Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy
Abstract
1. Introduction
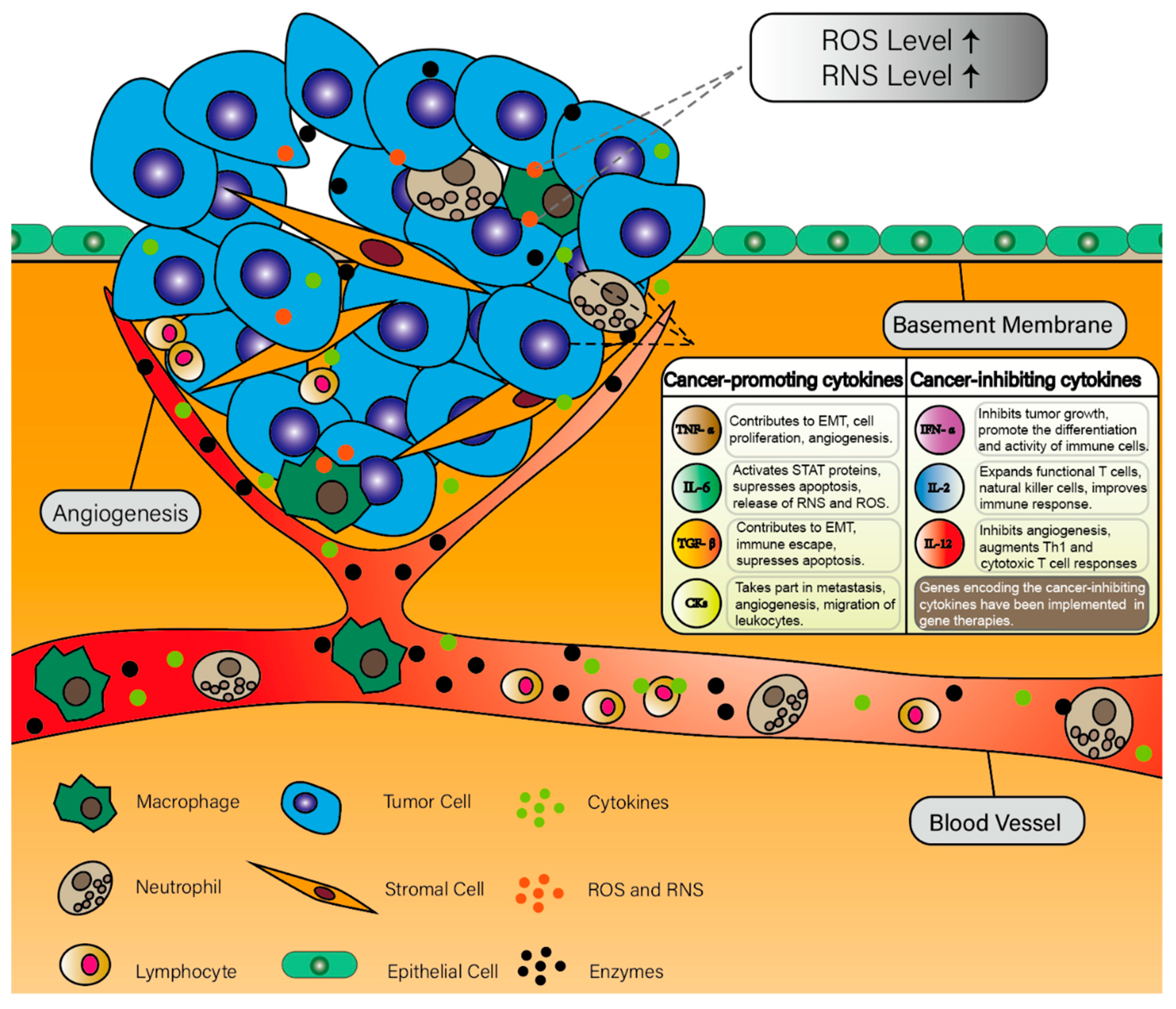
2. Inflammation and Cancer
2.1. Inflammation and Cancer Development
2.2. Cytokines in Cancer-Related Inflammation
2.2.1. Interleukins
2.2.2. Tumor Necrosis Factor Alpha
2.2.3. Transforming Growth Factor Beta
2.2.4. Chemokines
2.2.5. Interferons
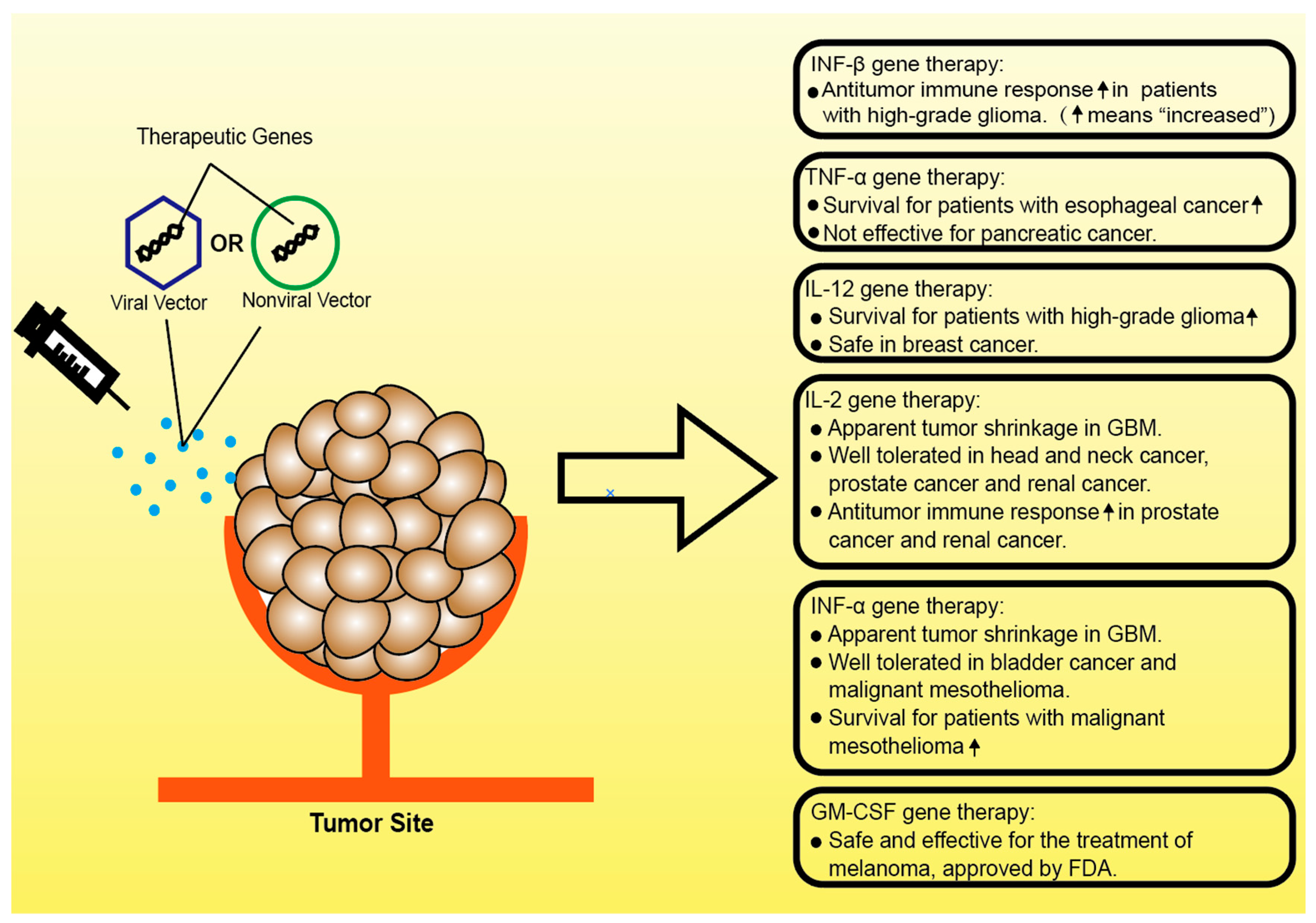
3. Clinical Studies of Gene Therapies Targeting Inflammatory Cytokines in Cancer
3.1. Gene Therapies Based on TNF-α
3.2. Gene Therapies Based on IL-12
3.3. Gene Therapies Based on IL-2
3.4. Gene Therapies Based on IFN-α
3.5. Gene Therapies Based on IFN-β
3.6. Gene Therapies Based on GM-CSF
4. Future Perspectives and Ongoing Preclinical Studies
4.1. CRISPR Targeting Cancer Inflammatory Environment
4.2. Novel Nonviral Vectors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Fleit, H.B. Chronic Inflammation. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- King, T.C. Inflammation, Inflammatory Mediators, and Immune-Mediated Disease-ScienceDirect. Elsevier’s Integrated Pathology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 21–57. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Attia, Z.I.; Galal, S.M. Acute inflammation induces immunomodulatory effects on myeloid cells associated with anti-tumor responses in a tumor mouse model. J. Adv. Res. 2016, 7, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Detering, E. An emerging role for anti-inflammatory agents for chemoprevention. Recent Results Cancer Res. 2013, 191, 1–5. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Gronke, K.; Hernández, P.P.; Zimmermann, J.; Klose, C.S.N.; Kofoed-Branzk, M.; Guendel, F.; Witkowski, M.; Tizian, C.; Amann, L.; Schumacher, F.; et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566, 249–253. [Google Scholar] [CrossRef]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Geismann, C.; Schäfer, H.; Gundlach, J.P.; Hauser, C.; Egberts, J.H.; Schneider, G.; Arlt, A. NF-κB Dependent Chemokine Signaling in Pancreatic Cancer. Cancers 2019, 11, 1445. [Google Scholar] [CrossRef]
- Putoczki, T.L.; Thiem, S.; Loving, A.; Busuttil, R.A.; Wilson, N.J.; Ziegler, P.K.; Nguyen, P.M.; Preaudet, A.; Farid, R.; Edwards, K.M.; et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013, 24, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Zaalberg, A.; Moradi Tuchayi, S.; Ameri, A.H.; Ngo, K.H.; Cunningham, T.J.; Eliane, J.P.; Livneh, M.; Horn, T.D.; Rosman, I.S.; Musiek, A.; et al. Chronic Inflammation Promotes Skin Carcinogenesis in Cancer-Prone Discoid Lupus Erythematosus. J. Investig. Derm. 2019, 139, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.M.; Shiri, S.; Farsinejad, S. Metastasis review: From bench to bedside. Tumor Biol. 2014, 35, 8483–8523. