1. Introduction
Oxadiazon (5-terbuthyl-3-(2,4-dichloro-5-isopropoxyphenyl)-1,3,4-oxadiazol-2-(3H)-one, is a herbicide widely used to control annual broad leaved weeds and grasses in fruit trees, turf, rice, soybean, sunflower, cotton, some flower species and ornamental plants. Oxadiazon is characterized by a high persistence in the soil with a DT
50 from three to six months [
1,
2], a low water solubility (0.57 mg/L) [
2,
3] and a strong adsorption on soil colloids and humus that confers a Koc in the range 1400-3200 mL/g [
3]. The degradation of the herbicide in different soils is low [
4,
5] and losses of oxadiazon by volatilization and leaching are considered negligible [
2,
4,
6].
Rice yield strictly depends on available water, which is delivered to fields by a vast network of rivers, channels and ditches. The risk of water contamination by Plant Protection Products (PPPs) is generally higher for surface water than for groundwater due to the interconnections between flooding waters and the network of ditches, channels and rivers that permeate rice areas [
7,
8]. This peculiarity makes water courses more vulnerable to contamination by PPPs applied during crop cultivation to protect crops. The presence of pesticides in surface and ground waters of rice-paddy regions is reported worldwide. In rice cultivation, crop protection is mostly represented by weed control. Hence, it is not unexpected that herbicides are the most frequently found contaminants in water. Despite its old introduction into the market, oxadiazon is still an herbicide largely applied in direct-seeded rice, mainly to control
Heteranthera spp. The presence of this herbicide in surface and ground waters is mentioned in different reports made by environmental authorities, as well as by several monitoring studies conducted in many countries of the world [
9,
10,
11,
12,
13,
14,
15,
16]. The monitoring campaigns conducted in 2016 by the Italian Institute for Environmental Protection and Research (ISPRA), found oxadiazon residues in 23.6% and 3.9% of the monitored surface waters and ground water sites, respectively. Out of the total samples monitored, the threshold limit established by the European legislation was exceeded in 1.5% and 0.6% of the cases for surface and ground waters, respectively [
16]. In Europe the current legislation on protection of water resources has set the maximum allowable concentrations in ground water and surface drinking water to 0.1 µg/L for any individual substance, and to 0.5 µg/L for total pesticide load, while more restrictive concentration limits have been set for the priority substances listed in the Directives 2008/105/CE and 2000/60/CE. At present, the frequency of detection of oxadiazon residues both in ground and surface waters has led to the adoption of restrictions of use in certain areas in order to prevent further contamination. For instance, the Lombardy region, in Northern Italy, has already adopted specific limitations of use of oxadiazon. By 2018, in dry-seeded rice fields, oxadiazon has to be applied only on 50% of the total farm area cultivated with rice. Furthermore, the herbicide application must be done with sprayers able to ensure a 30% drift reduction.
Rice is cultivated under a wide range of agronomic practices, and only few data are at present available on the effect of management practices on the environmental behavior of oxadiazon. Up to now no data explored the effect of the cultivation system on oxadiazon behavior in the paddy environment. The aim of this study was to assess the potential differences in oxadiazon dissipation in topsoil and its behavior in the water of paddy fields as affected by the type of rice cultivation system (conventional and dry systems). The hypothesis is that dissipation is faster in a conventional flooded system, as the herbicide has more chances to move from soil to water compartments. The continuous water flow in conventional systems should theoretically enhance dissipation via runoff transfer. Field experiments may usually provide most reliable data compared to laboratory studies because they are closest to field-like conditions than the latter. The result of this study will help to better understand the oxadiazon behavior in field conditions, thus allowing to reduce as much as possible product residues in surface waters.
2. Materials and Methods
2.1. Experimental Design
The study was performed in 2012 and 2013 at the experimental farm “Cascina Boschine” in the municipality of Vercelli (45°18′ N, 8°26′ E; WGS84), 130 m a.s.l., in the Piemonte region, Northern Italy (
Figure 1).
The experimental fields consisted of two hydraulically separated paddy fields of 1850 m
2 each, managed since 2002 under the two cultivation systems most commonly adopted in Italy. Each field was divided into three sections (a split plot with three blocks per field), namely top, middle and bottom, starting from the upper part of the field (paddy water entrance) to the lower part (paddy water exit). The assessments were carried out on the same fields during the 2012 and 2013 growing seasons, which were regarded as temporal replications. Water and soil monitoring were conducted by taking samples separately in each section of the field. The first system (CON) is the conventional system, in which soil is plowed in autumn to incorporate rice residues and rice is broadcast seeded during spring on flooded fields. The second system (DRY), which is adopted on about 33% of the total Italian rice area, is characterized by spring plowing for straw incorporation and drill-seeding of rice on a dry field followed by conventional flooding at the 2–3 leaf stage of the rice (12–13 on the BBCH scale) [
17].
