Author Contributions
M.Y., A.T., H.M., K.Y., and H.K. conceived and supervised research. T.N., M.S., and M.Y. designed experiments. T.N. conducted most experiments. M.S. and T.O. assisted and performed some experiments. M.S. analyzed data and wrote the manuscript. M.Y., A.T., H.M., K.Y., and H.K. supervised the preparation of the manuscript.
Figure 1.
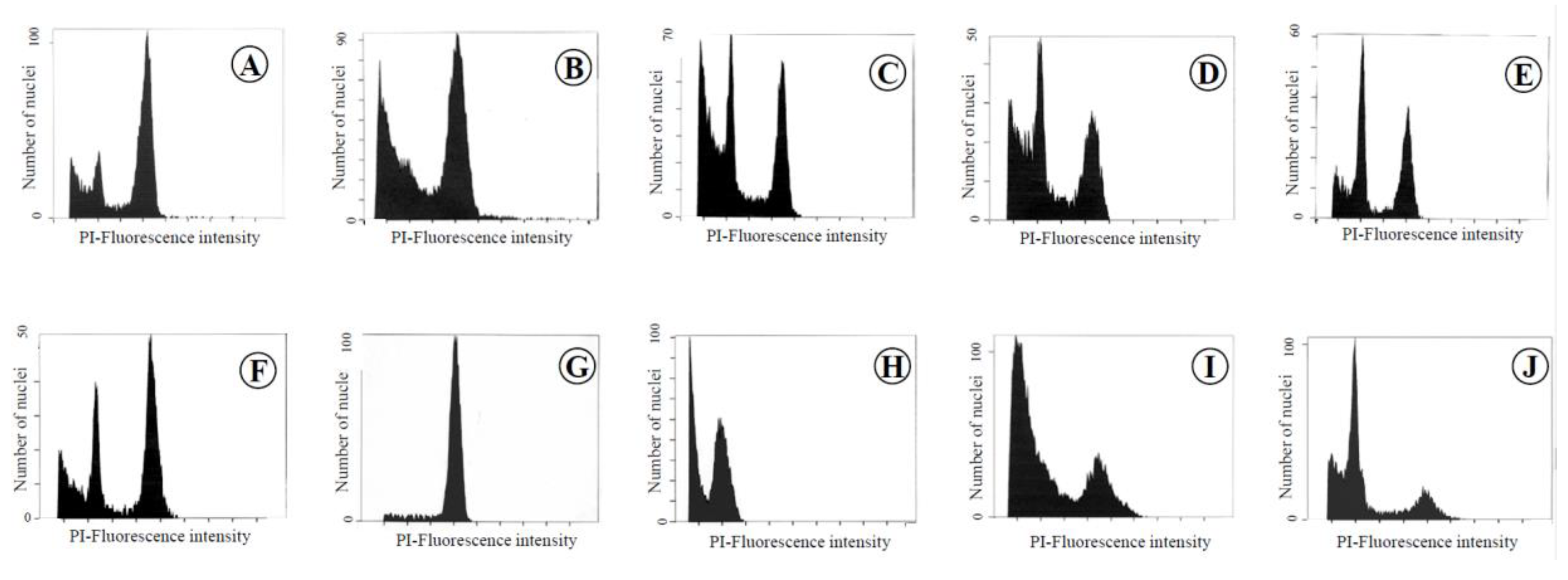
Flow cytometric analysis of each organ and tissue in leaves, flowers, seeds, and fruits of the chimera of the Meiwa kumquat. A: Leaf, B: Midrib, C: Petal, D: Filament, E: Style, F: Ovary, G: Seed, H: Juice sac, I: Albedo, J: Flavedo.
Figure 1.
Flow cytometric analysis of each organ and tissue in leaves, flowers, seeds, and fruits of the chimera of the Meiwa kumquat. A: Leaf, B: Midrib, C: Petal, D: Filament, E: Style, F: Ovary, G: Seed, H: Juice sac, I: Albedo, J: Flavedo.
