The Confirmation of a Ploidy Periclinal Chimera of the Meiwa Kumquat (Fortunella crassifolia Swingle) Induced by Colchicine Treatment to Nucellar Embryos and Its Morphological Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
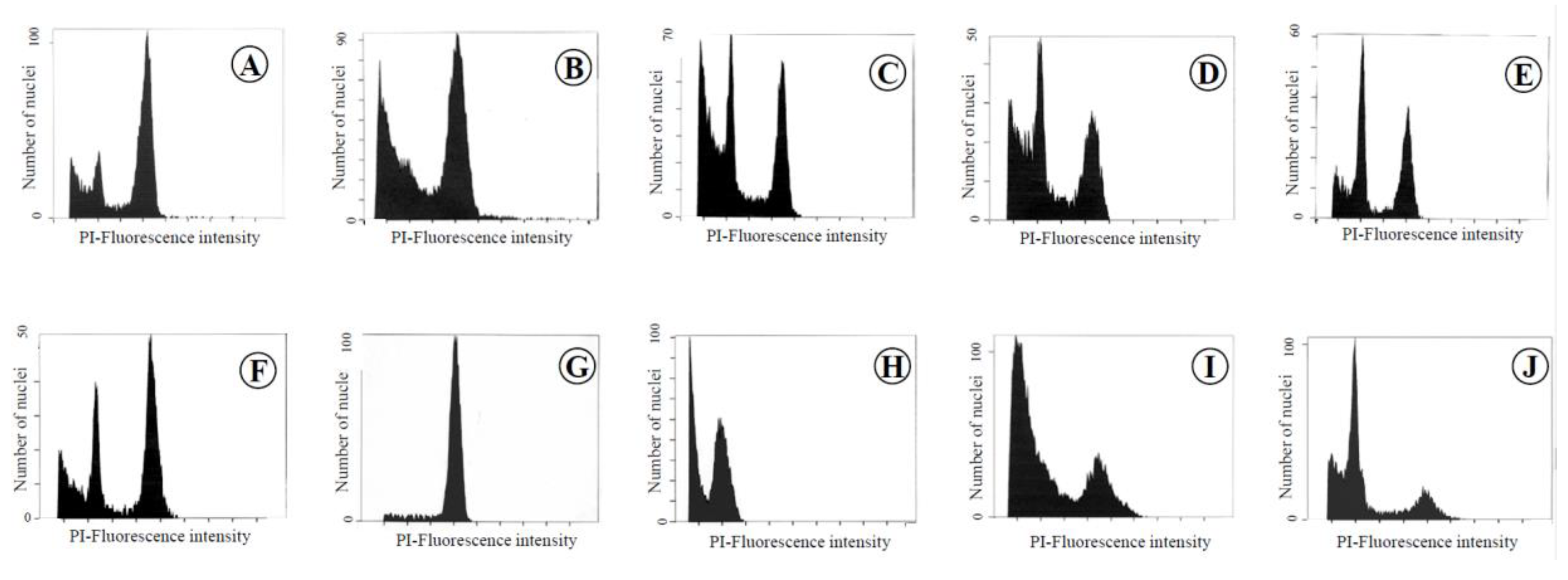
2.2. Confirmation of Ploidy Level by Flow Cyotometry
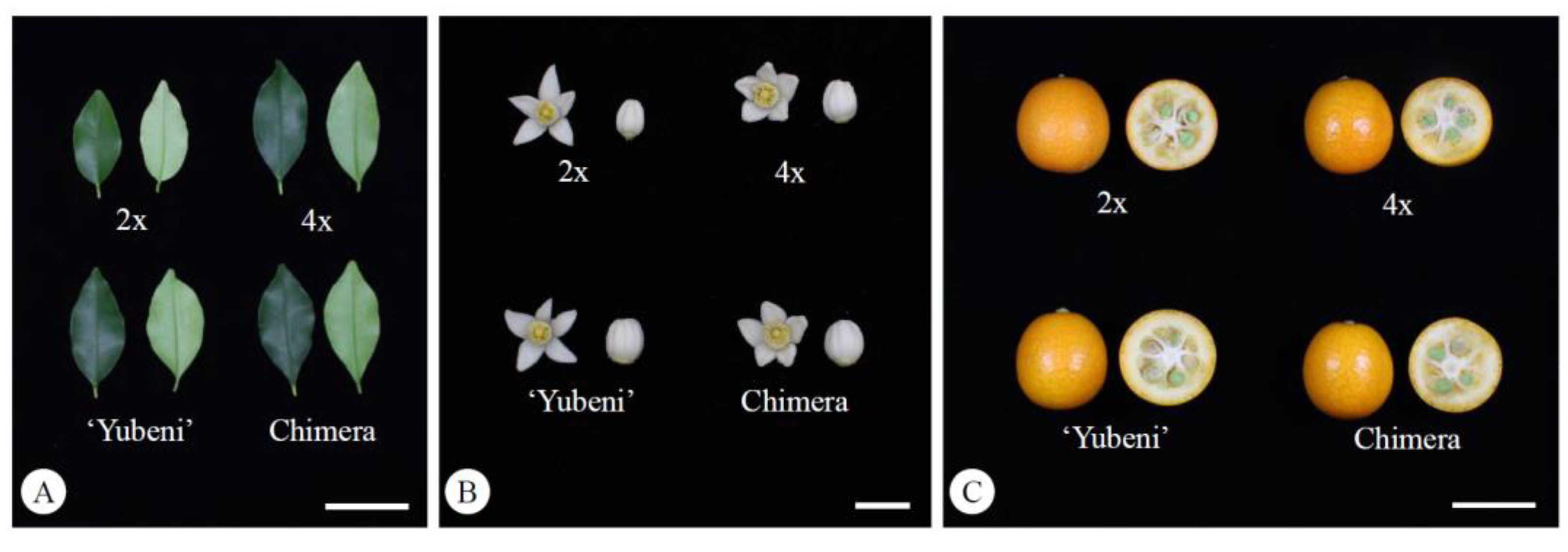
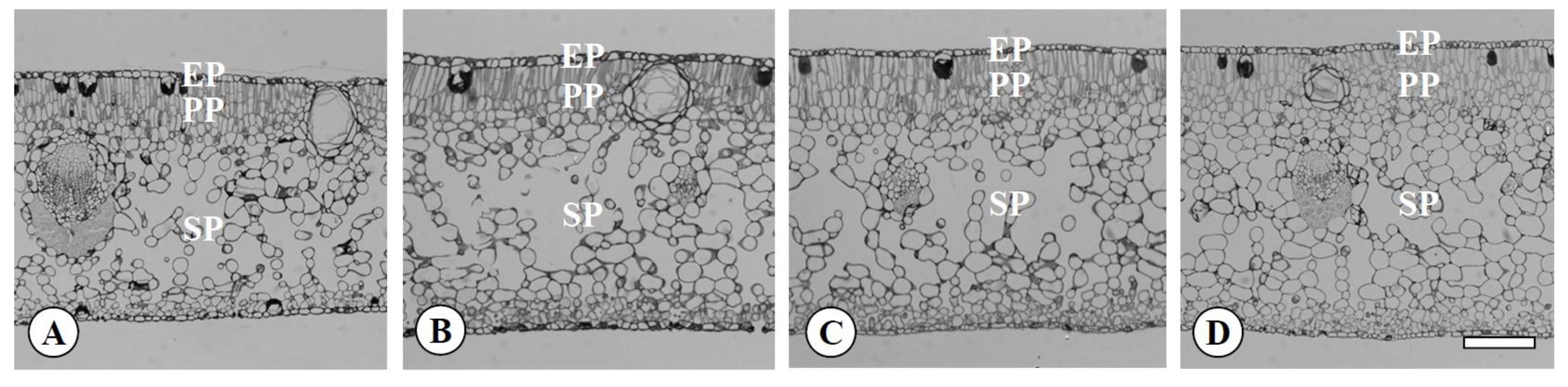
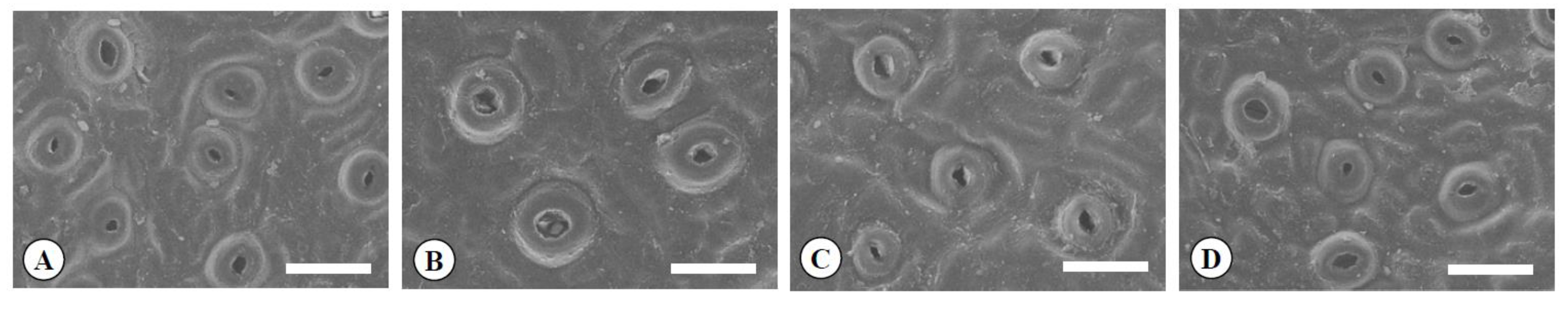
2.3. The Characteristics of Leaves, Flowers, Pollen, and Fruits in the Chimera
2.4. Crossing for the Evaluation of the Reproductive Organs of the Chimera
3. Results
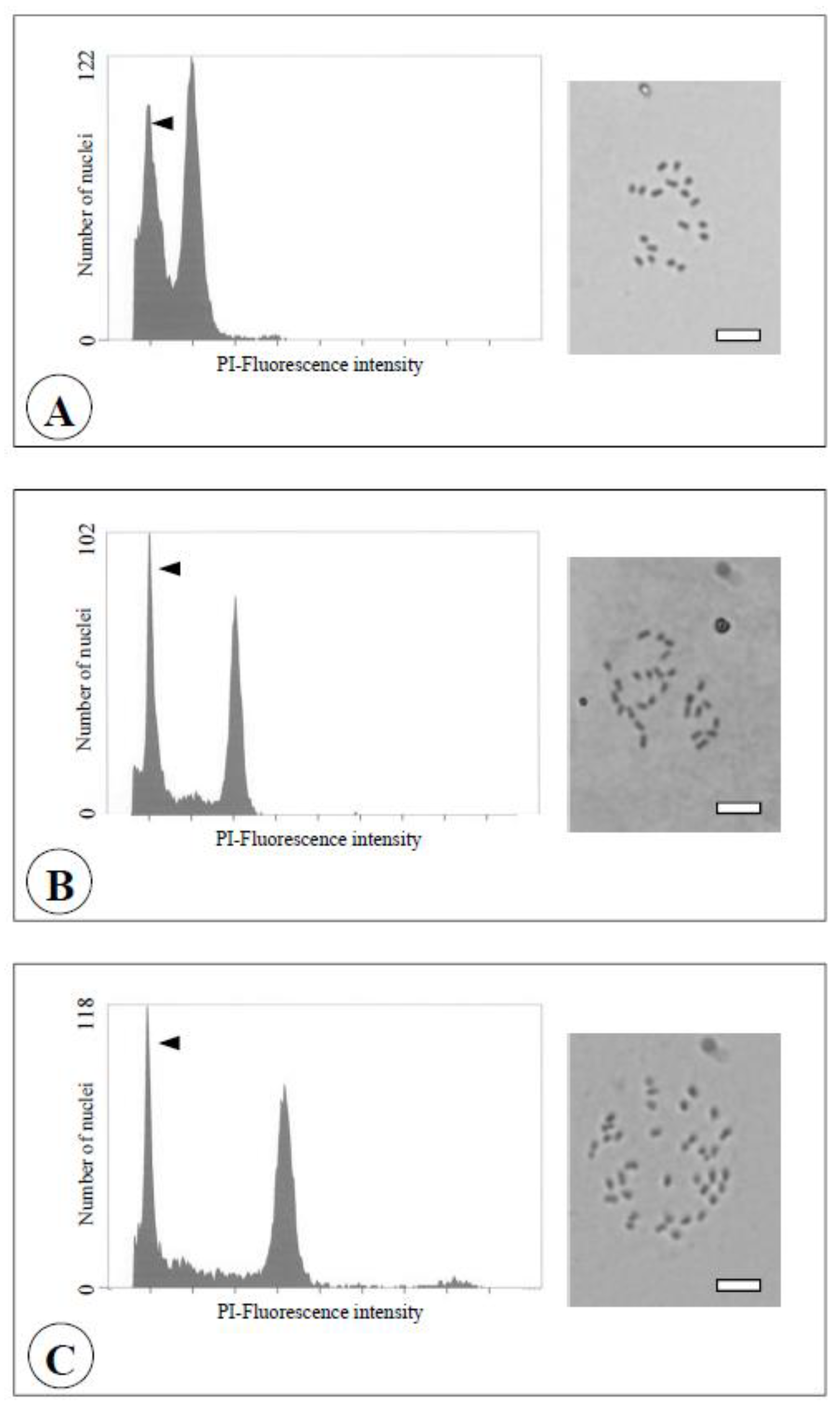
3.1. Confirmation of the Ploidy Level by FCM
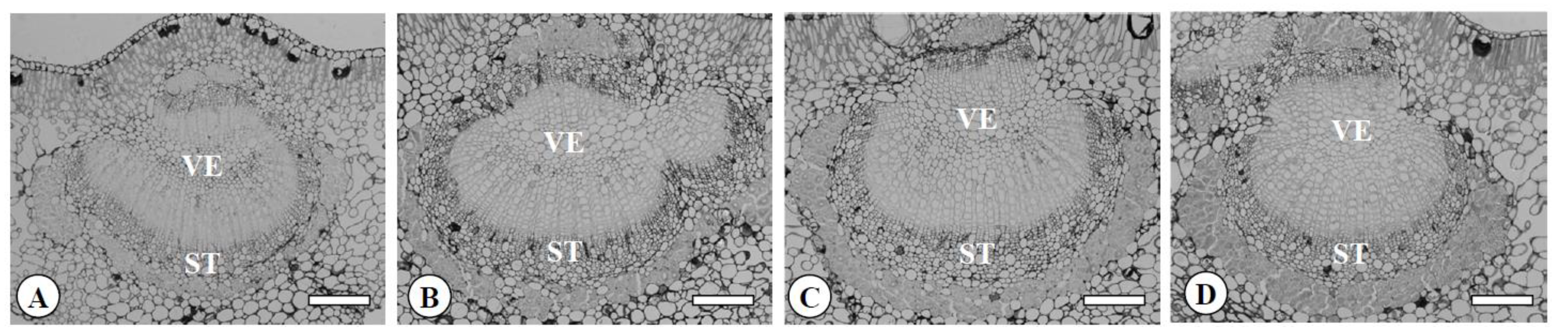
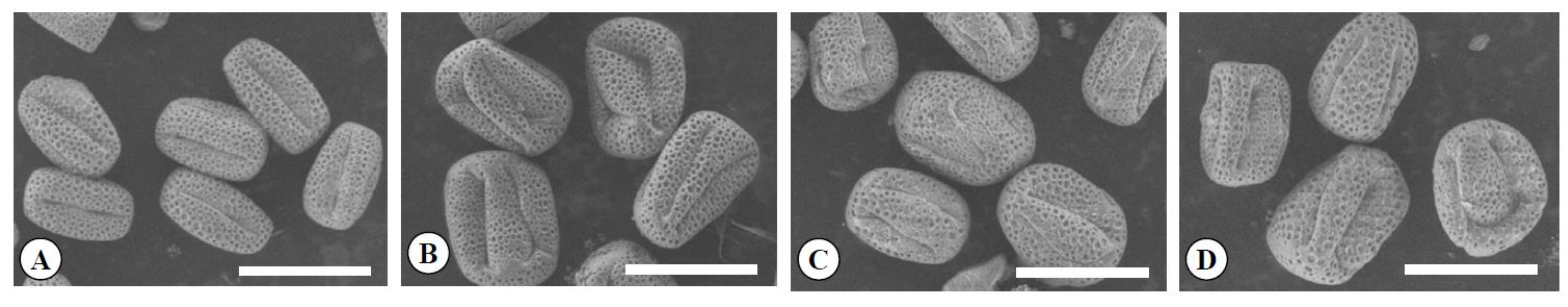
3.2. The Characteristics of Leaves, Flowers, Pollen, and Fruits in the Chimera
3.3. Crossing for the Evaluation of the Reproductive Organs of the Chimera
4. Discussion
Author Contributions
Conflicts of Interest
References
- Schmidts, A. Histologische studien an phanerogamen vegetationspunkten. Bot. Arch. 1924, 8, 345–404. [Google Scholar]
- Frost, H.B.; Krug, C.A. Diploid-tetraploid periclinal chimeras as bud variants in citrus. Genetics 1942, 27, 619–634. [Google Scholar] [PubMed]
- Cameron, J.W.; Frost, H.B. Genetics, breeding, and nucellar embryony. In The Citrus Industry vol. II; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California Press: Berkeley, CA, USA, 1968; pp. 325–370. [Google Scholar]
