Immune Cell-Associated Protein Expression Helps to Predict Survival in Muscle-Invasive Urothelial Bladder Cancer Patients after Radical Cystectomy and Optional Adjuvant Chemotherapy
Abstract
1. Introduction
2. Results
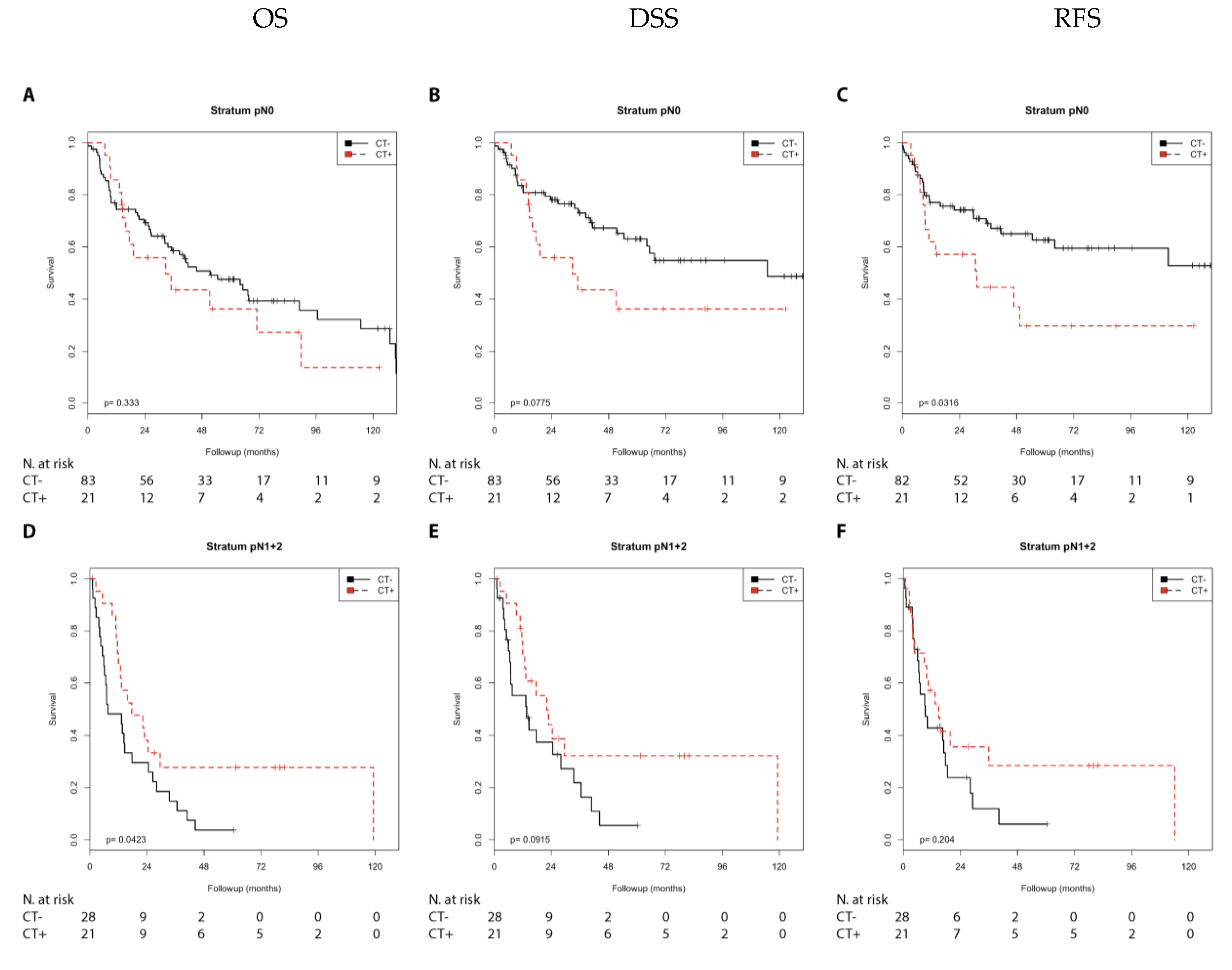
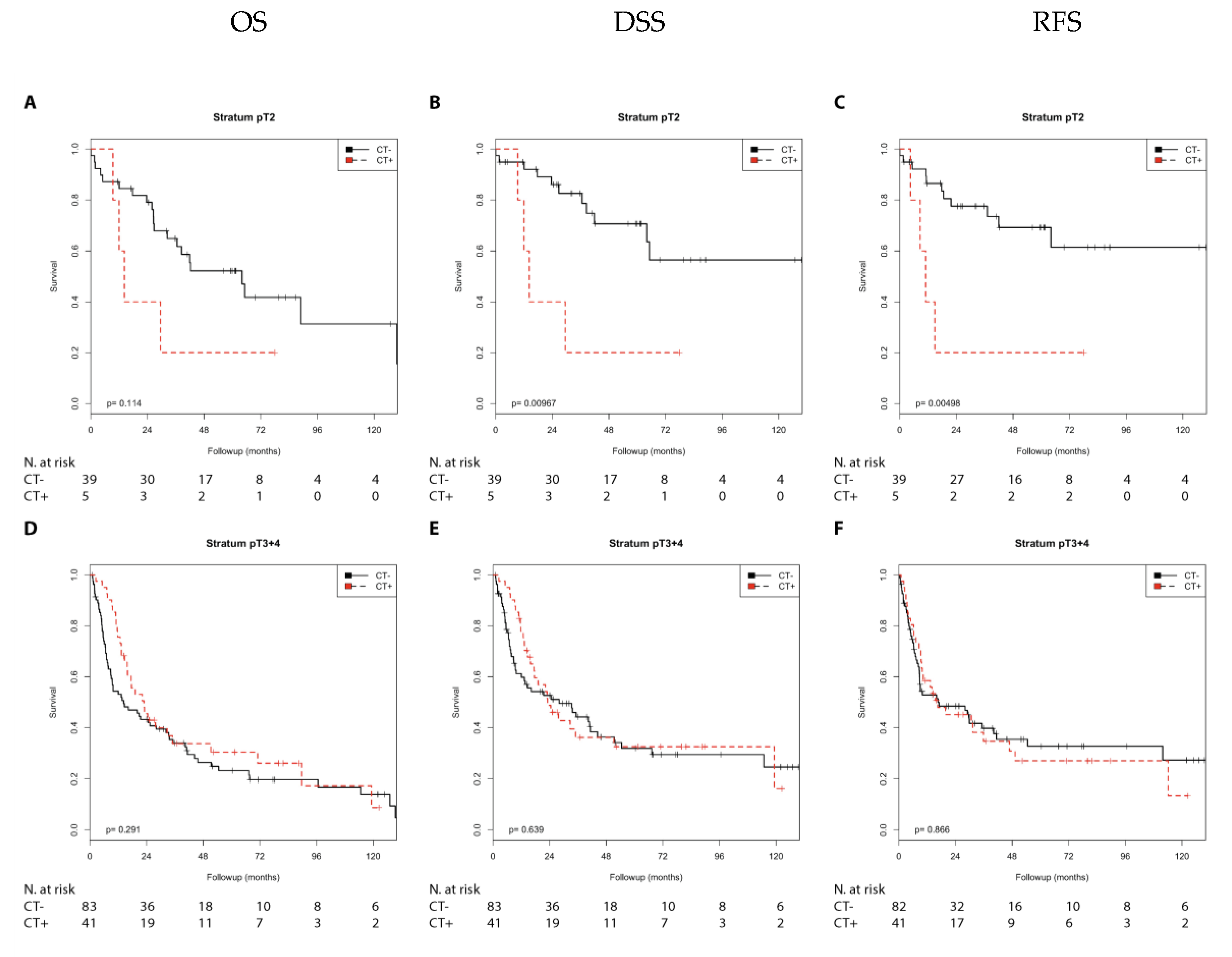
2.1. Association of Chemotherapy Treatment with Prognosis in Different Nodal Stage and Tumor Stage Groups of Patients with Muscle-Invasive Bladder Cancer
2.2. Association of Chemotherapy Treatment with Prognosis in Different Nodal Stage and Tumor Stage Groups Stratified by CCL2, CD68, and CD163 Expression in Muscle Invasive Bladder Cancer Patients
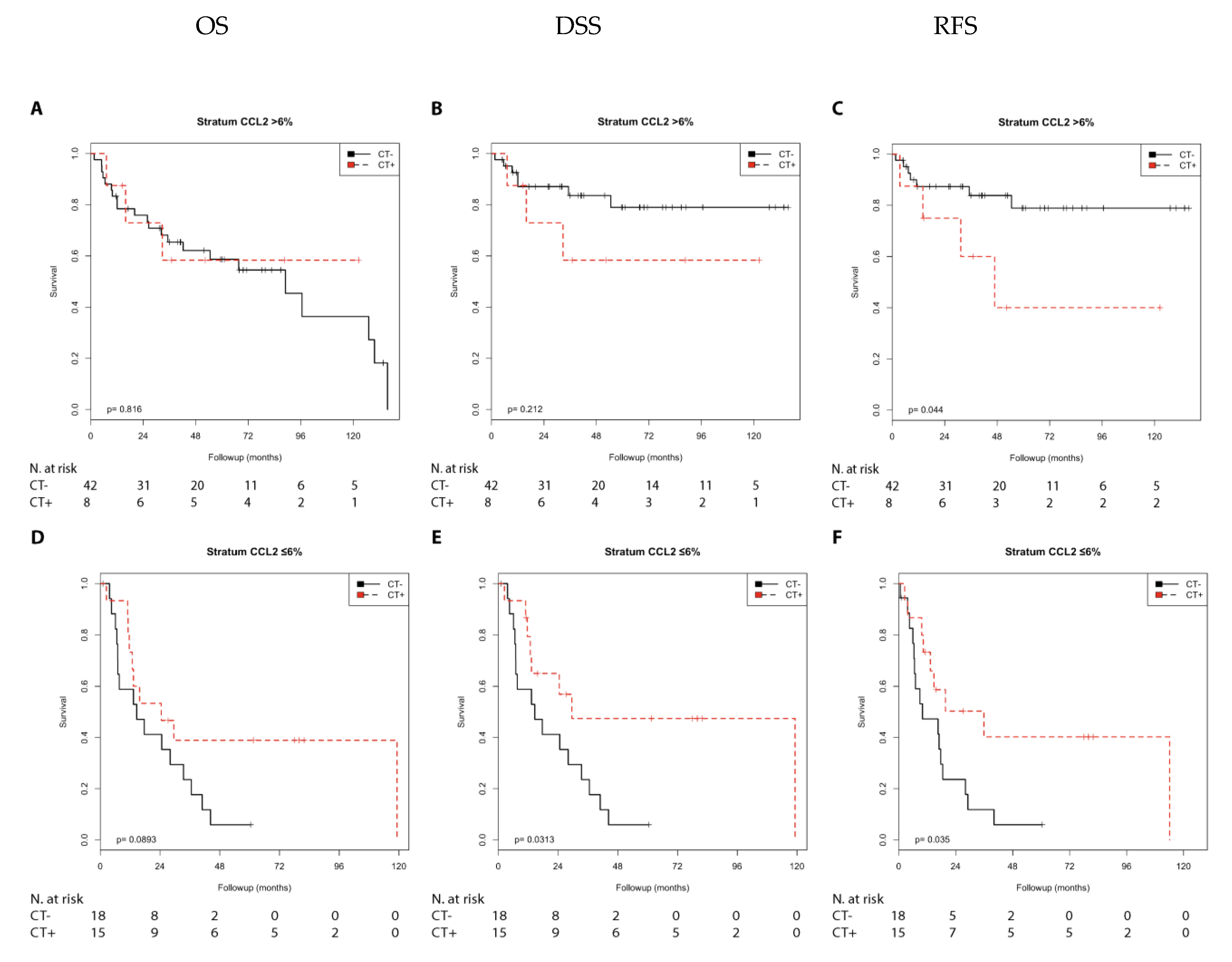
2.2.1. Immune Marker CCL2
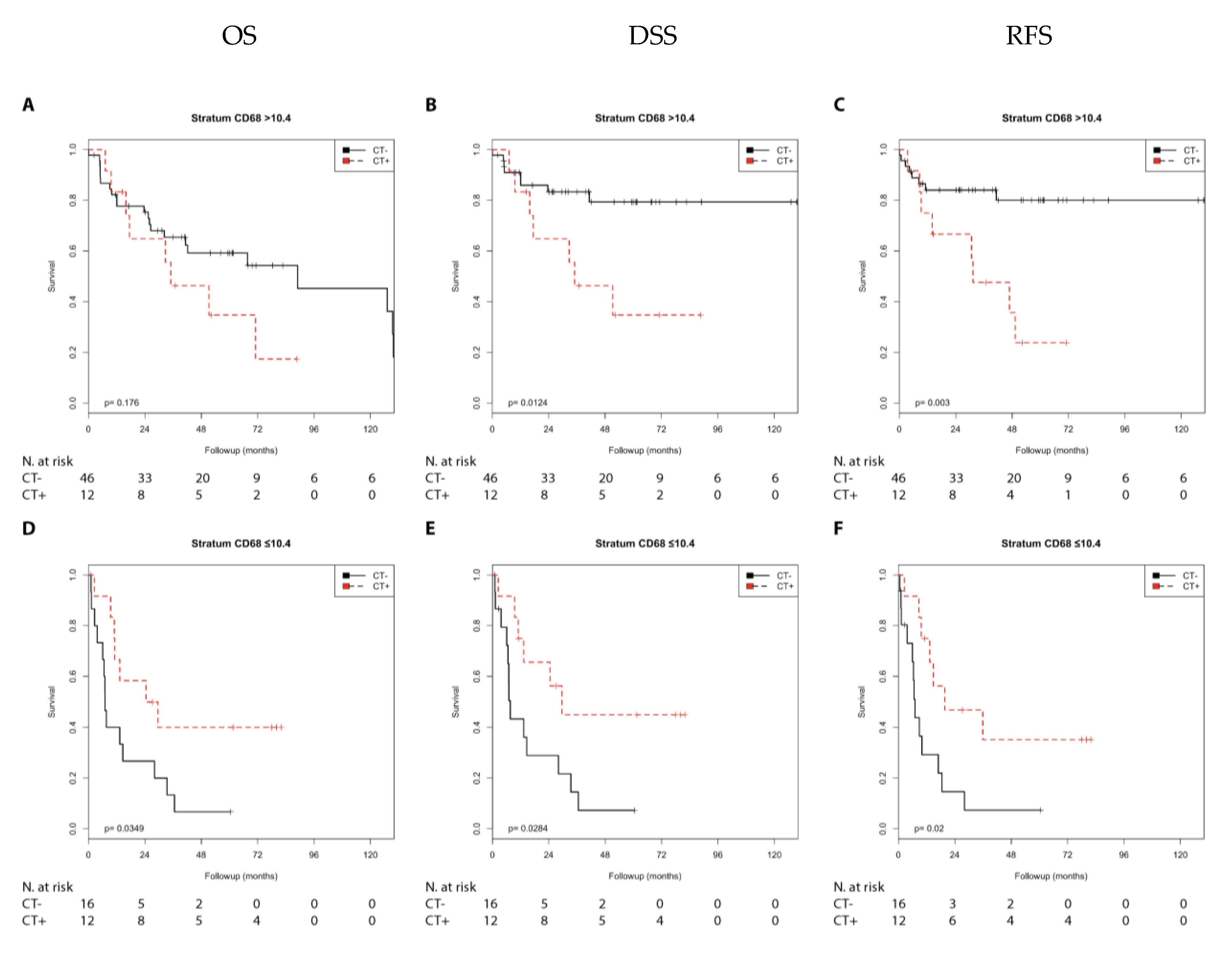
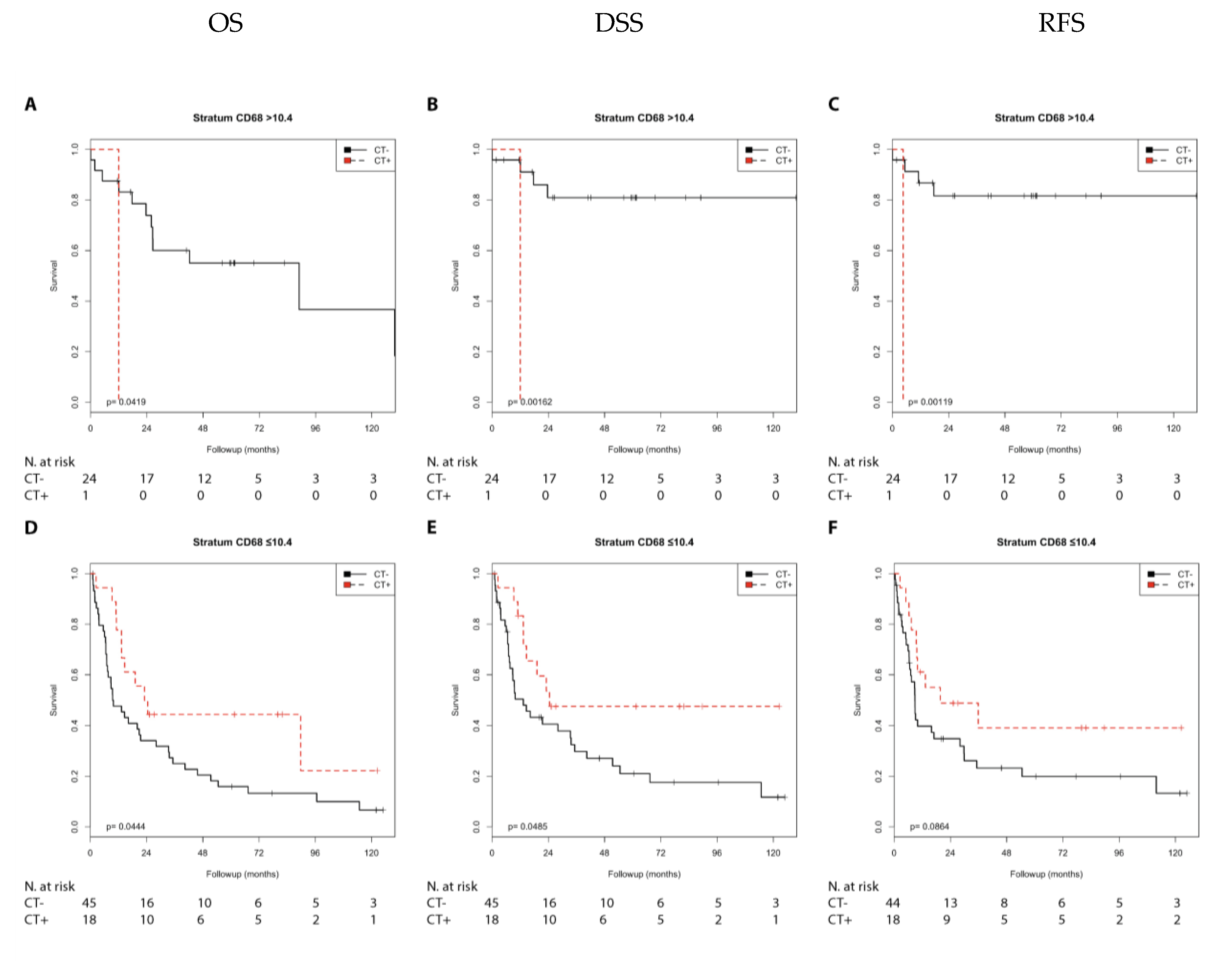
2.2.2. Immune Marker CD68
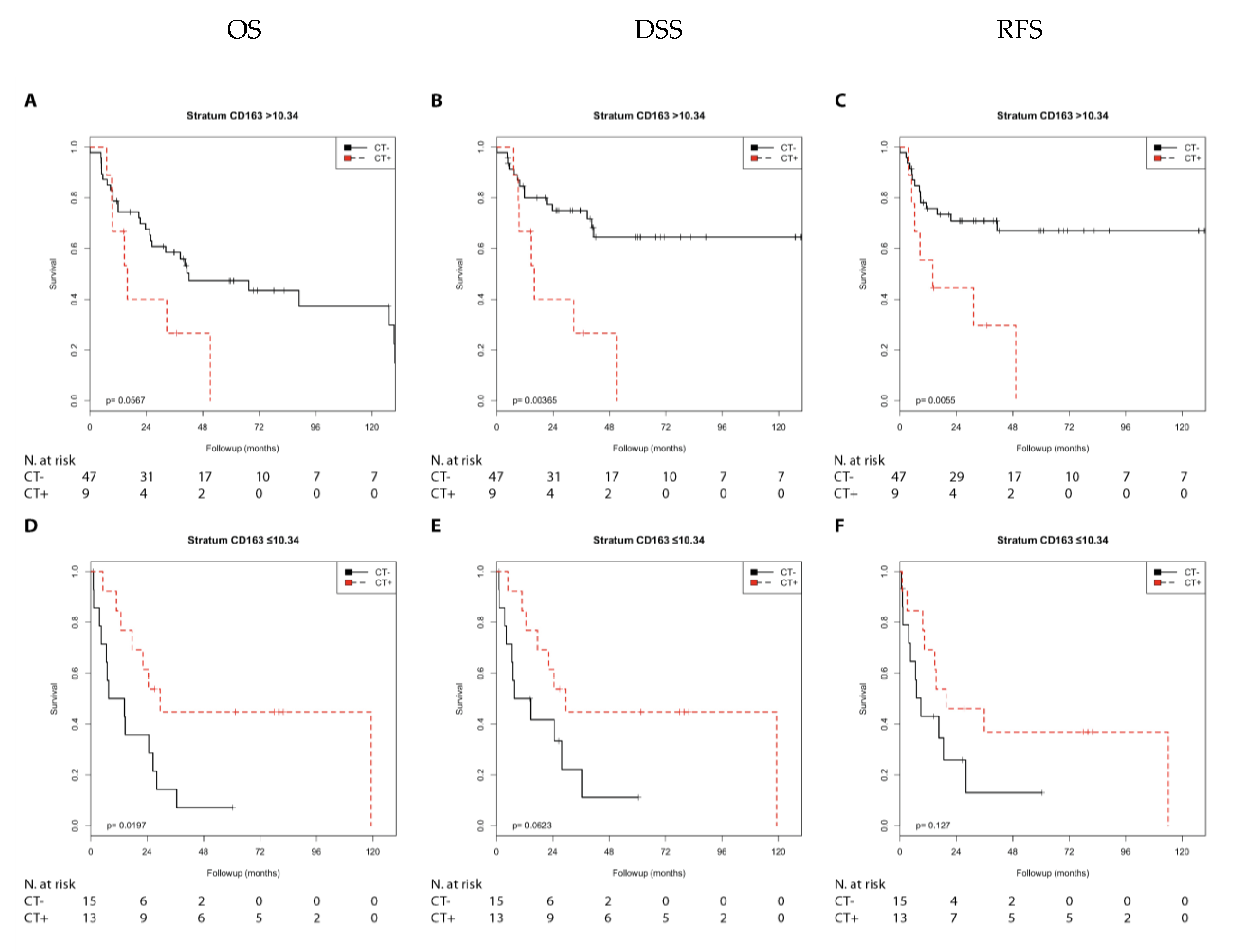
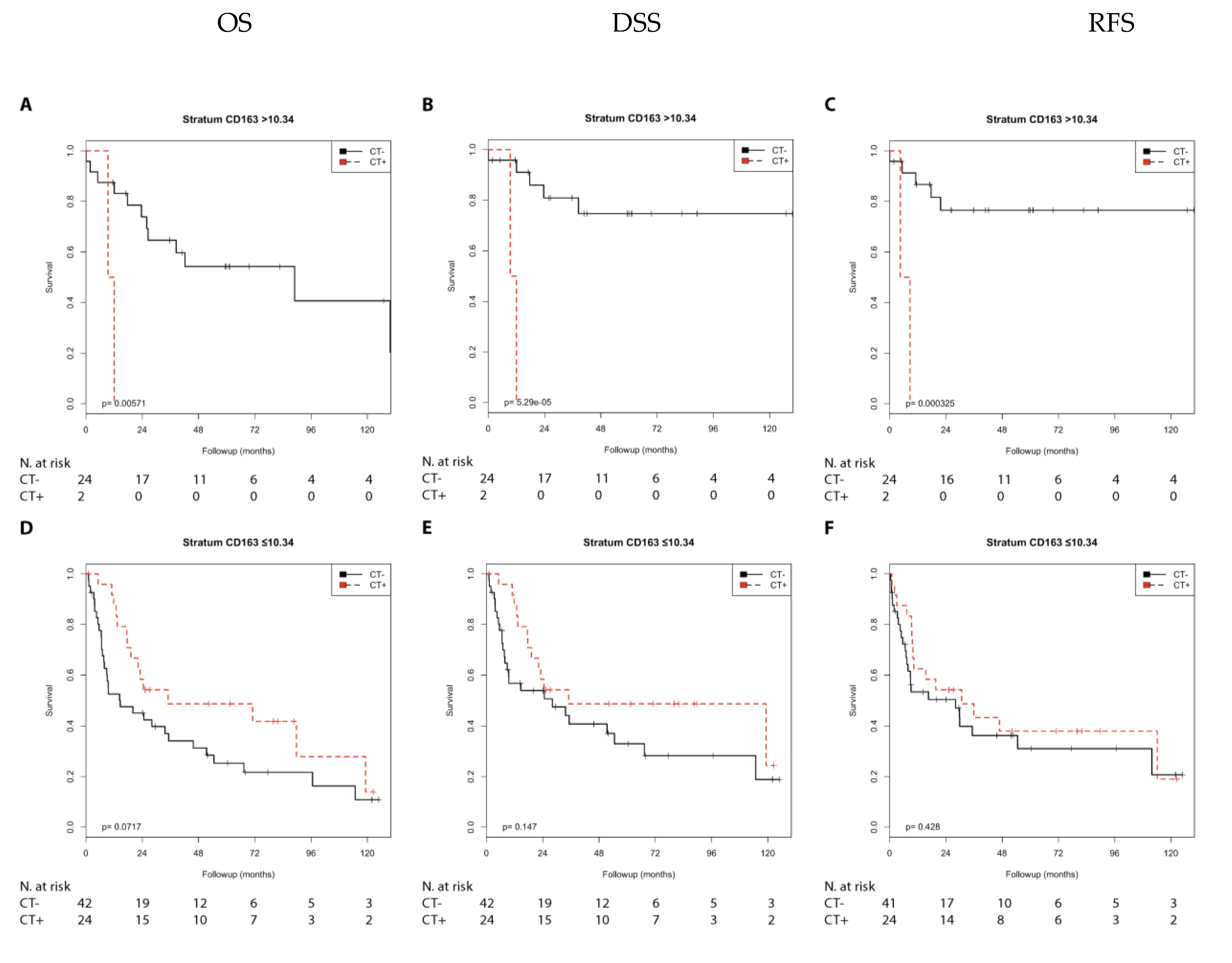
2.2.3. Immune Marker CD163
2.3. Association of Chemotherapy with Prognosis in Different Nodal Stage and Tumor Stage Groups (Adjusted for CCL2, CD68, and CD163 Expression) in Muscle Invasive Bladder Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Patients and Tumor Material
4.2. Immunohistochemistry
4.3. Immune Cell Quantification via Definiens Developer Software
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weaver, A.L.; Leibovich, B.C.; Ramnani, D.M.; Neumann, R.M.; Scherer, B.G.; Nehra, A.; Zincke, H.; Bostwick, D.G. Predicting the survival of bladder carcinoma patients treated with radical cystectomy. Cancer 2000, 88, 2326–2332. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020. [CrossRef]
- Frank, I.; Cheville, J.C.; Blute, M.L.; Lohse, C.M.; Karnes, R.J.; Weaver, A.L.; Sebo, T.J.; Nehra, A.; Zincke, H. Prognostic value of p53 and MIB-1 in transitional cell carcinoma of the urinary bladder with regional lymph node involvement. Cancer 2004, 101, 1803–1808. [Google Scholar] [CrossRef]