Figure 2.
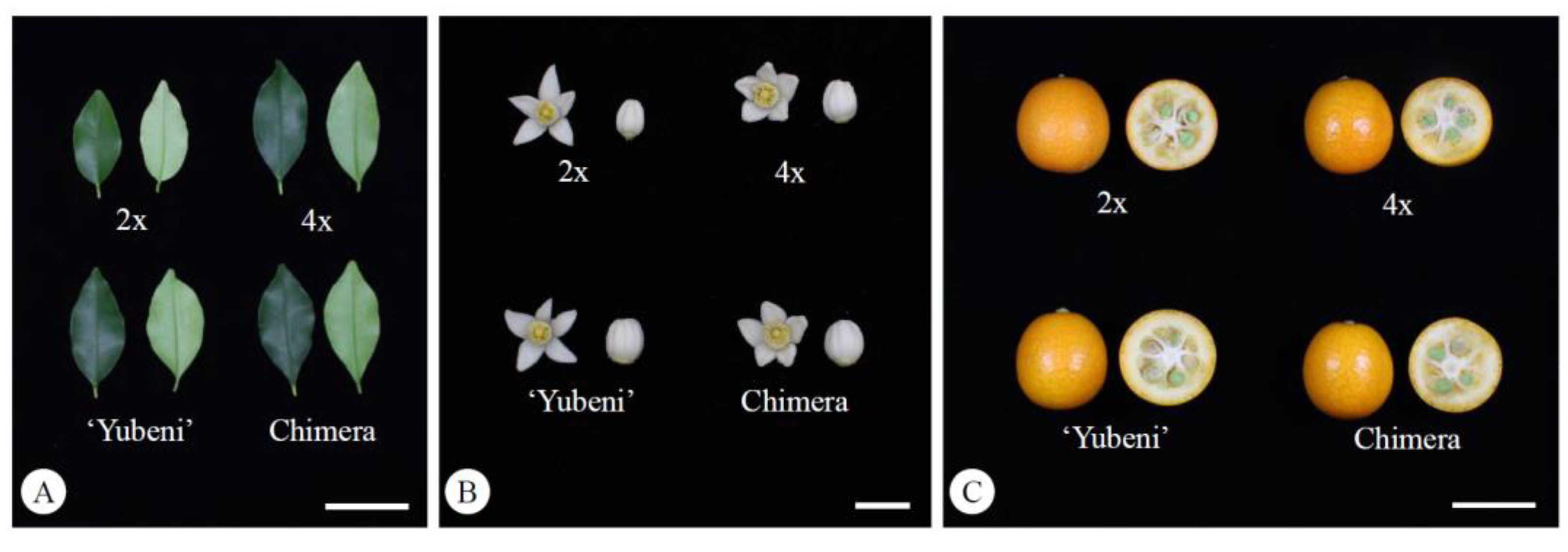
Comparison of the morphological characteristics of leaves (A, bar = 5 cm), flowers (B, bar = 1 cm), and fruits (C, bar = 3 cm) in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Figure 2.
Comparison of the morphological characteristics of leaves (A, bar = 5 cm), flowers (B, bar = 1 cm), and fruits (C, bar = 3 cm) in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Figure 3.