- Burge, G.K.; Morgan, E.R.; Seelye, J.F. Opportunities for synthetic plant chimeral breeding: Past and future. Plant Cell Tiss. Organ Cult. 2002, 70, 13–21. [Google Scholar] [CrossRef]
- Cameron, J.W.; Soost, R.K.; Olson, F.O. Chimeral basis for color in pink and red grapefruit. J. Her. 1964, 55, 23–28. [Google Scholar] [CrossRef]
- Nishiura, M.; Iwamasa, M. Chimeral basis for vegetative reversion in the Suzuki wase, an early maturing mutant of Satsuma mandarin. Bull. Hortic. Res. Stn. 1969, 9, 1–9. [Google Scholar]
- Yamashita, K. Chimerism of Kobayashi-mikan (Citrus natsudaidai×unshiu) judged from isozyme patterns in organs and tissues. J. Jpn. Soc. Hortic. Sci. 1983, 52, 223–230. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Deng, X.X.; Qin, C.; Chen, C.; Zhang, H.; Liu, Q.; Hu, Z.; Guo, L. Characterization of a new natural periclinal navel-satsuma chimera of Citrus: ‘Zaohong‘ navel orange. J. Am. Soc. Hortic. Sci. 2007, 132, 374–380. [Google Scholar] [CrossRef]
- Sugawara, K.; Wakizuka, T.; Oowada, A.; Moriguchi, T.; Omura, M. Histogenic identification by RAPD analysis of leaves and fruit of newly synthesized chimeric citrus. J. Am. Soc. Hortic. Sci. 2002, 127, 104–107. [Google Scholar] [CrossRef]
