Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Characterization of ASCs
2.3. Indirect Co-Culture Experiments
2.4. Statistical Analysis
3. Results
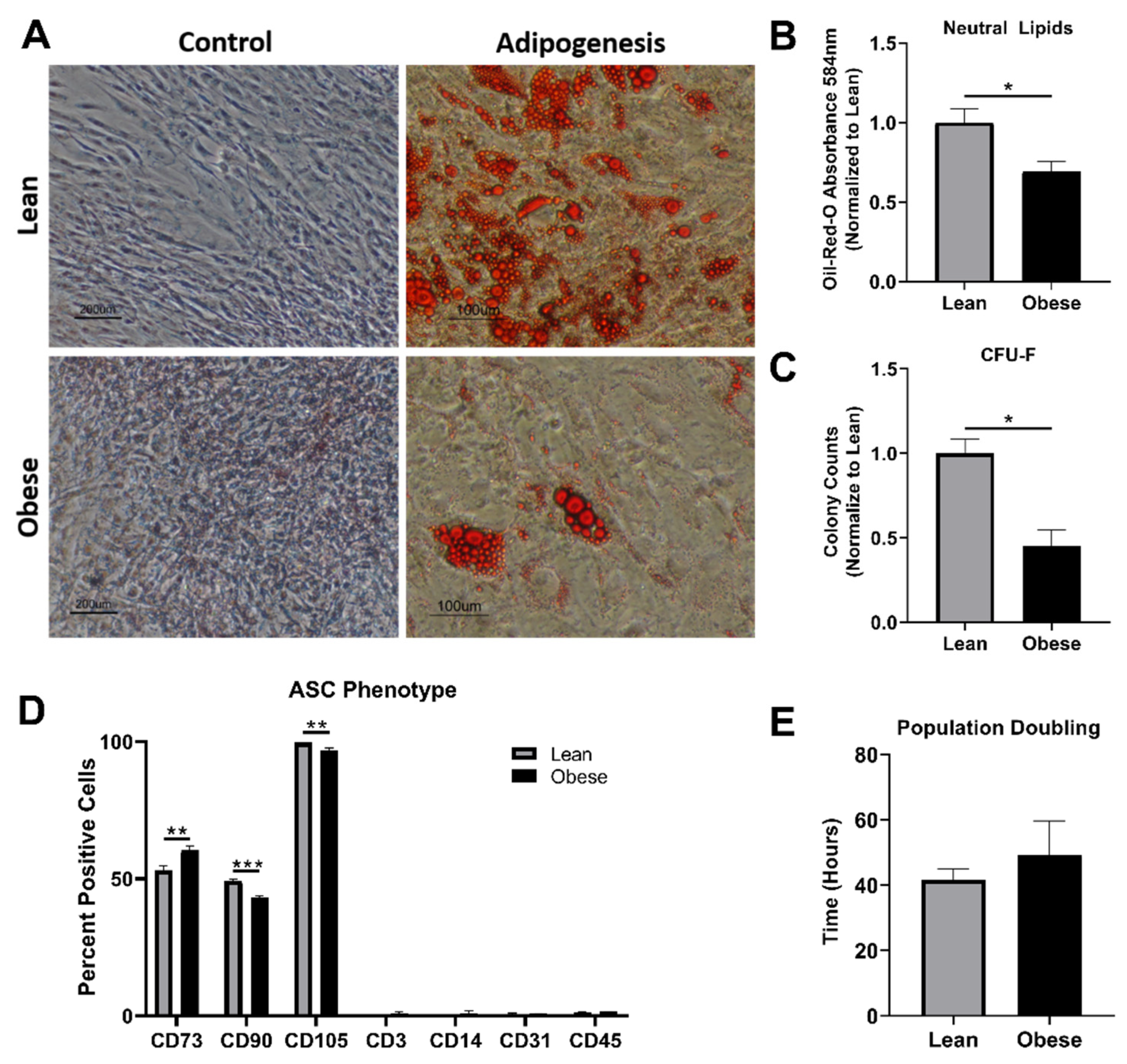
3.1. ObASCs Exhibit Reduced Stemness Characteristics When Compared with LnASCs
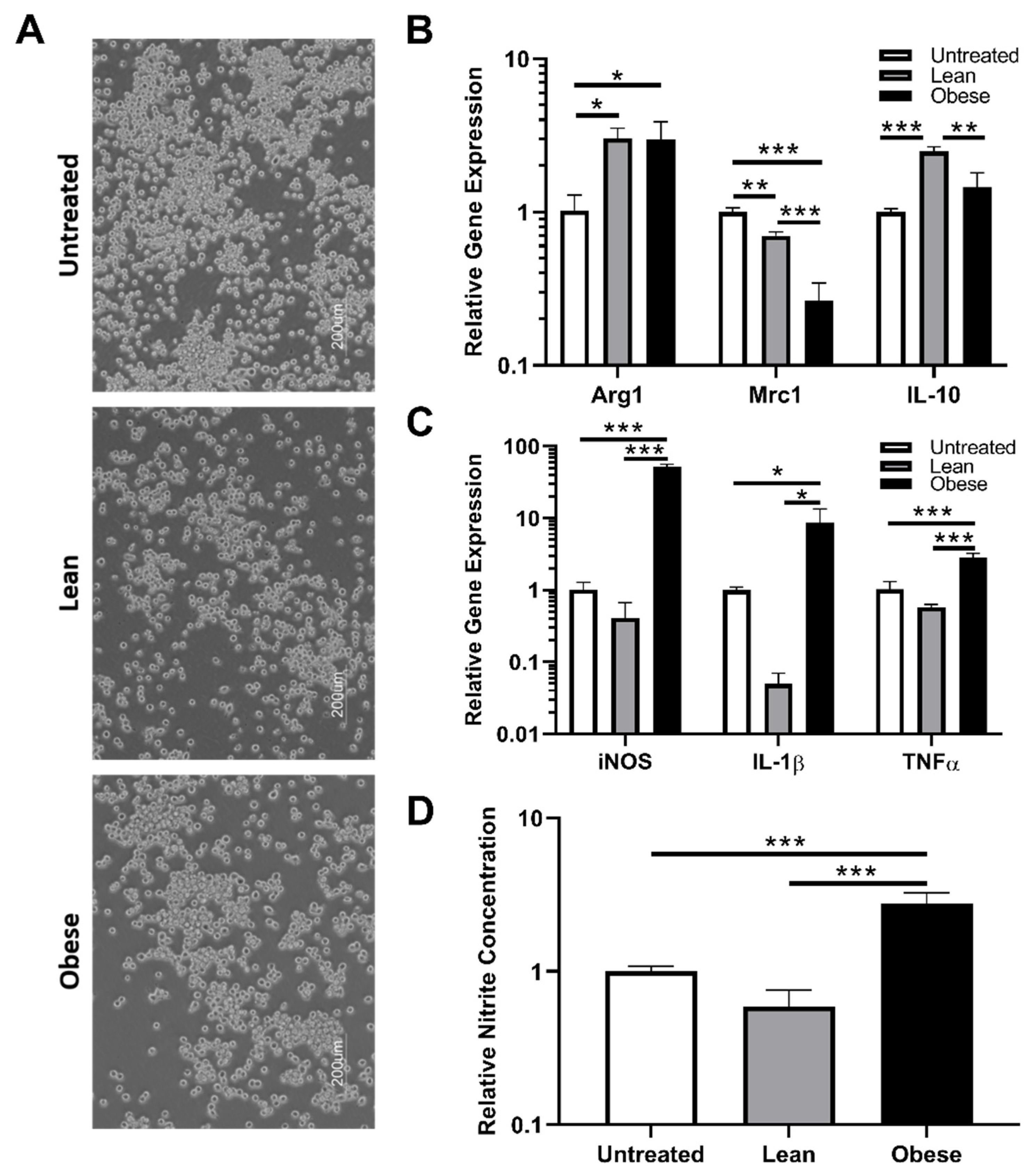
3.2. Indirect Co-Culture with ObASCs, But Not LnASCs, Induces Polarization toward M1 Phenotype in RAW264.7 Macrophages
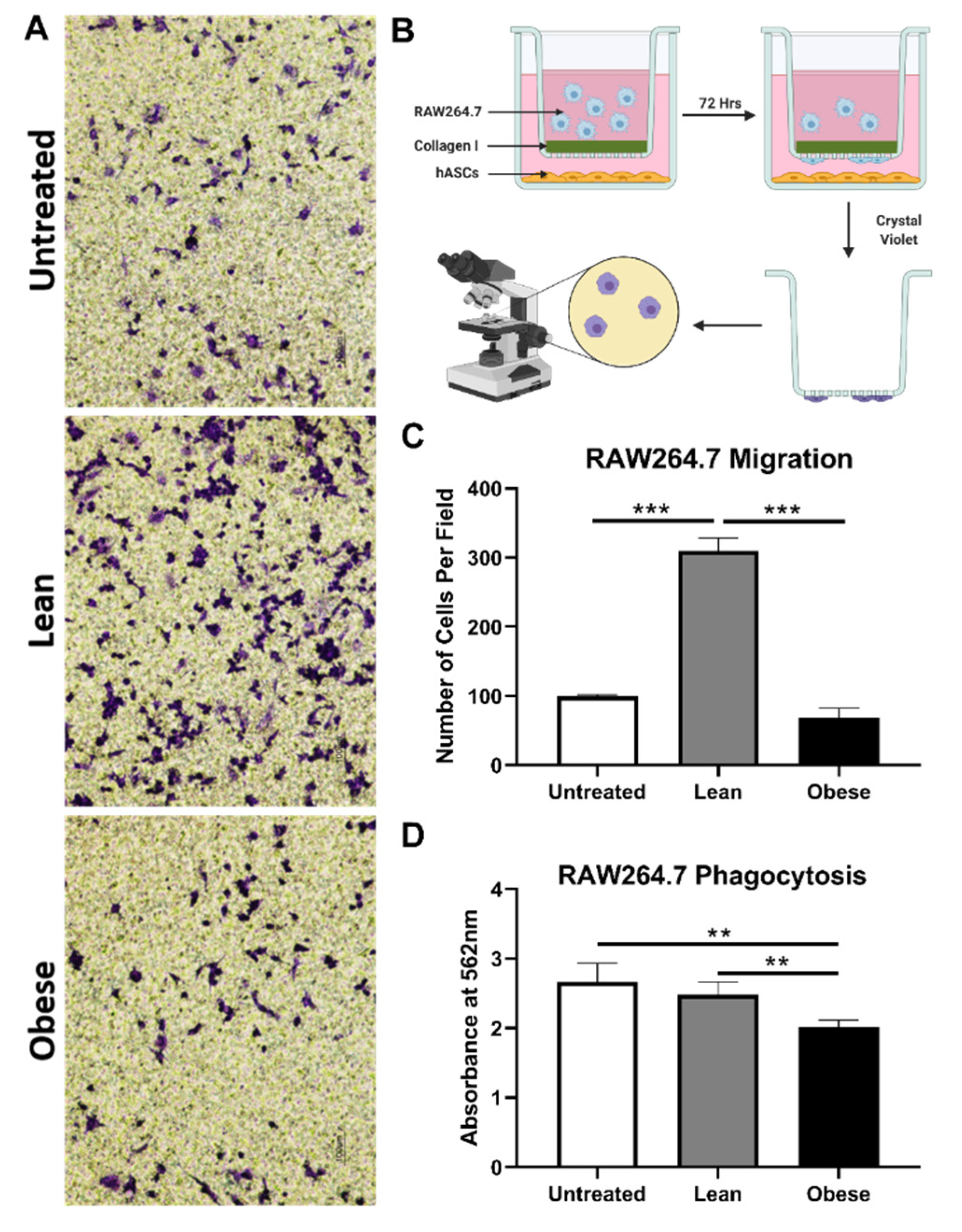
3.3. LnASCs and ObASCs Differentially Affect the Migration and Phagocytic Abilities of RAW264.7 Macrophages
3.4. Indirect Co-Culture with ObASCs, But Not LnASCs, Induces Polarization toward M1 Phenotype in SIM-A9 Microglia
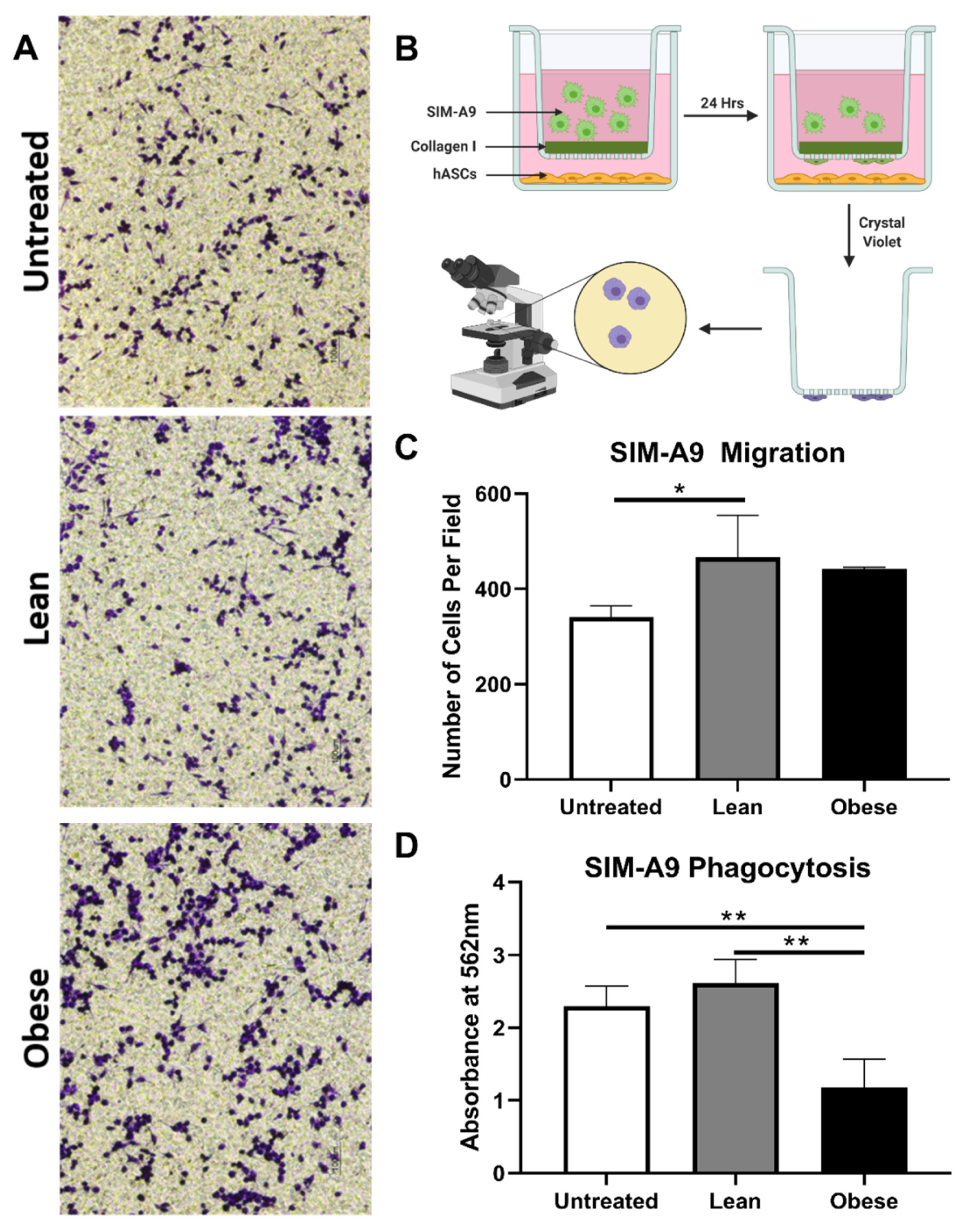
3.5. LnASCs and ObASCs Differentially Affect the Migration and Phagocytic Abilities of SIM-A9 Microglia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Gambari, L.; Piacentini, A.; Filardo, G.; Fleury-Cappellesso, S.; Barbero, A.; Murphy, M.; Lisignoli, G. Adipose Stromal Cells Mediated Switching of the Pro-Inflammatory Profile of M1-Like Macrophages Is Facilitated by Pge2: In Vitro Evaluation. Osteoarthr. Cartil. 2017, 25, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Cifu, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro Inflammatory Stimuli Enhance the Immunosuppressive Functions of Adipose Mesenchymal Stem Cells-Derived Exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. Tsg-6 Secreted by Human Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorates Dss-Induced Colitis by Inducing M2 Macrophage Polarization in Mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Ontoria-Oviedo, I.; Ricardo, C.P.; Harding, S.E.; Sacedon, R.; Varas, A.; Zapata, A.; Sepulveda, P.; Vicente, A. Overexpression of Hypoxia-Inducible Factor 1 Alpha Improves Immunomodulation by Dental Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 208. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-Gamma Does Not Break, but Promotes the Immunosuppressive Capacity of Adult Human Mesenchymal Stem Cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Liu, G.Y.; Liu, Y.; Lu, Y.; Qin, Y.R.; Di, G.H.; Lei, Y.H.; Liu, H.X.; Li, Y.Q.; Wu, C.; Hu, X.W.; et al. Short-Term Memory of Danger Signals or Environmental Stimuli in Mesenchymal Stem Cells: Implications for Therapeutic Potential. Cell Mol. Immunol. 2016, 13, 369–378. [Google Scholar] [CrossRef]
- Andras, I.E.; Leda, A.; Contreras, M.G.; Bertrand, L.; Park, M.; Skowronska, M.; Toborek, M. Extracellular Vesicles of the Blood-Brain Barrier: Role in the Hiv-1 Associated Amyloid Beta Pathology. Mol. Cell Neurosci. 2017, 79, 12–22. [Google Scholar] [CrossRef]
- Semon, J.A.; Maness, C.; Zhang, X.; Sharkey, S.A.; Beuttler, M.M.; Shah, F.S.; Pandey, A.C.; Gimble, J.M.; Zhang, S.; Scruggs, A.B.; et al. Comparison of Human Adult Stem Cells from Adipose Tissue and Bone Marrow in the Treatment of Experimental Autoimmune Encephalomyelitis. Stem Cell Res. Ther. 2014, 5, 2. [Google Scholar] [CrossRef]
- Bowles, A.C.; Strong, A.L.; Wise, R.M.; Thomas, R.C.; Gerstein, B.Y.; Dutreil, M.F.; Hunter, R.S.; Gimble, J.M.; Bunnell, B.A. Adipose Stromal Vascular Fraction-Mediated Improvements at Late-Stage Disease in a Murine Model of Multiple Sclerosis. Stem Cells 2017, 35, 532–544. [Google Scholar] [CrossRef]
- Zhao, P.T.; Zhang, L.J.; Shao, H.; Bai, L.L.; Yu, B.; Su, C.; Dong, L.J.; Liu, X.; Li, X.R.; Zhang, X.M. Therapeutic Effects of Mesenchymal Stem Cells Administered at Later Phase of Recurrent Experimental Autoimmune Uveitis. Int. J. Ophthalmol. 2016, 9, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 2017, 7, 4323. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Silva, M.; Semedo-Kuriki, P.; Donizetti-Oliveira, C.; Costa, P.B.; Cenedeze, M.A.; Hiyane, M.I.; Pacheco-Silva, A.; Camara, N.O. Adipose Tissue-Derived Stem Cells Reduce Acute and Chronic Kidney Damage in Mice. PLoS ONE 2015, 10, e0142183. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective Function of Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Kidney Injury through Sirt1 Pathway. Life Sci. 2020, 255, 117719. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Gonzalez-Rey, E.; Rico, L.; Buscher, D.; Delgado, M. Adipose-Derived Mesenchymal Stem Cells Alleviate Experimental Colitis by Inhibiting Inflammatory and Autoimmune Responses. Gastroenterology 2009, 136, 978–989. [Google Scholar] [CrossRef]