- Eckstein, M.; Strissel, P.; Strick, R.; Weyerer, V.; Wirtz, R.; Pfannstiel, C.; Wullweber, A.; Lange, F.; Erben, P.; Stoehr, R.; et al. Cytotoxic T-cell-related gene expression signature predicts improved survival in muscle-invasive urothelial bladder cancer patients after radical cystectomy and adjuvant chemotherapy. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Efstathiou, J.A.; Mouw, K.W.; Gibb, E.A.; Liu, Y.; Wu, C.L.; Drumm, M.R.; da Costa, J.B.; du Plessis, M.; Wang, N.Q.; Davicioni, E.; et al. Impact of Immune and Stromal Infiltration on Outcomes Following Bladder-Sparing Trimodality Therapy for Muscle-Invasive Bladder Cancer. Eur. Urol. 2019, 76, 59–68. [Google Scholar] [CrossRef]
- Pfannstiel, C.; Strissel, P.L.; Chiappinelli, K.B.; Sikic, D.; Wach, S.; Wirtz, R.M.; Wullweber, A.; Taubert, H.; Breyer, J.; Otto, W.; et al. The Tumor Immune Microenvironment Drives a Prognostic Relevance that Correlates with Bladder Cancer Subtypes. Cancer Immunol. Res. 2019, 7, 923–938. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef]
- Sjodahl, G.; Lovgren, K.; Lauss, M.; Chebil, G.; Patschan, O.; Gudjonsson, S.; Mansson, W.; Ferno, M.; Leandersson, K.; Lindgren, D.; et al. Infiltration of CD3(+) and CD68(+) cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 2014, 32, 791–797. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Q.; Zeng, H.; Zhang, H.; Liu, Z.; Huang, Q.; Xiong, Y.; Wang, J.; Chang, Y.; Bai, Q.; et al. Tumor-infiltrating IL-17A(+) cells determine favorable prognosis and adjuvant chemotherapeutic response in muscle-invasive bladder cancer. Oncoimmunology 2020, 9, 1747332. [Google Scholar] [CrossRef]