- Zhou, J.; Hirata, Y.; Nou, I.S.; Shiotani, H.; Ito, T. Interactions between different genotypic tissues in citrus graft chimeras. Euphytica 2002, 126, 355–364. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Kaneyoshi, J.; Furuta, T. Polyploid of pollen and fruit tissue, and utilization for triploid breeding in 2x−4x−4x ploidy chimera obtained by colchicine treatment of Citrus. Hortic. Res. 2017, 16, 249–257. [Google Scholar] [CrossRef]
- Yasuda, K.; Kunitake, H.; Nakagawa, S.; Kurogi, H.; Yahata, M.; Hirata, R.; Yoshikura, Y.; Kawakami, I.; Sugimoto, Y. The confirmation of ploidy periclinal chimera and its morphological characteristics in Meiwa kumquat ‘Yubeni’. Hortic. Res. 2008, 7, 165–171. [Google Scholar] [CrossRef][Green Version]
- Yahata, M.; Kashihara, Y.; Kurogi, H.; Kunitake, H.; Komatsu, H. Effect of colchicine and oryzalin treatment on tetraploid production of nucellar embryos in Meiwa kumquat (Fortunella crassifolia Swingle). Hortic. Res. 2004, 3, 11–16. [Google Scholar] [CrossRef]
- Nukaya, T.; Ohta, T.; Yasuda, K.; Yahata, M.; Kunitake, H.; Komatsu, H.; Nii, N.; Mukai, H.; Harada, H.; Takagi, T. Characteristics in polyploid Meiwa kumquat (Fortunella crassifolia Swingle) induced by colchicine treatment to nucellar embryos and their utilization for triploid breeding. Hortic. Res. 2011, 10, 1–8. [Google Scholar] [CrossRef][Green Version]
- Yahata, M.; Nukaya, T.; Sudo, M.; Ohta, T.; Yasuda, K.; Inagaki, H.; Mukai, H.; Harada, H.; Takagi, T.; Komatsu, H.; et al. Morphological characteristics of a doubled haploid line from ‘Banpeiyu’ pummelo [Citrus maxima (Burm.) Merr.] and its reproductive function. Hortic. J. 2015, 84, 30–36. [Google Scholar] [CrossRef]