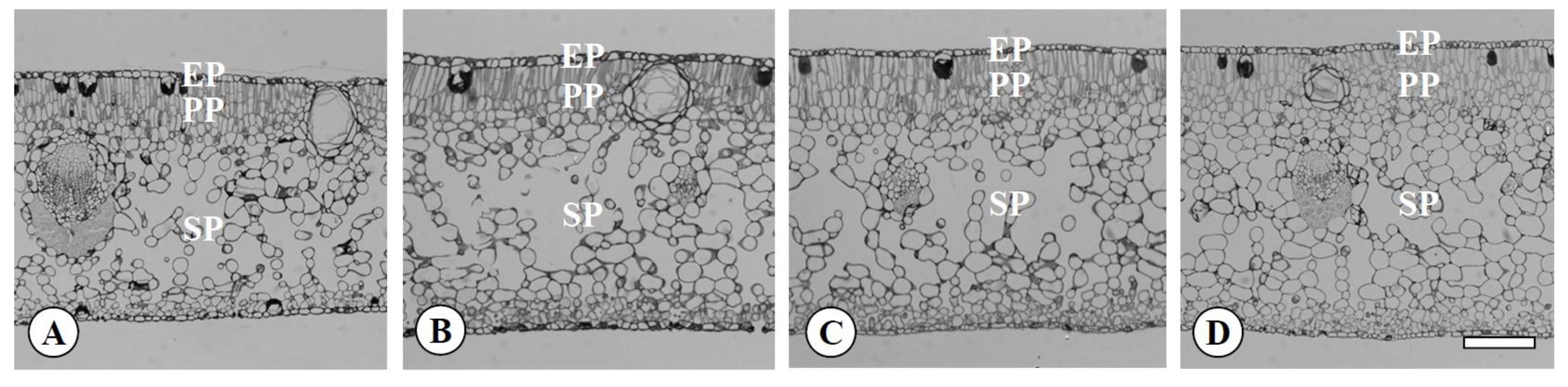
Transversal sections of leaves in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bar = 100 μm. EP: Epidermis, PP: Palisade parenchyma, SP: Spongy parenchyma.
Figure 3.
Transversal sections of leaves in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bar = 100 μm. EP: Epidermis, PP: Palisade parenchyma, SP: Spongy parenchyma.
Figure 4.
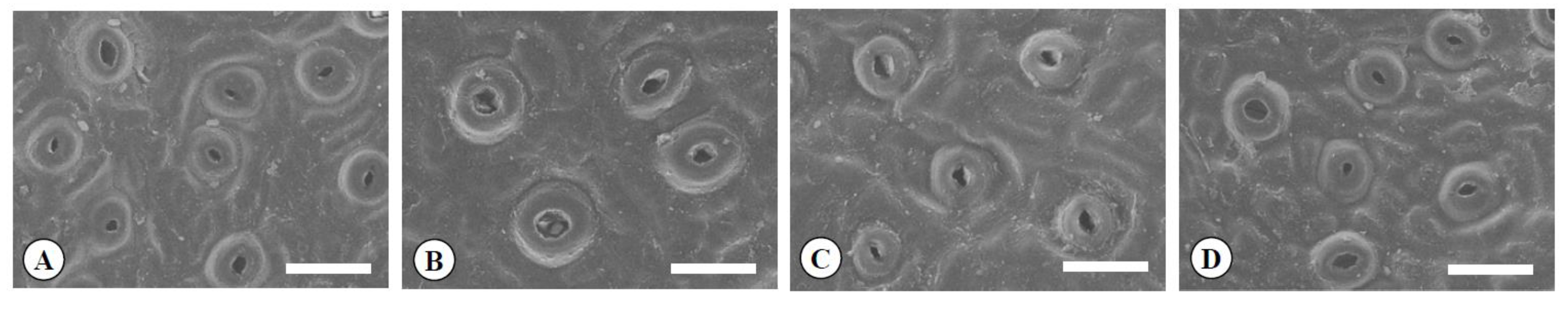
Scanning electron micrographs of guard cells in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 30 μm.
Figure 4.
Scanning electron micrographs of guard cells in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 30 μm.