- Eckstein, M.; Epple, E.; Jung, R.; Weigelt, K.; Lieb, V.; Sikic, D.; Stohr, R.; Geppert, C.; Weyerer, V.; Bertz, S.; et al. CCL2 Expression in Tumor Cells and Tumor-Infiltrating Immune Cells Shows Divergent Prognostic Potential for Bladder Cancer Patients Depending on Lymph Node Stage. Cancers 2020, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Johanns, U.; Kugler, A.; Reffelmann, M.; Radzun, H.J. Quantification and in situ localization of MCP-1 mRNA and its relation to the immune response of renal cell carcinoma. Cytokine 2001, 13, 227–233. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Liu, L.; Gong, C.Y.; Shi, H.S.; Zeng, Y.H.; Wang, X.Z.; Zhao, Y.W.; Wei, Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Borre, A.; Wang, J.M.; Tattanelli, M.; Maddalena, F.; Polentarutti, N.; Peri, G.; Mantovani, A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J. Immunol. 1992, 148, 760–765. [Google Scholar]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef]
- Bello, D.M.; Russell, C.; McCullough, D.; Tierno, M.; Morrow, M. Lymph Node Status in Breast Cancer Does Not Predict Tumor Biology. Ann. Surg. Oncol. 2018, 25, 2884–2889. [Google Scholar] [CrossRef]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Kiselyov, A.; Bunimovich-Mendrazitsky, S.; Startsev, V. Key signaling pathways in the muscle-invasive bladder carcinoma: Clinical markers for disease modeling and optimized treatment. Int. J. Cancer 2016, 138, 2562–2569. [Google Scholar] [CrossRef]
- Rodel, C.; Grabenbauer, G.G.; Rodel, F.; Birkenhake, S.; Kuhn, R.; Martus, P.; Zorcher, T.; Fursich, D.; Papadopoulos, T.; Dunst, J.; et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: Possible predictors for response to radiochemotherapy and successful bladder preservation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1213–1221. [Google Scholar] [CrossRef]
- Tanabe, K.; Yoshida, S.; Koga, F.; Inoue, M.; Kobayashi, S.; Ishioka, J.; Tamura, T.; Sugawara, E.; Saito, K.; Akashi, T.; et al. High Ki-67 Expression Predicts Favorable Survival in Muscle-Invasive Bladder Cancer Patients Treated With Chemoradiation-Based Bladder-Sparing Protocol. Clin. Genitourin. Cancer 2015, 13, e243–e251. [Google Scholar] [CrossRef]