- Jung, W.Y.; Kang, J.H.; Kim, K.G.; Kim, H.S.; Jang, B.I.; Park, Y.H.; Song, I.H. Human Adipose-Derived Stem Cells Attenuate Inflammatory Bowel Disease in Il-10 Knockout Mice. Tissue Cell 2015, 47, 86–93. [Google Scholar] [CrossRef]
- Kawata, Y.; Tsuchiya, A.; Seino, S.; Watanabe, Y.; Kojima, Y.; Ikarashi, S.; Tominaga, K.; Yokoyama, J.; Yamagiwa, S.; Terai, S. Early Injection of Human Adipose Tissue-Derived Mesenchymal Stem Cell after Inflammation Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice through the Induction of M2 Macrophages and Regulatory T Cells. Cell Tissue Res. 2019, 376, 257–271. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Cell Proliferation, Migration, and Inhibit Cell Apoptosis Via Wnt/Beta-Catenin Signaling in Cutaneous Wound Healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef]
- Sun, Y.; Song, L.; Zhang, Y.; Wang, H.; Dong, X. Adipose Stem Cells from Type 2 Diabetic Mice Exhibit Therapeutic Potential in Wound Healing. Stem Cell Res. Ther. 2020, 11, 298. [Google Scholar] [CrossRef]
- Skubis-Sikora, A.; Sikora, B.; Witkowska, A.; Mazurek, U.; Gola, J. Osteogenesis of Adipose-Derived Stem Cells from Patients with Glucose Metabolism Disorders. Mol. Med. 2020, 26, 67. [Google Scholar] [CrossRef]
- Payr, S.; Schuseil, T.; Unger, M.; Seeliger, C.; Tiefenboeck, T.; Balmayor, E.R.; Griensven, M.V. Effect of Donor Age and 3d-Cultivation on Osteogenic Differentiation Capacity of Adipose-Derived Mesenchymal Stem Cells. Sci. Rep. 2020, 10, 10408. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, H.; Liang, X.; Hong, Y.; Mao, M.; Han, Q.; He, H.; Tao, W.; Jiang, G.; Zhang, Y.; et al. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Abdominal Aortic Aneurysm Exhibit Senescence Phenomena. Oxid. Med. Cell. Longev. 