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Marusyk, A.; Tabassum, D.P.; Altrock, P.M.; Almendro, V.; Michor, F.; Polyak, K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature 2014, 514, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Siegel, R. 9-Cytokines and cytokine receptors. In Clinical Immunology, 4th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: London, UK, 2013; pp. 108–135. [Google Scholar] [CrossRef]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R. Chapter 17-Cytokines. In Systemic Lupus Erythematosus; Tsokos, G.C., Ed.; Academic Press: Boston, MA, USA, 2016. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhang, L.; Dai, Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac. Cancer 2020, 11, 835–839. [Google Scholar] [CrossRef]
- Sun, X.; Qu, Q.; Lao, Y.; Zhang, M.; Yin, X.; Zhu, H.; Wang, Y.; Yang, J.; Yi, J.; Hao, M. Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer. BMC Cancer 2019, 19, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Rose-John, S.; Schooltink, H. Cytokines are a therapeutic target for the prevention of inflammation-induced cancers. Recent Results Cancer Res. 2007, 174, 57–66. [Google Scholar] [CrossRef]
- Marson, A.; Levine, S.S.; Cole, M.F.; Frampton, G.M.; Brambrink, T.; Johnstone, S.; Guenther, M.G.; Johnston, W.K.; Wernig, M.; Newman, J.; et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 2008, 134, 521–533. [Google Scholar] [CrossRef]
- Hecht, N.; Pappo, O.; Shouval, D.; Rose-John, S.; Galun, E.; Axelrod, J.H. Hyper-IL-6 gene therapy reverses fulminant hepatic failure. Mol. Ther. 2001, 3, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xu, M.; Wang, W.; Zhang, F.; Li, D.; Xu, X.; Gu, J.; Hoffman, R.M. IL-2 gene therapy of advanced lung cancer patients. Anticancer Res. 1996, 16, 1993–1998. [Google Scholar] [PubMed]
- Chiocca, E.A.; Yu, J.S.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M.; et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- de Rham, C.; Ferrari-Lacraz, S.; Jendly, S.; Schneiter, G.; Dayer, J.M.; Villard, J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res. 2007, 9, R125. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Sarzi-Puttini, P. Tumor Necrosis Factor. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, MA, USA, 2013; pp. 229–231. [Google Scholar] [CrossRef]
- Cao, Y. Tumor angiogenesis and therapy. Biomed. Pharmacother. 2005, 59 (Suppl. 2), S340–S343. [Google Scholar] [CrossRef]
- Kumar, M.; Allison, D.F.; Baranova, N.N.; Wamsley, J.J.; Katz, A.J.; Bekiranov, S.; Jones, D.R.; Mayo, M.W. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS ONE 2013, 8, e68597. [Google Scholar] [CrossRef]