- Wang, B.; Xie, H.; Ma, C.; Zhang, G.; Gan, H.; Wang, Q.; Liu, X.; Zhu, Y.; Zhu, Y.; Shi, G.; et al. Expression of ARID1B Is Associated With Poor Outcomes and Predicts the Benefit from Adjuvant Chemotherapy in Bladder Urothelial Carcinoma. J. Cancer 2017, 8, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.C.; Wild, P.; Leicht, C.; Bertz, S.; Danenberg, K.D.; Danenberg, P.V.; Stohr, R.; Stockle, M.; Lehmann, J.; Schuler, M.; et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010, 12, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Sung, J.Y.; Park, S.H.; Kwon, G.Y.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Jo, J.; Choi, H.Y.; et al. ERCC1 as a biomarker for bladder cancer patients likely to benefit from adjuvant chemotherapy. BMC Cancer 2012, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Rinaldetti, S.; Wirtz, R.M.; Worst, T.S.; Eckstein, M.; Weiss, C.A.; Breyer, J.; Otto, W.; Bolenz, C.; Hartmann, A.; Erben, P. FOXM1 predicts overall and disease specific survival in muscle-invasive urothelial carcinoma and presents a differential expression between bladder cancer subtypes. Oncotarget 2017, 8, 47595–47606. [Google Scholar] [CrossRef] [PubMed]
- Teh, M.T. FOXM1 coming of age: Time for translation into clinical benefits? Front. Oncol 2012, 2, 146. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J., Jr.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 18, 14016–14021. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, Y.; Wang, Y.; Liu, Z.; Zhang, J.; Xie, H.; Fu, Q.; Dai, B.; Ye, D.; Xu, J. Identification and Validation of Stromal Immunotype Predict Survival and Benefit from Adjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2018, 24, 3069–3078. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Nie, L.; Jin, D.; Jin, K.; Zheng, X.; Yan, G.L.; Wei, Q. Classification of Muscle-Invasive Bladder Cancer Based on Immunogenomic Profiling. Front. Oncol. 2020, 18, 1429. [Google Scholar] [CrossRef]