2019, 2019, 1305049. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Liang, X.; Zou, J.; Liu, N.; Liu, T.; Wang, G.; Ding, X.; Liu, Y.; Zhang, B.; et al. Adipose Tissue-Derived Stem Cells from Type 2 Diabetics Reveal Conservative Alterations in Multidimensional Characteristics. Int. J. Stem Cells 2020, 13, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Volk, C.; Marga, C.; Santos, A.N.; Jung, M.; Rujescu, D.; Santos, A.N. Adipose-Derived Stem/Stromal Cells Recapitulate Aging Biomarkers and Show Reduced Stem Cell Plasticity Affecting Their Adipogenic Differentiation Capacity. Cell. Reprogram. 2019, 21, 187–199. [Google Scholar] [CrossRef]
- Scruggs, B.A.; Semon, J.A.; Zhang, X.; Zhang, S.; Bowles, A.C.; Pandey, A.C.; Imhof, K.M.; Kalueff, A.V.; Gimble, J.M.; Bunnell, B.A. Age of the Donor Reduces the Ability of Human Adipose-Derived Stem Cells to Alleviate Symptoms in the Experimental Autoimmune Encephalomyelitis Mouse Model. Stem Cells Transl. Med. 2013, 2, 797–807. [Google Scholar] [CrossRef]
- Strong, A.L.; Bowles, A.C.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; Gimble, J.M.; Bunnell, B.A. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef]
- Zhang, X.; Bowles, A.C.; Semon, J.A.; Scruggs, B.A.; Zhang, S.; Strong, A.L.; Gimble, J.M.; Bunnell, B.A. Transplantation of Autologous Adipose Stem Cells Lacks Therapeutic Efficacy in the Experimental Autoimmune Encephalomyelitis Model. PLoS ONE 2014, 9, e85007. [Google Scholar] [CrossRef]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased Adipocyte O2 Consumption Triggers Hif-1alpha, Causing Inflammation and Insulin Resistance in Obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.R.; Liechocki, S.; Carneiro, J.R.; Claudio-da-Silva, C.; Maya-Monteiro, C.M.; Borojevic, R.; Baptista, L.S. Stromal-Vascular Fraction Content and Adipose Stem Cell Behavior Are Altered in Morbid Obese and Post Bariatric Surgery Ex-Obese Women. Stem Cell Res. Ther. 2015, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Onate, B.; Vilahur, G.; Camino-Lopez, S.; Diez-Caballero, A.; Ballesta-Lopez, C.; Ybarra, J.; Moscatiello, F.; Herrero, J.; Badimon, L. Stem Cells Isolated from Adipose Tissue of Obese Patients Show Changes in Their Transcriptomic Profile That Indicate Loss in Stemcellness and Increased Commitment to an Adipocyte-Like Phenotype. BMC Genom. 