- Li, B.; Vincent, A.; Cates, J.; Brantley-Sieders, D.M.; Polk, D.B.; Young, P.P. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009, 69, 338–348. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Jo, E.; Jang, H.J.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.S.; Jang, I.S.; Park, S.J. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complementary Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Schröder, S.K.; Asimakopoulou, A.; Tillmann, S.; Koschmieder, S.; Weiskirchen, R. TNF-α controls Lipocalin-2 expression in PC-3 prostate cancer cells. Cytokine 2020, 135, 155214. [Google Scholar] [CrossRef]
- Zhang, G.P.; Yue, X.; Li, S.Q. Cathepsin C Interacts with TNF-α/p38 MAPK Signaling Pathway to Promote Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 10–23. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, R.; ten Hagen, T.L.M.; Eggermont, A.M.M. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. Oncology 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, R.; Yu, Z.; Shi, R.; Zhang, J.; Gao, S.; Shao, M.; Cui, S.; Gao, Z.; Xu, J.; et al. Tumor Necrosis Factor α Reduces SNAP29 Dependent Autolysosome Formation to Increase Prion Protein Level and Promote Tumor Cell Migration. Virol. Sin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-beta signaling in cancer treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Liu, J.; Lin, P.; Shi, T.; Jain, R.K.; Xu, L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. 2011, 17, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Sirotkovic-Skerlev, M.; Kulić, A.; Bradić, L.; Cacev, T. Protumor effects of proinflammatory mediators in breast cancer. Period. Biol. 2012, 114, 489. [Google Scholar]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of Macrophage Targeting in the Antitumor Activity of Trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef]
- Germano, G.; Mantovani, A.; Allavena, P. Targeting of the innate immunity/inflammation as complementary anti-tumor therapies. Ann. Med. 2011, 43, 581–593. [Google Scholar] [CrossRef]
- Onuffer, J.J.; Horuk, R. Chemokines, chemokine receptors and small-molecule antagonists: Recent developments. Trends Pharmacol. Sci. 2002, 23, 459–467. [Google Scholar] [CrossRef]
- Ryan, C.W.; Desai, J. The past, present, and future of cytotoxic chemotherapy and pathway-directed targeted agents for soft tissue sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2013. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J.; et al. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8(+) T-cell Infiltration. Clin. Cancer Res. 2017, 23, 4556–4568. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Klement, J.D.; Ibrahim, M.L.; Xiao, W.; Redd, P.S.; Nayak-Kapoor, A.; Zhou, G.; Liu, K. Type I interferon suppresses tumor growth through activating the STAT3-granzyme B pathway in tumor-infiltrating cytotoxic T lymphocytes. J. Immunother. Cancer 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kerner, Z.J.; Hong, H.; Sun, J. Targeted Cancer Therapy with Tumor Necrosis Factor-Alpha. Biochem. Insights 2008, 2008, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Wei, J.; Ma, L.; Lai, Y.-H.; Zhang, R.; Li, H.; Li, C.; Lin, J. Bazedoxifene as a novel GP130 inhibitor for Colon Cancer therapy. J. Exp. Clin. Cancer Res. 2019, 38, 63. [Google Scholar] [CrossRef]
- O’Malley, B.W., Jr.; Li, D.; McQuone, S.J.; Ralston, R. Combination Nonviral Interleukin-2 Gene Immunotherapy For Head and Neck Cancer: From Bench Top to Bedside. Laryngoscope 2005, 115, 391–404. [Google Scholar] [CrossRef]
- Trudel, S.; Trachtenberg, J.; Toi, A.; Sweet, J.; Hua Li, Z.; Jewett, M.; Tshilias, J.; Zhuang, L.H.; Hitt, M.; Wan, Y.; et al. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 2003, 10, 755–763. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Belldegrun, A.S. Phase I clinical trial of interleukin 2 (IL-2) gene therapy for prostate cancer. Curr. Urol. Rep. 2001, 2, 9. [Google Scholar] [CrossRef]
- Galanis, E.; Burch, P.A.; Richardson, R.L.; Lewis, B.; Pitot, H.C.; Frytak, S.; Spier, C.; Akporiaye, E.T.; Peethambaram, P.P.; Kaur, J.S.; et al. Intratumoral administration of a 1,2-dimyristyloxypropyl-3- dimethylhydroxyethyl ammonium bromide/dioleoylphosphatidylethanolamine formulation of the human interleukin-2 gene in the treatment of metastatic renal cell carcinoma. Cancer 2004, 101, 2557–2566. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Boorjian, S.A.; Canter, D.J.; Ogan, K.; Karsh, L.I.; Downs, T.M.; Gomella, L.G.; Kamat, A.M.; Lotan, Y.; Svatek, R.S.; et al. Intravesical rAd-IFN alpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J. Clin. Oncol. 2017, 35, 30. [Google Scholar] [CrossRef] [PubMed]
- Sterman, D.H.; Alley, E.; Stevenson, J.P.; Friedberg, J.; Metzger, S.; Recio, A.; Moon, E.K.; Haas, A.R.; Vachani, A.; Katz, S.I.; et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin. Cancer Res. 2016, 22, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Natsume, A.; Hashizume, Y.; Fujii, M.; Mizuno, M.; Yoshida, J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: Novel findings from gene expression profiling and autopsy. J. Gene Med. 2008, 10, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.J.; Maguire, H.C.; Eisenlohr, L.C.; Laughlin, C.E.; Monken, C.E.; McCue, P.A.; Kovatich, A.J.; Lattime, E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999, 6, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Coffin, R.S.; Davis, C.J.; Graham, N.J.; Groves, N.; Guest, P.J.; Harrington, K.J.; James, N.D.; Love, C.A.; McNeish, I.; et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006, 12, 6737–6747. [Google Scholar] [CrossRef] [PubMed]