- Eckstein, M.; Wirtz, R.M.; Pfannstil, C.; Wach, S.; Stoehr, R.; Breyer, J.; Erlmeier, F.; Gunes, C.; Nitschke, K.; Weichert, W.; et al. A multicenter round robin test of PD-L1 expression assessment in urothelial bladder cancer by immunohistochemistry and RT-qPCR with emphasis on prognosis prediction after radical cystectomy. Oncotarget 2018, 9, 15001–15014. [Google Scholar] [CrossRef]
- Nolte, S.; Zlobec, I.; Lugli, A.; Hohenberger, W.; Croner, R.; Merkel, S.; Hartmann, A.; Geppert, C.I.; Rau, T.T. Construction and analysis of tissue microarrays in the era of digital pathology: A pilot study targeting CDX1 and CDX2 in a colon cancer cohort of 612 patients. J. Pathol. Clin. Res. 2017, 3, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Taubert, H.; Wach, S.; Jung, R.; Pugia, M.; Keck, B.; Bertz, S.; Nolte, E.; Stoehr, R.; Lehmann, J.; Ohlmann, C.H.; et al. Piwil 2 expression is correlated with disease-specific and progression-free survival of chemotherapy-treated bladder cancer patients. Mol. Med. 2015, 21, 371–380. [Google Scholar] [CrossRef] [PubMed]

| CT+ vs. CT− | Overall Survival (OS) | Disease-Specific Survival (DSS) | Recurrence-Free-Survival (RFS) | |||||
|---|---|---|---|---|---|---|---|---|
| RR (95%CI) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | |||
| Nodal stage | ||||||||
| N0 | n.s. | n.s. | 1.82 (0.93–3.59) | (0.082) | 2.03 (1.05–3.94) | 0.035 | ||
| N1 + 2 | 0.52 (0.28–0.99) | 0.046 | 0.55 (0.28–1.11) | (0.097) | n.s. | n.s. | ||
| NX | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Tumor stage | ||||||||
| pT2 | n.s. | n.s. | 4.10 (1.29–13.04) | 0.016 | 4.61 (1.43–14.84) | 0.010 | ||
| pT3 + 4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Combination of nodal and tumor stage | ||||||||
| N0/T2 | 6.21 (0.72–53.18) | (0.096) | 10.28 (1.07–98.98) | 0.044 | 10.47 (1.09–100.70) | 0.042 | ||