- Nii, N.; Pang, C.X.; Ogawa, Y.; Cui, S.M. Anatomical features of the cell wall ingrowth in the cortical cells outside the endodermis and the development of the casparian strip in loquat trees. J. Jpn. Soc. Hortic. Sci. 2004, 73, 411–414. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Fukui, K. Plant chromosome at mitosis. In Plant Chromosome. Laboratory Methods; Fukui, K., Nakayama, S., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 1–17. [Google Scholar]
- Yahata, M.; Kunitake, H.; Yabuya, T.; Yamashita, K.; Kashihara, Y.; Komatsu, H. Production of a doubled haploid from a haploid pummelo using colchicine treatment of axillary shoot buds. J. Am. Soc. Hortic. Sci. 2005, 130, 899–903. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Kaneyoshi, J.; Furuta, T.; Kitano, T. Characteristics of tetraploid and ploidy chimera induced by colchicine treatment in Citrus. Hortic. Res. 2012, 11, 449–457. [Google Scholar] [CrossRef][Green Version]
- Nii, N.; Coombe, B.G. Anatomical aspects of juice sacs of Satsuma mandarin in relation to translocation. J. Jpn. Soc. Hortic. Sci. 1998, 56, 375–381. [Google Scholar] [CrossRef][Green Version]
- Nukaya, T.; Sudo, M.; Yahata, M.; Nakajo, Y.; Ohta, T.; Yasuda, K.; Tominaga, A.; Mukai, H.; Kunitake, H. Characteristics in autotetraploid kumquats (Fortunella spp.) induced by colchicine treatment to nucellar embryos and their utilization for triploid breeding. Sci. Hortic 2019, 245, 210–217. [Google Scholar] [CrossRef]