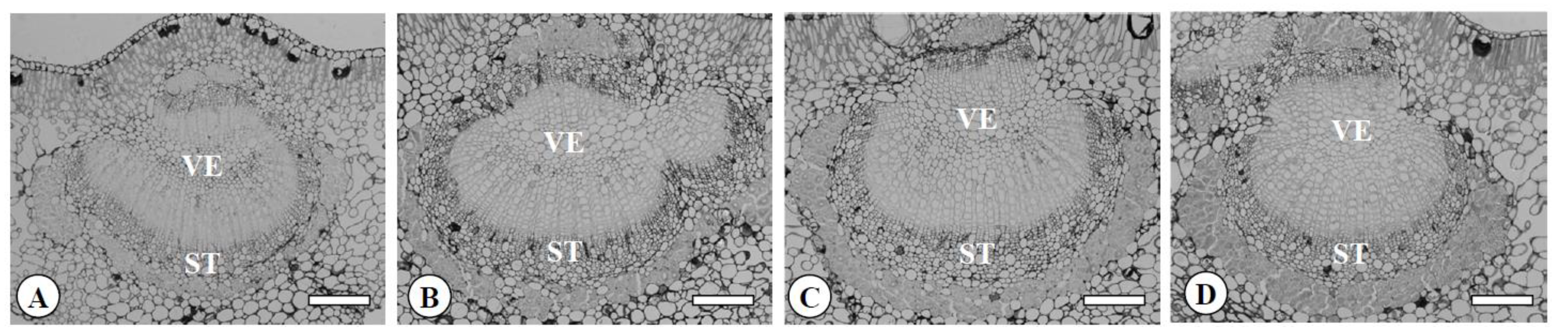
Figure 5.
Transversal sections of midribs in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 100 μm. VE: Vessel, ST: Sieve tube.
Figure 5.
Transversal sections of midribs in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 100 μm. VE: Vessel, ST: Sieve tube.
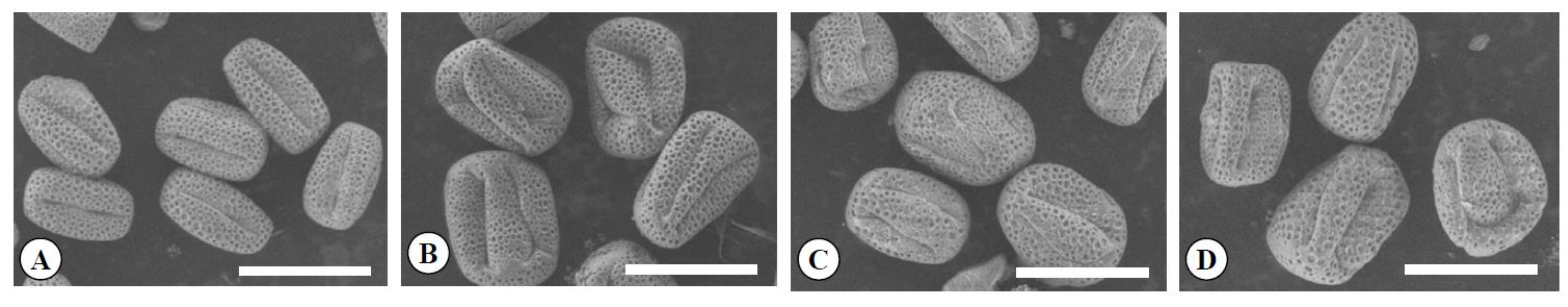
Figure 6.
Scanning electron micrographs of pollen grains in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 30 μm.
Figure 6.
Scanning electron micrographs of pollen grains in the diploid (A), the tetraploid (B), the Yubeni (C), and the chimera (D) of the Meiwa kumquat. Bars = 30 μm.