| N0/T3 + 4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| N1 + 2/T2 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| N1 + 2/T3 + 4 | 0.48 (0.24–0.97) | 0.042 | 0.48 (0.22–1.05) | (0.066) | n.s. | n.s. | ||
| CT+ vs. CT− | OS | DSS | RFS | ||||
|---|---|---|---|---|---|---|---|
| Parameter | RR (95%CI) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | |
| Nodal stage | |||||||
| N0 | CCL2 ≤ 6% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N0 | CCL2 > 6% | n.s. | n.s. | n.s. | n.s. | 3.33 (0.96–11.51 | (0.057) |
| N0 | CD68 ≤ 10.4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N0 | CD68 > 10.4 | n.s. | n.s. | 3.38 (1.22–9.33) | 0.019 | 3.97 (1.48–10.61) | 0.006 |
| N0 | CD163 ≤ 10.34 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N0 | CD163 > 10.34 | 2.25 (0.95–5.32) | (0.064) | 3.62 (0.28–9.17) | 0.007 | 3.99 (0.29–8.51) | 0.009 |
| N1 + 2 | CCL2 ≤ 6% | 0.50 (0.22–1.13) | (0.096) | 0.39 (0.16–0.95) | 0.038 | 0.41 (0.17–0.96) | 0.041 |
| N1 + 2 | CCL2 > 6% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N1 + 2 | CD68 ≤ 10.4 | 0.39 (0.16–0.97) | 0.042 | 0.35 (0.13–0.94) | 0.036 | 0.34 (0.13–0.88) | 0.026 |
| N1 + 2 | CD68 > 10.4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N1 + 2 | CD163 ≤ 10.34 | 0.35 (0.14–0.88) | 0.025 | 0.41 (0.16–1.08) | (0.071) | n.s. | n.s. |
| N1 + 2 | CD163 > 10.34 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CT+ vs. CT− | OS | DSS | RFS | ||||
|---|---|---|---|---|---|---|---|
| Parameter | RR (95%CI) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | |
| Tumor stage | |||||||
| pT2 | CCL2 ≤ 6% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| pT2 | CCL2 > 6% | 11.56 (1.60–83.37) | 0.015 | 24.61 (2.18–277.50) | 0.009 | 25.51 (2.26–288.20) | 0.009 |
| pT2 | CD68 ≤ 10.4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| pT2 | CD68 > 10.4 | 7.39 (0.77–71.13) | (0.083) | 21.91 (1.37–351.30) | 0.029 | 22.98 (1.44–367.60) | 0.027 |