- Nukaya, T.; Yahata, M.; Suzuki, K.; Yasuda, K.; Kunitake, H.; Komatsu, H.; Mukai, H.; Harada, H.; Takagi, T. Fruit characteristics in autotetraploid Meiwa kumquat induced by colchicine treatment to nucellar embryos. Hortic. Environ. Biotechnol. 2009, 50, 188–190. [Google Scholar]

| Leaf | Flower | Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole | Midrib | Petal | Filament | Style | Ovary | Seed | Juice Sac | Albedo | Flavedo | |
| Diploid | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x |
| Tetraploid | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x | 4x |
| Yubeni | 2x+4x | 4x | 2x+4x | 2x+4x | 2x+4x | 2x+4x | 4x | 2x | 4x | 2x+4x |
| Chimera | 2x+4x | 4x | 2x+4x | 2x+4x | 2x+4x | 2x+4x | 4x | 2x | 4x | 2x+4x |
| Ploidy Level | Leaf Blade (mm) | Shape Index of Leaf y | Leaf Thickness (μm) | Guard Cell (μm) | Guard Cell Density (No./mm2) | |||
|---|---|---|---|---|---|---|---|---|
| Length | Width | Length | Width | |||||
| Diploid | 2x | 75.2 a z | 27.7 c | 2.7 a | 437.0 b | 22.0 c | 19.6 b | 397.4 a |
| Tetraploid | 4x | 70.0 b | 32.3 b | 2.2 b | 519.6 a | 27.6 a | 23.9 a | 289.5 b |
| Yubeni | 2x+4x | 72 a b | 32.9 b | 2.2 b | 532.3 a | 24.2 b | 19.3 b | 419.3 a |
| Chimera | 2x+4x | 75.8 a | 39.6 a | 1.9 c | 523.7 a | 23.2 c | 19.8 b | 375.8 a |
| Ploidy Level | Epidermis (μm) | Palisade Parenchyma (μm) | Spongy Parenchyma (μm) | Vessel (μm) | Sieve Tube (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis | Major Axis | Minor Axis | ||
