%) |
|---|---|---|
| Neutropenia | 102 (63.7) | |
| Thrombocytopenia | 119 (74.4) | |
| Anemia | 75 (46.9) | |
| Neutropenic Sepsis | 2 (1.25) | |
| Hepatic Toxicity on 160 PHPs | ||
| Elevated AST | 23 (14.4) | |
| Elevated ALT | 11 (6.9) | |
| Elevated Bilirubinemia | 16 (10) | |
| Elevated ALP | 9 (5.6) | |
| Liver Failure | 1 (0.62) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrucci, P.F.; Cocorocchio, E.; Bonomo, G.; Varano, G.M.; Della Vigna, P.; Orsi, F. A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System. Cells 2021, 10, 70. https://doi.org/10.3390/cells10010070
Ferrucci PF, Cocorocchio E, Bonomo G, Varano GM, Della Vigna P, Orsi F. A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System. Cells. 2021; 10(1):70. https://doi.org/10.3390/cells10010070
Chicago/Turabian StyleFerrucci, Pier Francesco, Emilia Cocorocchio, Guido Bonomo, Gianluca Maria Varano, Paolo Della Vigna, and Franco Orsi. 2021. "A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System" Cells 10, no. 1: 70. https://doi.org/10.3390/cells10010070
APA StyleFerrucci, P. F., Cocorocchio, E., Bonomo, G., Varano, G. M., Della Vigna, P., & Orsi, F. (2021). A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System. Cells, 10(1), 70. https://doi.org/10.3390/cells10010070