| pT2 | CD163 ≤ 10.34 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| pT2 | CD163 > 10.34 | 8.35 (1.38–50.52) | 0.021 | 27.55 (2.44–310.40) | 0.007 | 15.35 (2.12–111.20) | 0.007 |
| pT3 + 4 | CCL2 ≤ 6% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| pT3 + 4 | CCL2 > 6% | n.s. | n.s. | n.s. | n.s. | 2.52 (1.08–5.84) | 0.032 |
| pT3 + 4 | CD68 ≤ 10.4 | 0.51 (0.26–0.99) | 0.049 | 0.48 (0.23–1.0) | (0.053) | 0.54 (0.27–1.10) | (0.091) |
| pT3 + 4 | CD68 > 10.4 | n.s. | n.s. | n.s. | n.s. | 2.14 (1.07–4.24) | 0.031 |
| pT3 + 4 | CD163 ≤ 10.34 | 0.57 (0.31–1.06) | (0.075) | n.s. | n.s. | n.s. | n.s. |
| pT3 + 4 | CD163 > 10.34 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CT+ vs. CT− | OS | DSS | RFS | |||||
|---|---|---|---|---|---|---|---|---|
| N | RR (95%CI) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | ||
| N0/T2 | 35 | 4.46 (0.45–41.57) | (0.200) | 5.24 (0.48–57.48) | (0.175) | 5.09 (0.19–55.48) | (0.182) | |
| N0/T3 + 4 | 69 | 1.24 (0.64–2.43) | (0.523) | 1.60 (0.75–3.42) | (0.226) | 1.88 (0.89–3.99) | (0.099) * | |
| N1 + 2/T2 | 7 | 0.71 (0.04–11.79) | (0.809) | 0.71 (0.04–11.79) | (0.809) | 3.21 (0.15–70.80) | (0.459) | |
| N1 + 2/T3 + 4 | 42 | 0.48 (0.23–1.03) | (0.058) * | 0.47 (0.21–1.06) | (0.067) * | 0.65 (0.29–1.44) | (0.286) | |
| Immunological Marker | OS | DSS | RFS | |||||
|---|---|---|---|---|---|---|---|---|
| N | RR (95%CI) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | ||
| N0/T2 | CCL2 > 6% vs. ≤6% | 35 | n.s. | n.s. | 0.20 (0.75–31.76) | (0.095) | 0.21 (0.74–31.18) | (0.099) |
| N0/T3 + 4 | CCL2 > 6% vs. ≤6% | 69 | n.s. | n.s. | 0.41 (0.17–1.02) | (0.054) | 0.43 (0.18–1.00) | (0.051) |
| N0/T3 + 4 | CD68 > 10.4 vs. ≤10.4 | 69 | 0.49 (0.22–1.07) | (0.072) | 0.41 (0.17–1.03) | (0.057) | 0.35 (0.14–0.88) | 0.027 |
| N0/T3 + 4 | CD163 > 10.34 vs. ≤10.34 | 69 | 2.44 (1.18–5.02) | 0.016 | 3.02 (1.33–6.86) | 0.008 | 3.25 (1.37–7.69) | 0.007 |