The experimental fields were characterized by short evolved, hydromorphic and poorly drained soils. Flooding water was delivered to each field by means of a common ditch running along the upper part of the fields. Each field has its own inlet and outlet prefabricated concrete housing floodgates, to allow submersion water entering and leaving the paddies.
In both years, rice (variety Loto from the SA.PI.SE Seed Company, Vercelli, Italy) was seeded at the same rate (180 kg ha−1) in each field, but at different times in the various fields: during the first ten days of May in system DRY, and in the last ten days of May in system CON. Fields were fertilized in three different periods: pre-seeding, tillering and panicle differentiation. In both systems the same amounts of fertilizers were distributed, without any difference among the two years.
2.2. Weed Control
The weed flora generally detected in the area was mainly represented by Heteranthera reniformis Ruiz and Pavon, Echinochloa crus-galli Beauv., Rotala ramosior L., Lindernia dubia L., Cyperus diffomis L. and Ammania coccinea Rottb. The strategy adopted to control weeds consisted of two different applications carried out in pre-emergence and in post-emergence. Pre-emergence control of weeds was carried out by applying oxadiazon (Ronstar ®, Bayer CropScience Italia) at the rate of 0.9 L/ha (342 g ha−1 of active ingredient), mainly to control infestations of Heteranthera spp. On system DRY, pendimethalin (Stomp ®, Basf Italia) was added to the mixture at a rate of 2.5 L/ha (1137.5 g/ha of active ingredient) to better control grass weeds during the first period when the field was not flooded. Oxadiazon and pendimethalin were applied on May 9 and May 12 in 2012 and 2013, respectively. Fields were then flooded within a few days after treatment in system CON and about 30 days after treatment in system DRY.
Post-emergence weed control was done by using a mixture of penoxsulam (ViperTM, Dow AgroScience Italia, Milano, Italy) at 2 L/ha (40.8 g/L of active ingredient) and halosulfuron-methyl (Permit®, Nissan Chemicals) at 30 g/ha (22.5 g/L of active ingredient). Herbicides were applied on June 19 in 2012 and 2013. Fields were then flooded within 2–3 days after treatment. The water level within the field was maintained at 6–8 cm until panicle initiation and then increased to 10–13 cm until the beginning of rice ripening. Water temperature varied from 13–15.5 °C in May–June to 16–21 °C in July.
2.3. Oxadiazon Dissipation in Paddy Fields
The environmental behavior of oxadiazon in paddy fields was studied by assessing its dissipation in topsoil, paddy waters and in inlet and outlet waters.
2.3.1. Soil Sampling
Soil samples were collected randomly in three different sections in each field (top, middle and bottom). Each sample (0.8 kg each) was derived from 8 sub-samples pooled together. Each subsample was taken from the top 2 cm of soil using a stainless-steel shovel. The sampling was wittingly limited to the upper few centimeters of the soil because in rice paddies there is a strong interaction between the top -soil layers and the flood water. A blank sample (of about 2 kg) was collected a day before the treatment within the field to assess residues of oxadiazon from the previous season. Sampling was done at 0, 2, 4, 10, 27, 47, 68 and 129 days after treatment (DAT) in 2012, and at 0, 2, 4, 10, 27, 47, 68 and 270 DAT in 2013. Soil samples were put into polyethylene graduated squared bottles with a wide neck (Kartell, Noviglio, Italia) and stored at −25 °C until analysis. Blotting paper was used to draw away excess water on the saturated samples during the extraction. To establish the moisture level of the soil at each sampling date, a small amount of the original sample (about 25 g) was taken, weighed and then placed into a stove at 105 °C for 24 h.
2.3.2. Water Sampling
Paddy Water
In the CON system, samples of paddy water were collected in 2012 at 4, 6, 10, 27, 47 and 68 DAT; in 2013 samples were collected at 4, 6, 10, 16, 40, and 47 DAT. In the DRY system, as the paddy field was flooded only from about 30 DAT onwards, samples were collected only at 47 and 68 DAT in 2012 and at 47 DAT in 2013. Paddy water samples (three samples of 0.5 L each) were gathered by randomly filling polyethylene graduated squared bottles with wide necks from three different sections in each field (top, middle and bottom). After the collection, the samples were immediately stored in a cold room at −25 °C until extraction.