| Diploid | 2x | 19.6 b z | 13.5 b | 27.5 b | 8.9 b | 35.5 b | 25.5 b | 18.6 b | 16.9 b | 19.0 b | 15.1 b |
| Tetraploid | 4x | 27.4 a | 15.9 a | 41.7 a | 13.0 a | 60.4 a | 40.2 a | 26.2 a | 22.6 a | 22.0 a | 18.0 a |
| Yubeni | 2x+4x | 16.0 c | 11.9 c | 42.6 a | 11.5 a | 54.2 a | 34.9 a | 26.3 a | 22.0 a | 24.4 a | 18.8 a |
| Chimera | 2x+4x | 16.8 c | 11.4 c | 46.0 a | 11.6 a | 54.2 a | 35.0 a | 25.8 a | 22.5 a | 22.2 a | 18.3 a |
| Ploidy Level | Flower Bud (mm) | No. of Petal | Petal (mm) | Length of Pistil (mm) | Ovary (mm) | No. of Stamens | Pollen Grain (μm) | Shape Index of Pollen Grain y | Pollen Stability Rate (%) | Pollen Germination Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Length | Width | Diameter | Height | Length | Width | ||||||||
| Diploid | 2x | 8.8 cz | 5.9 b | 5.2 | 8.9 b | 4.1 b | 5.3 b | 2.0 b | 2.1 c | 16.3 | 29.5 c | 17.4 c | 1.7 a | 97.5 a | 37.2 a |
| Tetraploid | 4x | 10.1 b | 7.5 a | 5.1 | 9.7 b | 4.8 a | 5.5 b | 2.4 a | 2.5 b | 17.9 | 38.1 a | 29.1 a | 1.3 b | 85.8 b | 18.0 b |
| Yubeni | 2x+4x | 11.7 a | 7.5 a | 5.1 | 11.0 a | 5.3 a | 6.1 a | 2.5 a | 3.0 a | 18.5 | 36.6 ab | 25.2 b | 1.4 b | 72.4 c | 12.5 b |
| Chimera | 2x+4x | 10.9 ab | 7.1 a | 4.9 | 10.8 a | 5.1 a | 5.6 b | 2.4 a | 2.5 b | 16.9 | 35.2 b | 25.4 b | 1.4 b | 80.2 bc | 14.2 b |