- Senzer, N.N.; Kaufman, H.L.; Amatruda, T.; Nemunaitis, M.; Reid, T.; Daniels, G.; Gonzalez, R.; Glaspy, J.; Whitman, E.; Harrington, K.; et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009, 27, 5763–5771. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Fazle Akbar, S.M.; Abe, M.; Yoshida, O.; Murakami, H.; Onji, M. Dendritic cell-based therapy as a multidisciplinary approach to cancer treatment: Present limitations and future scopes. Curr. Med. Chem. 2006, 13, 3113–3119. [Google Scholar] [CrossRef]
- Gao, J.Q.; Eto, Y.; Yoshioka, Y.; Sekiguchi, F.; Kurachi, S.; Morishige, T.; Yao, X.; Watanabe, H.; Asavatanabodee, R.; Sakurai, F.; et al. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J. Control. Release 2007, 122, 102–110. [Google Scholar] [CrossRef]
- Wright, P.; Zheng, C.; Moyana, T.; Xiang, J. Intratumoral vaccination of adenoviruses expressing fusion protein RM4/tumor necrosis factor (TNF)-alpha induces significant tumor regression. Cancer Gene Ther. 1998, 5, 371–379. [Google Scholar] [PubMed]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.-P.; Wang, Z.-F.; Liang, W.-Y.; Chen, L.; Chen, D.; Wang, A.-X.; Zhang, Z.-Q. In vitro and in vivo effect of 5-FC combined gene therapy with TNF-α and CD suicide gene on human laryngeal carcinoma cell line Hep-2. PLoS ONE 2013, 8, e61136. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Wild, A.T.; Wang, H.; Tran, P.T.; Chang, K.J.; Taylor, G.E.; Donehower, R.C.; Pawlik, T.M.; Ziegler, M.A.; Cai, H.; et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J. Clin. Oncol. 2013, 31, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Reid, T.; Senzer, N.; Swisher, S.; Pinto, H.; Hanna, N.; Chak, A.; Soetikno, R. Phase I evaluation of TNFerade biologic plus chemoradiotherapy before esophagectomy for locally advanced resectable esophageal cancer. Gastrointest. Endosc. 2012, 75, 1139–1146.e1132. [Google Scholar] [CrossRef]
- Balasubbramanian, D.; Goodlett, B.L.; Mitchell, B.M. Is IL-12 pro-inflammatory or anti-inflammatory? Depends on the blood pressure. Cardiovasc. Res. 2019, 115, 998–999. [Google Scholar] [CrossRef]
- Xinjie, L. Impact of IL-12 in Cancer. Curr. Cancer Drug Targets 2017, 17, 682–697. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Palù, G.; Cavaggioni, A.; Calvi, P.; Franchin, E.; Pizzato, M.; Boschetto, R.; Parolin, C.; Chilosi, M.; Ferrini, S.; Zanusso, A.; et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: A pilot study in humans. Gene Ther. 1999, 6, 330–337. [Google Scholar] [CrossRef]