Inlet and Outlet Water
To assess the transport of oxadiazon in the fields with the irrigation waters, samples of inlet water were collected at 4, 6, 10, 27, 47 and 68 DAT in 2012 and at 4, 6, 10, 16, 40 and 47 DAT in 2013. Outlet water was collected in 2012 at 4, 6, 10, 27, 47 and 68 DAT, and at 4, 6, 10, 16, 40 and 47 DAT in 2013. At each sampling date, a bulk of 10 L of water was collected from the ditch that fed both fields (inlet water) and from the outlet floodgates (outlet water). From each bulk, gathered in a tank during the sampling, a 1 L sample was then obtained. After the collection, the samples were immediately stored in a cold room at −25 °C until extraction.
2.3.3. GC Analysis
Analysis was performed by GC by using a Perkin Elmer 8500 GC split injector (PerkinElmer, Waltham, MA, USA), equipped with a SPB column (30 m × 0.25 µm). The temperature was set at 250 °C for the injector and at 315 °C for the ECD detector. The gas carrier was helium. Retention time was 7.3 min.
2.3.4. Recovery and Detection Limits
Oxadiazon concentrations were determined relative to the peak of the analytical standard solutions. Chlorpyrifos-methyl was used as internal standard. Both standards were purchased from Sigma Aldrich, Germany. The mean recovery of oxadiazon in water and soil was 98% (±1.8,
n = 12) and 85% (±5.2,
n = 12), respectively. The quantification limit (LOQ) achieved in water samples was 0.10 µg/L, while in soil it was 4 µg/kg. The LOQ was calculated by using the signal-to-noise method. The noise magnitude was measured manually. A signal to noise ratio (S/N) of ten was used for estimating LOQ [
18].
2.4. Data Analysis
Oxadiazon dissipation in the soil was fitted to a 2-parameter exponential decay model:
where
Ct is the concentration at time
t,
C0 is the initial concentration,
t is time, and k is the rate constant.
Model fitting was performed using the function
drm of the add-on package
drc of the R software version 3.5.2 [
19]. The soil data were subjected to analysis of co-variance (ANCOVA,
p ≤ 0.05) to assess any field or year effect, using SPSS version 25.00 (SPSS, IBM Corporation, 2008, Armonk, NY, USA). As the analysis revealed a non-significant effect of the system and of the year, data were pooled together to fit into a single model. Soil half-life (T
1/2) for oxadiazon was calculated using the function
ED in the package
drc.
Paddy water concentrations recorded in system CON in 2012 and 2013 were subjected to analysis of co-variance (ANCOVA, p ≤ 0.05) with days after treatment as the covariate using SPSS, version 25.00. The analysis revealed a non-significant effect for year. Data of 2012 and 2013 were thus pooled together to fit into a single model. As for the soil, model fitting was performed using the function drm in the add-on package drc of the R software. Oxadiazon dissipation in the water was fitted to the same 2-parameter exponential decay model used for soil.