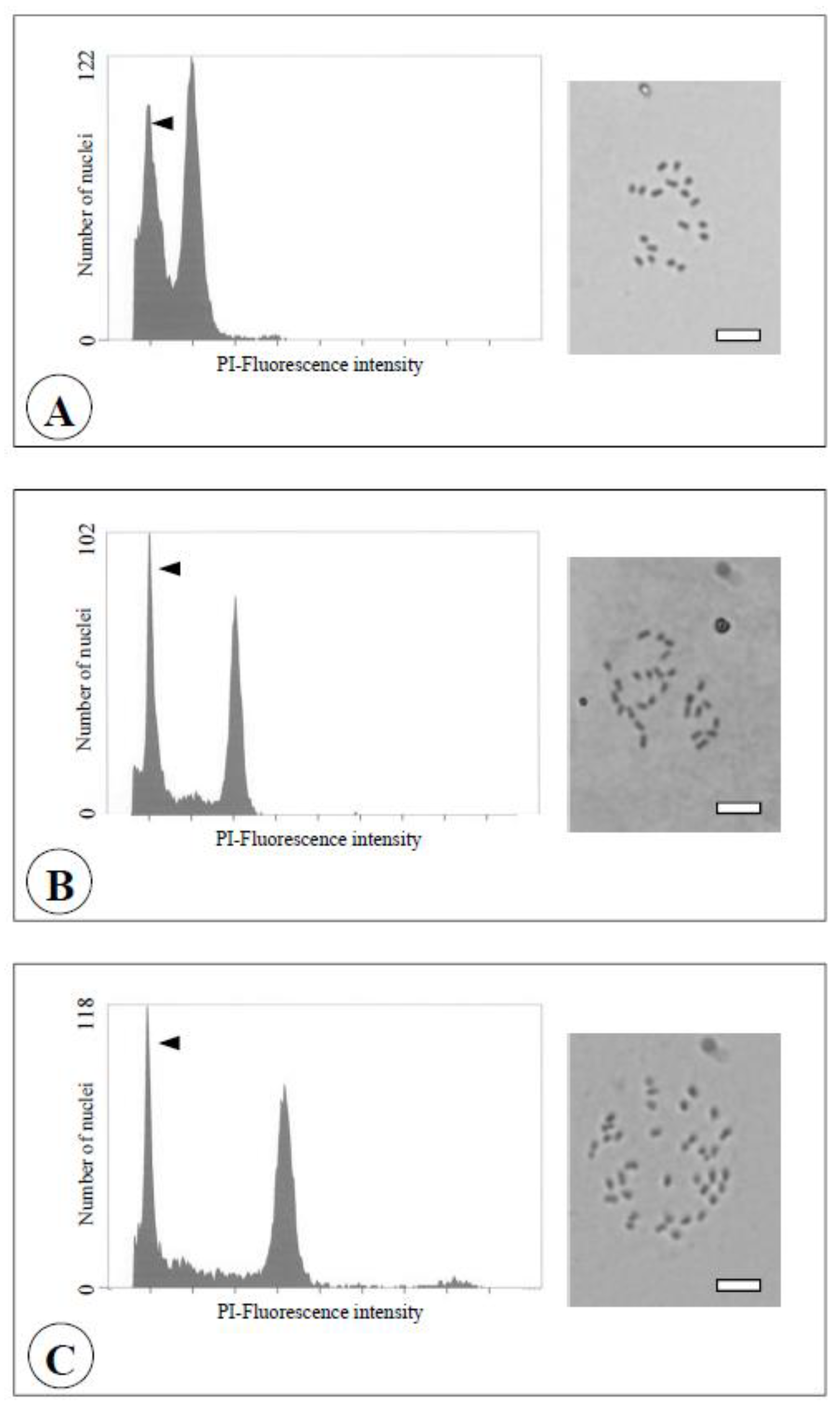
Figure 7.
Flow cytometric analysis (left) and chromosome observation (right) of the seedlings obtained from reciprocal crosses between the diploid and the chimera of the Meiwa kumquat. Bars = 10 μm.
A: Diploid (2n = 2x = 18).
B: Triploid (2n = 3x = 27).
C: Tetraploid (2n = 4x = 36). Arrows indicate the relative fluorescence of internal standard (the haploid pummelo (2n = x = 9, Yahata et al., [
19])).
Figure 7.
Flow cytometric analysis (left) and chromosome observation (right) of the seedlings obtained from reciprocal crosses between the diploid and the chimera of the Meiwa kumquat. Bars = 10 μm.
A: Diploid (2n = 2x = 18).
B: Triploid (2n = 3x = 27).