- Broz, P.; Monack, D.M. Noncanonical Inflammasomes: Caspase-11 Activation and Effector Mechanisms. PLoS Pathog. 2013, 9, e1003144. [Google Scholar] [CrossRef]
- Bolívar, S.; Anfossi, R.; Humeres, C.; Vivar, R.; Boza, P.; Muñoz, C.; Pardo-Jimenez, V.; Olivares-Silva, F.; Díaz-Araya, G. IFN-β Plays Both Pro- and Anti-inflammatory Roles in the Rat Cardiac Fibroblast Through Differential STAT Protein Activation. Front. Pharm. 2018, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hashimoto, M.; Ito, Y.; Matsuura, M.; Ito, H.; Tanaka, M.; Watanabe, H.; Kondoh, G.; Tanaka, A.; Yasuda, K.; et al. Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis. Immunity 2018, 48, 1220–1232.e1225. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Kroemer, G.; Galluzzi, L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology 2016, 5, e1115641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Eshraghian, E.A.; Jammal, O.A.; Zhang, Z.; Zhu, X. CRISPR technology: The engine that drives cancer therapy. Biomed. Pharmacother. 2020, 133, 111007. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.; ElMallah, M.; Flotte, T. Gene Therapy 2017: Progress and Future Directions. Clin. Transl. Sci. 2017, 10, 242–248. [Google Scholar] [CrossRef]
- Wei, X.; Wei, Y. Opportunities and challenges in the nanoparticles for nucleic acid therapeutics: The first approval of an RNAi nanoparticle for treatment of a rare disease. Natl. Sci. Rev. 2019, 6, 1105–1106. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Q.; Wang, X. PLK1, A Potential Target for Cancer Therapy. Transl. Oncol. 2017, 10, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Xu, C.-F.; Li, H.-J.; Cao, Z.-T.; Liu, J.; Wang, J.-L.; Du, X.-J.; Yang, X.-Z.; Gu, Z.; Wang, J. Macrophage-Specific in Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef]
- Xu, C.; Lu, Z.; Luo, Y.; Liu, Y.; Cao, Z.; Shen, S.; Li, H.; Liu, J.; Chen, K.; Chen, Z.; et al. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat. Commun. 2018, 9, 4092. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gu, Z.; Zhong, J.; Wen, D.; Chen, G.; He, L.; Wu, J.; Gu, Z. Advances in glycosylation-mediated cancer-targeted drug delivery. Drug Discov. Today 2018, 23, 1126–1138. [Google Scholar] [CrossRef]
- Fan, D.Y.; Tian, Y.; Liu, Z.J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, I.V.; Snezhkov, E.V.; Chernov, I.P.; Pleshkan, V.V.; Potapov, V.K.; Sass, A.V.; Monastyrskaya, G.S.; Kopantzev, E.P.; Vinogradova, T.V.; Khramtsov, Y.V.; et al. Therapeutic properties of a vector carrying the HSV thymidine kinase and GM-CSF genes and delivered as a complex with a cationic copolymer. J. Transl. Med. 2015, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, Y.; Chen, L.; Hu, L.; Zhu, F.; He, Q. High iodine induces DNA damage in autoimmune thyroiditis partially by inhibiting the DNA repair protein MTH1. Cell Immunol. 2019, 344, 103948. [Google Scholar] [CrossRef] [PubMed]