| Ploidy Level | Fruit Wt. (g) | Fruit (mm) | Shape Index of Fruit z | Pericarp Wt. (g) | Pericarp Wt./Fruit Wt. (%) | No. of Locules | No. of Developed Seeds/Fruit | No. of Undeveloped Seeds/Fruit | Developed Seeds/Fruit (%) | SSC (°Brix) | TA of Pericarp (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter | Height | Pericarp | Juice Sac | |||||||||||
| Diploid | 2x | 22.2 | 33.6 | 35.9 | 93.7 | 12.6 b y | 62.3 c | 5.7 | 5.5 a | 0.9 | 86.8 ab | 16.3 c | 14.0 b | 0.31 |
| Tetraploid | 4x | 22.7 | 33.7 | 35.1 | 96.1 | 15.9 a | 74.5 a | 6.4 | 3.8 b | 1.5 | 74.2 b | 19.8 ab | 17.8 a | 0.26 |
| Yubeni | 2x+4x | 24.8 | 35.0 | 36.2 | 96.3 | 18.3 a | 68.5 b | 6.1 | 2.1 c | 0 | 100 a | 19.5 b | 16.0 ab | 0.30 |
| Chimera | 2x+4x | 23.4 | 33.8 | 35.6 | 94.8 | 16.3 a | 70.4 b | 6.1 | 2.6 c | 0.3 | 98.3 a | 21.3 a | 17.8 a | 0.27 |
| Cross Combination | No. of Fruits | No. of Seeds | No. of Developed Seeds/Fruit | Developed Seeds (%) y | Av. Developed Seed Weight | No. of Seedlings Examined | Ploidy Level | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed Parent | Pollen Parent | Developed | Undeveloped | 2x | 3x | 4x | |||||
| Diploid | Self-pollination | 10 | 55 | 9 | 5.5 | 86.8 | 122.0 | 50 | 50 | 0 | 0 |
| Chimera | 20 | 27 | 90 | 1.4 | 23.1 | 97.5 | 77 | 68 | 8 | 1 | |
| * | * | * | |||||||||
| Chimera | Self-pollination | 10 | 33 | 4 | 3.3 | 89.2 | 148.9 | 50 | 50 | 0 | 0 |
| Diploid | 10 | 26 | 4 | 2.6 | 86.7 | 64.9 | 72 | 0 | 12 | 60 | |