| N1 + 2/T2 | CD163 > 10.34 vs. ≤10.34 | 7 | n.s. | n.s. | n.s. | n.s. | 14.3 (0.73 –282.72) | (0.080) |
| N1 + 2/T3 + 4 | CCL2 > 6% vs. ≤6% | 42 | 5.38 (1.92–15.03) | 0.001 | 4.12 (1.31–12.98) | 0.016 | 4.26 (1.40–12.93) | 0.011 |
| N1 + 2/T3 + 4 | CD68 > 10.4 vs. ≤10.4 | 42 | 0.43 (0.17–1.13) | (0.088) | n.s. | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taubert, H.; Eckstein, M.; Epple, E.; Jung, R.; Weigelt, K.; Lieb, V.; Sikic, D.; Stöhr, R.; Geppert, C.; Weyerer, V.; et al. Immune Cell-Associated Protein Expression Helps to Predict Survival in Muscle-Invasive Urothelial Bladder Cancer Patients after Radical Cystectomy and Optional Adjuvant Chemotherapy. Cells 2021, 10, 159. https://doi.org/10.3390/cells10010159
Taubert H, Eckstein M, Epple E, Jung R, Weigelt K, Lieb V, Sikic D, Stöhr R, Geppert C, Weyerer V, et al. Immune Cell-Associated Protein Expression Helps to Predict Survival in Muscle-Invasive Urothelial Bladder Cancer Patients after Radical Cystectomy and Optional Adjuvant Chemotherapy. Cells. 2021; 10(1):159. https://doi.org/10.3390/cells10010159
Chicago/Turabian StyleTaubert, Helge, Markus Eckstein, Elena Epple, Rudolf Jung, Katrin Weigelt, Verena Lieb, Danijel Sikic, Robert Stöhr, Carol Geppert, Veronika Weyerer, and et al. 2021. "Immune Cell-Associated Protein Expression Helps to Predict Survival in Muscle-Invasive Urothelial Bladder Cancer Patients after Radical Cystectomy and Optional Adjuvant Chemotherapy" Cells 10, no. 1: 159. https://doi.org/10.3390/cells10010159
APA StyleTaubert, H., Eckstein, M., Epple, E., Jung, R., Weigelt, K., Lieb, V., Sikic, D., Stöhr, R., Geppert, C., Weyerer, V., Bertz, S., Kehlen, A., Hartmann, A., Wullich, B., & Wach, S. (2021). Immune Cell-Associated Protein Expression Helps to Predict Survival in Muscle-Invasive Urothelial Bladder Cancer Patients after Radical Cystectomy and Optional Adjuvant Chemotherapy. Cells, 10(1), 159. https://doi.org/10.3390/cells10010159