C: Tetraploid (2n = 4x = 36). Arrows indicate the relative fluorescence of internal standard (the haploid pummelo (2n = x = 9, Yahata et al., [
19])).
Figure 8.
The triploid seedling obtained from the cross between the diploid and the chimera of the Meiwa kumquat (bar = 1 cm).
Figure 8.
The triploid seedling obtained from the cross between the diploid and the chimera of the Meiwa kumquat (bar = 1 cm).
Table 1.
Flow cytometric analysis of each organ and tissue in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Table 1.
Flow cytometric analysis of each organ and tissue in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
| | Leaf | Flower | Fruit |
|---|
| Whole | Midrib | Petal | Filament | Style | Ovary | Seed | Juice Sac | Albedo | Flavedo |
|---|
| Diploid | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x |
| Tetraploid | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x |
| Yubeni | 2x+4x | 4x | 2x+4x | 2x+4x | 2x+4x | 2x+4x | 4x | 2x | 4x | 2x+4x |
| Chimera | 2x+4x | 4x | 2x+4x | 2x+4x | 2x+4x | 2x+4x | 4x | 2x | 4x | 2x+4x |
Table 2.
Comparison of morphological characteristics of leaves in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Table 2.
Comparison of morphological characteristics of leaves in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
| | Ploidy Level | Leaf Blade (mm) | Shape Index of Leaf y | Leaf Thickness (μm) | Guard Cell (μm) | Guard Cell Density (No./mm2) |
|---|
| Length | Width | Length | Width |
|---|
| Diploid | 2x | 75.2 a z | 27.7 c | 2.7 a | 437.0 b | 22.0 c | 19.6 b | 397.4 a |
| Tetraploid | 4x | 70.0 b | 32.3 b | 2.2 b | 519.6 a | 27.6 a | 23.9 a | 289.5 b |
| Yubeni | 2x+4x | 72 a b | 32.9 b | 2.2 b | 532.3 a | 24.2 b | 19.3 b | 419.3 a |
| Chimera | 2x+4x | 75.8 a | 39.6 a | 1.9 c | 523.7 a | 23.2 c | 19.8 b | 375.8 a |
Table 3.
Comparison of the cell sizes of leaves in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Table 3.
Comparison of the cell sizes of leaves in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
| | Ploidy Level | Epidermis (μm) | Palisade Parenchyma (μm) | Spongy Parenchyma (μm) | Vessel (μm) | Sieve Tube (μm) |
|---|
| Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis |
|---|
| Diploid | 2x | 19.6 b z | 13.5 b | 27.5 b | 8.9 b | 35.5 b | 25.5 b | 18.6 b | 16.9 b | 19.0 b | 15.1 b |
| Tetraploid | 4x | 27.4 a | 15.9 a | 41.7 a | 13.0 a | 60.4 a | 40.2 a | 26.2 a | 22.6 a | 22.0 a | 18.0 a |
| Yubeni | 2x+4x | 16.0 c | 11.9 c | 42.6 a | 11.5 a | 54.2 a | 34.9 a | 26.3 a | 22.0 a | 24.4 a | 18.8 a |
| Chimera | 2x+4x | 16.8 c | 11.4 c | 46.0 a | 11.6 a | 54.2 a | 35.0 a | 25.8 a | 22.5 a | 22.2 a | 18.3 a |
Table 4.
Comparison of morphological characteristics of flowers and pollen grains in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Table 4.