| * | NS z | * | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nukaya, T.; Sudo, M.; Yahata, M.; Ohta, T.; Tominaga, A.; Mukai, H.; Yasuda, K.; Kunitake, H. The Confirmation of a Ploidy Periclinal Chimera of the Meiwa Kumquat (Fortunella crassifolia Swingle) Induced by Colchicine Treatment to Nucellar Embryos and Its Morphological Characteristics. Agronomy 2019, 9, 562. https://doi.org/10.3390/agronomy9090562
Nukaya T, Sudo M, Yahata M, Ohta T, Tominaga A, Mukai H, Yasuda K, Kunitake H. The Confirmation of a Ploidy Periclinal Chimera of the Meiwa Kumquat (Fortunella crassifolia Swingle) Induced by Colchicine Treatment to Nucellar Embryos and Its Morphological Characteristics. Agronomy. 2019; 9(9):562. https://doi.org/10.3390/agronomy9090562
Chicago/Turabian StyleNukaya, Tsunaki, Miki Sudo, Masaki Yahata, Tomohiro Ohta, Akiyoshi Tominaga, Hiroo Mukai, Kiichi Yasuda, and Hisato Kunitake. 2019. "The Confirmation of a Ploidy Periclinal Chimera of the Meiwa Kumquat (Fortunella crassifolia Swingle) Induced by Colchicine Treatment to Nucellar Embryos and Its Morphological Characteristics" Agronomy 9, no. 9: 562. https://doi.org/10.3390/agronomy9090562
APA StyleNukaya, T., Sudo, M., Yahata, M., Ohta, T., Tominaga, A., Mukai, H., Yasuda, K., & Kunitake, H. (2019). The Confirmation of a Ploidy Periclinal Chimera of the Meiwa Kumquat (Fortunella crassifolia Swingle) Induced by Colchicine Treatment to Nucellar Embryos and Its Morphological Characteristics. Agronomy, 9(9), 562. https://doi.org/10.3390/agronomy9090562