- Kumagae, Y.; Hirahashi, M.; Takizawa, K.; Yamamoto, H.; Gushima, M.; Esaki, M.; Matsumoto, T.; Nakamura, M.; Kitazono, T.; Oda, Y. Overexpression of MTH1 and OGG1 proteins in ulcerative colitis-associated carcinogenesis. Oncol. Lett. 2018, 16, 1765–1776. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Liu, X.; Yang, X.; Li, X.; He, T.; Wang, N.; Yang, S.; Yu, C.; Yin, T.; et al. Artificial Virus Delivers CRISPR-Cas9 System for Genome Editing of Cells in Mice. ACS Nano 2017, 11, 95–111. [Google Scholar] [CrossRef]
| Cytokine Genes | Transfer Vector | Cancer Applications | Phase | Key Publication or clinicaltrials.gov No. | |
|---|---|---|---|---|---|
| TNF | TNF-α | Adenovirus | Prostate cancer | II | NCT01048151 |
| TNF-α | Adenovirus | Pancreatic cancer | III | NCT00051467 | |
| TNF-α | Adenovirus | Esophagus cancer | II | NCT00051480 | |
| TNF-α | Adenovirus | Head and neck cancer | I/II | NCT00496535 | |
| TNF-α | Adenovirus | Rectal cancer | II | NCT00137878 | |
| TNF-α | Adenovirus | Melanoma | II | NCT00261404 | |
| TNF-α and IL-2 | Adenovirus | Melanoma | I | NCT04217473 | |
| IL | IL-12 | Adenovirus | Glioma | I | NCT02026271 [36] |
| IL-12 | Adenovirus | Breast cancer | II | NCT04095689 | |
| IL-2 | Plasmid | Head and neck cancer | I | [73] | |
| IL-2 | Adenovirus | Prostate cancer | I | [74] | |
| IL-2 | Plasmid | Prostate cancer | I | [75] | |
| IL-2 | Cationic lipid | Renal cancer | I/II | [76] | |
| IFN | IFN-α2b | Adenovirus | Bladder cancer | II | [77] NCT01687244 |
| IFN-α2b | Adenovirus | Mesothelioma | I | [78] NCT01119664 | |
| IFN-β | Adenovirus | Glioma | I | [79] | |
| IFN-β | Adenovirus | Pleural malignancies | I | NCT00299962 | |
| GM-CSF | GM-CSF | Vaccinia virus | Melanoma | Not reported | [80] |
| GM-CSF | Oncolytic virus | Head and neck cancer; breast cancer; melanoma; gastrointestinal cancer | I | [81] | |
| GM-CSF | Oncolytic virus | Melanoma | II | [82] | |
| GM-CSF | Oncolytic virus | Melanoma | III | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. https://doi.org/10.3390/cells10010100
Lan T, Chen L, Wei X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells. 2021; 10(1):100. https://doi.org/10.3390/cells10010100
Chicago/Turabian StyleLan, Tianxia, Li Chen, and Xiawei Wei. 2021. "Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy" Cells 10, no. 1: 100. https://doi.org/10.3390/cells10010100
APA StyleLan, T., Chen, L., & Wei, X. (2021). Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells, 10(1), 100. https://doi.org/10.3390/cells10010100