Comparison of morphological characteristics of flowers and pollen grains in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
| | Ploidy Level | Flower Bud (mm) | No. of Petal | Petal (mm) | Length of Pistil (mm) | Ovary (mm) | No. of Stamens | Pollen Grain (μm) | Shape Index of Pollen Grain y | Pollen Stability Rate (%) | Pollen Germination Rate (%) |
|---|
| Length | Width | Length | Width | Diameter | Height | Length | Width |
|---|
| Diploid | 2x | 8.8 cz | 5.9 b | 5.2 | 8.9 b | 4.1 b | 5.3 b | 2.0 b | 2.1 c | 16.3 | 29.5 c | 17.4 c | 1.7 a | 97.5 a | 37.2 a |
| Tetraploid | 4x | 10.1 b | 7.5 a | 5.1 | 9.7 b | 4.8 a | 5.5 b | 2.4 a | 2.5 b | 17.9 | 38.1 a | 29.1 a | 1.3 b | 85.8 b | 18.0 b |
| Yubeni | 2x+4x | 11.7 a | 7.5 a | 5.1 | 11.0 a | 5.3 a | 6.1 a | 2.5 a | 3.0 a | 18.5 | 36.6 ab | 25.2 b | 1.4 b | 72.4 c | 12.5 b |
| Chimera | 2x+4x | 10.9 ab | 7.1 a | 4.9 | 10.8 a | 5.1 a | 5.6 b | 2.4 a | 2.5 b | 16.9 | 35.2 b | 25.4 b | 1.4 b | 80.2 bc | 14.2 b |
Table 5.
Comparison of morphological characteristics of fruits in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
Table 5.
Comparison of morphological characteristics of fruits in the diploid, the tetraploid, the Yubeni, and the chimera of the Meiwa kumquat.
| | Ploidy Level | Fruit Wt. (g) | Fruit (mm) | Shape Index of Fruit z | Pericarp Wt. (g) | Pericarp Wt./Fruit Wt. (%) | No. of Locules | No. of Developed Seeds/Fruit | No. of Undeveloped Seeds/Fruit | Developed Seeds/Fruit (%) | SSC (°Brix) | TA of Pericarp (%) |
|---|
| Diameter | Height | Pericarp | Juice Sac |
|---|
| Diploid | 2x | 22.2 | 33.6 | 35.9 | 93.7 | 12.6 b y | 62.3 c | 5.7 | 5.5 a | 0.9 | 86.8 ab | 16.3 c | 14.0 b | 0.31 |
| Tetraploid | 4x | 22.7 | 33.7 | 35.1 | 96.1 | 15.9 a | 74.5 a | 6.4 | 3.8 b | 1.5 | 74.2 b | 19.8 ab | 17.8 a | 0.26 |
| Yubeni | 2x+4x | 24.8 | 35.0 | 36.2 | 96.3 | 18.3 a | 68.5 b | 6.1 | 2.1 c | 0 | 100 a | 19.5 b | 16.0 ab | 0.30 |
| Chimera | 2x+4x | 23.4 | 33.8 | 35.6 | 94.8 | 16.3 a | 70.4 b | 6.1 | 2.6 c | 0.3 | 98.3 a | 21.3 a | 17.8 a | 0.27 |
Table 6.
Seed content and ploidy level of the seedlings obtained from the reciprocal crosses between the diploid and the chimera of the Meiwa kumquat.
Table 6.
Seed content and ploidy level of the seedlings obtained from the reciprocal crosses between the diploid and the chimera of the Meiwa kumquat.
| Cross Combination | No. of Fruits | No. of Seeds | No. of Developed Seeds/Fruit | Developed Seeds (%) y | Av. Developed Seed Weight | No. of Seedlings Examined | Ploidy Level |
|---|
| Seed Parent | Pollen Parent | Developed | Undeveloped | 2x | 3x | 4x |
|---|
| Diploid | Self-pollination | 10 | 55 | 9 | 5.5 | 86.8 | 122.0 | 50 | 50 | 0 | 0 |
| Chimera | 20 | 27 | 90 | 1.4 | 23.1 | 97.5 | 77 | 68 | 8 | 1 |
| | | | | * | * | * | | | | |
| Chimera | Self-pollination | 10 | 33 | 4 | 3.3 | 89.2 | 148.9 | 50 | 50 | 0 | 0 |
| Diploid | 10 | 26 | 4 | 2.6 | 86.7 | 64.9 | 72 | 0 | 12 | 60 |
| | | | | * | NS z | * | | | | |