2013, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.; Gimble, J.; Bunnell, B. Analysis of the Pro- and Anti-Inflammatory Cytokines Secreted by Adult Stem Cells During Differentiation. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Girolamo, L.; Stanco, D.; Salvatori, L.; Coroniti, G.; Arrigoni, E.; Silecchia, G.; Russo, M.A.; Niada, S.; Petrangeli, E.; Brini, A.T. Stemness and Osteogenic and Adipogenic Potential Are Differently Impaired in Subcutaneous and Visceral Adipose Derived Stem Cells (Ascs) Isolated from Obese Donors. Int. J. Immunopathol. Pharmacol. 2013, 26, 11–21. [Google Scholar] [CrossRef]
- Patel, R.S.; Carter, G.; el Bassit, G.; Patel, A.A.; Cooper, D.R.; Murr, M.; Patel, N.A. Adipose-Derived Stem Cells from Lean and Obese Humans Show Depot Specific Differences in Their Stem Cell Markers, Exosome Contents and Senescence: Role of Protein Kinase C Delta (Pkcdelta) in Adipose Stem Cell Niche. Stem Cell Investig. 2016, 3, 2. [Google Scholar] [CrossRef]
- Frazier, T.P.; Gimble, J.M.; Devay, J.W.; Tucker, H.A.; Chiu, E.S.; Rowan, B.G. Body Mass Index Affects Proliferation and Osteogenic Differentiation of Human Subcutaneous Adipose Tissue-Derived Stem Cells.(Report). BMC Cell Biol. 2013, 14. [Google Scholar] [CrossRef]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafre, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nunez-Roa, C.; Vendrell, J.; Fernandez-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of Macrophage Polarization in Autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage Activation and Polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; RHunter, S.; Jones, R.B.; Bowles, A.C.; Dutreil, M.F.; Gaupp, D.; Hayes, D.J.; Gimble, J.M.; Levi, B.; McNulty, M.A.; et al. Obesity Inhibits the Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J. Transl. Med. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Sabol, R.A.; Beighley, A.; Giacomelli, P.; Wise, R.M.; Harrison, M.A.A.; O’Donnnell, B.A.; Sullivan, B.N.; Lampenfeld, J.D.; Matossian, M.D.; Bratton, M.R.; et al. Obesity-Altered Adipose Stem Cells Promote Er(+) Breast Cancer Metastasis through Estrogen Independent Pathways. Int. J. Mol. Sci. 2019, 20, 1419. [Google Scholar] [CrossRef] [PubMed]
- Sabol, R.A.; Villela, V.A.; Denys, A.; Freeman, B.T.; Hartono, A.B.; Wise, R.M.; Harrison, M.A.A.; Sandler, M.B.; Hossain, F.; Miele, L.; et al. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. Int. J. Mol. Sci. 2020, 21, 2722. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Kim, E.J.; Kim, J.M.; Kam, E.H.; Ko, B.W.; Koo, B.N. Regulation of Microglia and Macrophage Polarization Via Apoptosis Signal-Regulating Kinase 1 Silencing after Ischemic/Hypoxic Injury. Front. Mol. Neurosci. 2017, 10, 261. [Google Scholar] [CrossRef]
- Ning, K.; Liu, W.W.; Huang, J.L.; Lu, H.T.; Sun, X.J. Effects of Hydrogen on Polarization of Macrophages and Microglia in a Stroke Model. Med. Gas. Res. 2018, 8, 154–159. [Google Scholar] [CrossRef]
- Bureta, C.; Setoguchi, T.; Saitoh, Y.; Tominaga, H.; Maeda, S.; Nagano, S.; Komiya, S.; Yamamoto, T.; Taniguchi, N. Tgf-Beta Promotes the Proliferation of Microglia in Vitro. Brain Sci. 2019, 10, 20. [Google Scholar] [CrossRef]
- Nagamoto-Combs, K.; Kulas, J.; Combs, C.K. A Novel Cell Line from Spontaneously Immortalized Murine Microglia. J. Neurosci. Methods 2014, 233, 187–198. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint statement of the International Federation for Adipose Therapeutics and Science (Ifats) and the International Society for Cellular Therapy (Isct). Cytotherapy 2013, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, O.A.; O’Donnell, B.T.; Poche, J.N.; Iftikhar, R.; Wise, R.M.; Motherwell, J.M.; Campbell, B.; Savkovic, S.D.; Bunnell, B.A.; Hayes, D.J.; et al. Human Adipose-Derived Hydrogel Characterization Based on in Vitro Asc Biocompatibility and Differentiation. Stem Cells Int. 2019, 2019, 9276398. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, R.X.; He, X.T.; Xu, X.Y.; Wang, J.; Chen, F.M. Influences of Age-Related Changes in Mesenchymal Stem Cells on Macrophages During in-Vitro Culture. Stem Cell Res. Ther. 2017, 8, 153. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Kang, F.; Chen, Z.; Meng, Y.; Dai, M. Propofol Attenuates Inflammatory Damage on Neurons Following Cerebral Infarction by Inhibiting Excessive Activation of Microglia. Int. J. Mol. Med. 2019, 43, 452–460. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using in Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, H.K.; Park, H.Y.; Jung, J.S.; Jeon, S.G.; Kim, Y.K.; Roh, H.J. Ifats Collection: Immunomodulatory Effects of Adipose Tissue-Derived Stem Cells in an Allergic Rhinitis Mouse Model. Stem Cells 2009, 27, 259–265. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Skurk, T.; Rohrig, K.; Lee, Y.M.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of Bmi and Age on Adipose Tissue Cellularity and Differentiation Capacity in Women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 889–895. [Google Scholar] [CrossRef]
- Pachon-Pena, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simo, R.; Tinahones, F.J.; Fernandez-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells from Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Roldan, M.; Macias-Gonzalez, M.; Garcia, R.; Tinahones, F.J.; Martin, M. Obesity Short-Circuits Stemness Gene Network in Human Adipose Multipotent Stem Cells. FASEB J. 2011, 25, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation During Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by Alphadbeta2 and Alphambeta2 Integrin-Mediated Adhesion. Front. Immunol. 2018, 9, 2650. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Heijnen, P.D.; Breur, M.; de Vries, H.E.; Tool, A.T.; Amor, S.; Dijkstra, C.D. Macrophages Migrate in an Activation-Dependent Manner to Chemokines Involved in Neuroinflammation. J. Neuroinflamm. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. The Microglial Activation State Regulates Migration and Roles of Matrix-Dissolving Enzymes for Invasion. J. Neuroinflamm. 2013, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Frausto-Del-Rio, D.; Soto-Cruz, I.; Garay-Canales, C.; Ambriz, X.; Soldevila, G.; Carretero-Ortega, J.; Vazquez-Prado, J.; Ortega, E. Interferon Gamma Induces Actin Polymerization, Rac1 Activation and Down Regulates Phagocytosis in Human Monocytic Cells. Cytokine 2012, 57, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]

| Target Gene | Forward | Reverse |
|---|---|---|
| iNOS | 5′-GCCACCAACAATGGCAACA-3′ | 5′-CGTACCGGATGAGCTGTGAATT-3′ |
| IL-1β | 5′-CCTGCAGCTGGAGAGTGTGGAT-3′ | 5′-TGTGCTCTGCTTGTGAGGTGCT-3′ |
| TNFα | 5′-ATGGCCTCCCTCTCATCAGTTC-3′ | 5′-TTGGTGGTTTGCTACGACGTG-3′ |
| Arg1 | 5′-GTGAAGAACCCACGGTCTGT-3′ | 5′-CCAGCACCACACTGACTCTT-3′ |
| Mrc1 | 5′-GTGGAGTGATGGAACCCCAG-3′ | 5′-CTGTCCGCCCAGTATCCATC-3′ |
| IL-10 | 5′-GCTCTTGCACTACCAAAGCC-3′ | 5′-CTGCTGATCCTCATGCCAGT-3′ |
| RPS29 | 5′-TTCCTTTCTCCTCGTTGGGC-3′ | 5′-TTCAGCCCGTATTTGCGGAT-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, M.A.A.; Wise, R.M.; Benjamin, B.P.; Hochreiner, E.M.; Mohiuddin, O.A.; Bunnell, B.A. Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells 2021, 10, 26. https://doi.org/10.3390/cells10010026
Harrison MAA, Wise RM, Benjamin BP, Hochreiner EM, Mohiuddin OA, Bunnell BA. Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells. 2021; 10(1):26. https://doi.org/10.3390/cells10010026
Chicago/Turabian StyleHarrison, Mark A. A., Rachel M. Wise, Brooke P. Benjamin, Emily M. Hochreiner, Omair A. Mohiuddin, and Bruce A. Bunnell. 2021. "Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype" Cells 10, no. 1: 26. https://doi.org/10.3390/cells10010026
APA StyleHarrison, M. A. A., Wise, R. M., Benjamin, B. P., Hochreiner, E. M., Mohiuddin, O. A., & Bunnell, B. A. (2021). Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells, 10(1), 26. https://doi.org/10.3390/cells10010026