The same model was also adopted to fit data of oxadiazon concentrations in inlet and outlet water. Outlet data from system DRY were not analyzed, due to a limited number of samplings available. As no replicates were available for each sampling date, the effect of year was tested by analyzing the data first separately for each year and then pooled to fit into a single model. The ANOVA function of R was used to compute a likelihood ratio test to verify if the pooled dataset was significantly better explained by two curves, separately fitting 2012 and 2013 data, than by a single model fitting all data.
3. Results
3.1. Oxadiazon Dissipation in Soil
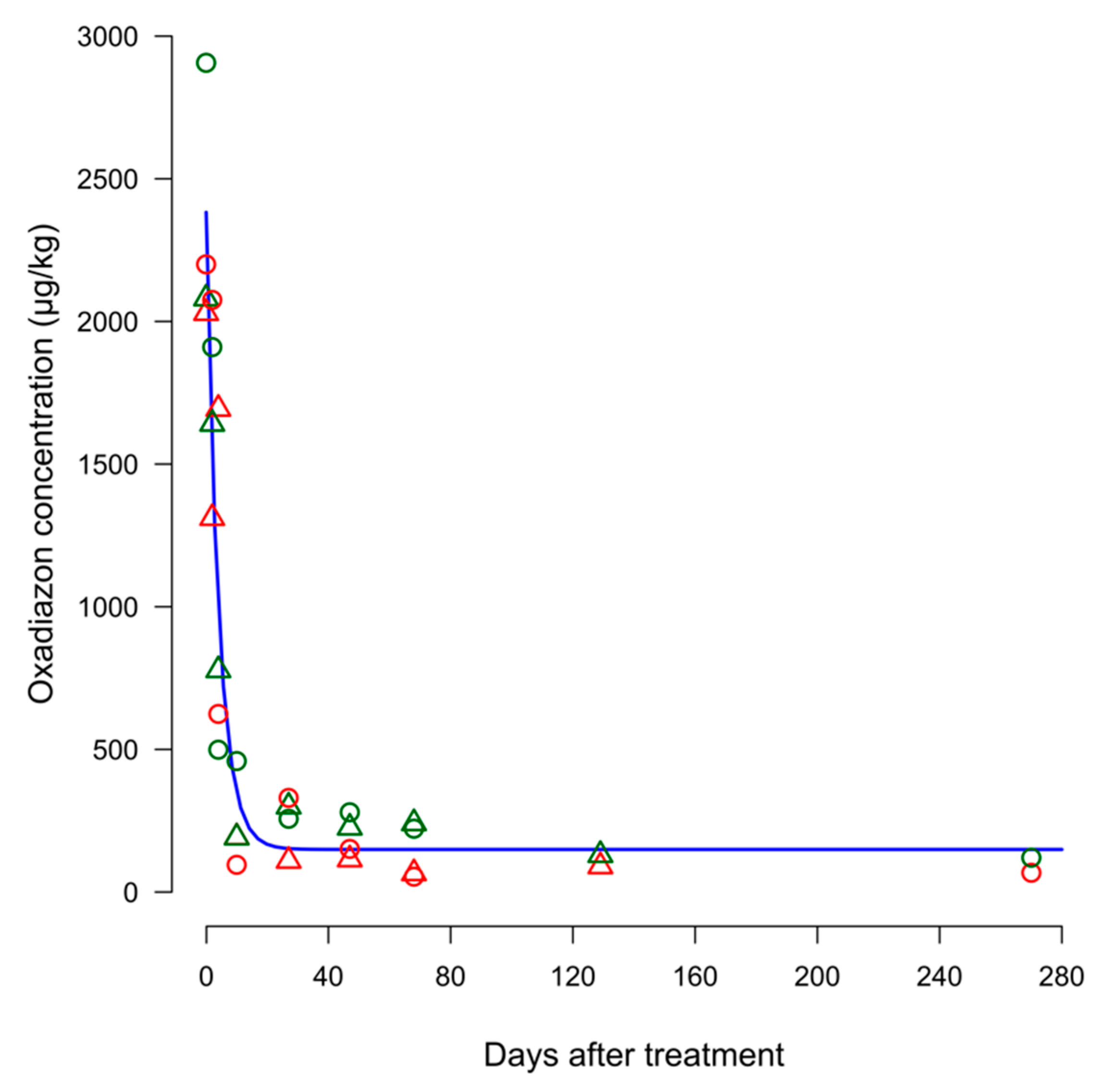
In both seasons and systems, the concentrations of oxadiazon found in soil decreased quickly after treatment, following a similar trend (
Figure 2).
During 2012 in the soil samples collected the day before the treatment, oxadiazon residues were found at concentrations ranging from 23.44 µg/kg (system CON) to 37.71 µg/kg (system DRY). These values must be put in relation to the application of oxadiazon that occurred in the previous season. Immediately after the spraying of the herbicide, the concentration detected in the soil was 2029.5 µg/kg in system CON and 2080.5 µg/kg in system DRY. The soil concentration of oxadiazon rapidly declined in both systems and at 10 DAT less than 10% of the initial concentration was detected. At 30 DAT the residues concentrations of oxadiazon found in system DRY were higher than those observed in the CON system. The same behavior was observed in the following sampling date, and four months after herbicide spraying the soil concentration of the herbicide was higher in system DRY (129.70 µg/kg) than in system CON (90.29 µg/kg).
In the following growing season (2013), the oxadiazon concentration found in soil samples collected the day before the treatment was 28.91 µg/kg and 40.30 µg/kg in the CON and DRY systems, respectively. The concentration of oxadiazon found in the soil after herbicide spraying was similar to that observed in the previous year at the same sampling time and ranged from 2200.17 µg/kg in system CON to 2906.50 µg/kg in system DRY. At 10 days after treatment oxadiazon concentration was higher in system DRY. A similar trend was observed for the following samplings. One month after spraying, oxadiazon residues in the soil varied from 330.13 µg/kg in system CON to 282.81 µg/kg in system DRY. At almost 70 DAT, the oxadiazon concentration was higher in system DRY (222.09 µg/kg) than in system CON (54.00 µg/kg). A more pronounced difference in terms of concentration was observed at the last soil sampling (270 DAT). The half-life calculated from the model fitting all the concentration values was 3.27 ± 0.46 days.
3.2. Oxadiazon Dissipation in Paddy Water
Due to the difference in the management practices of the two systems, the sampling of the water into the field started a few days after herbicide treatment in system CON, and about thirty days later in system DRY, letting to collect a limited number of samplings in the latter. In particular, concentrations recorded in DRY in 2012 were 3.08 ± 0.99 µg/L and 0.96 ± 0.56 µg/L at 47 and 68 DAT, respectively, and 2.57 ± 0.79 µg/L at 47 DAT in 2013.
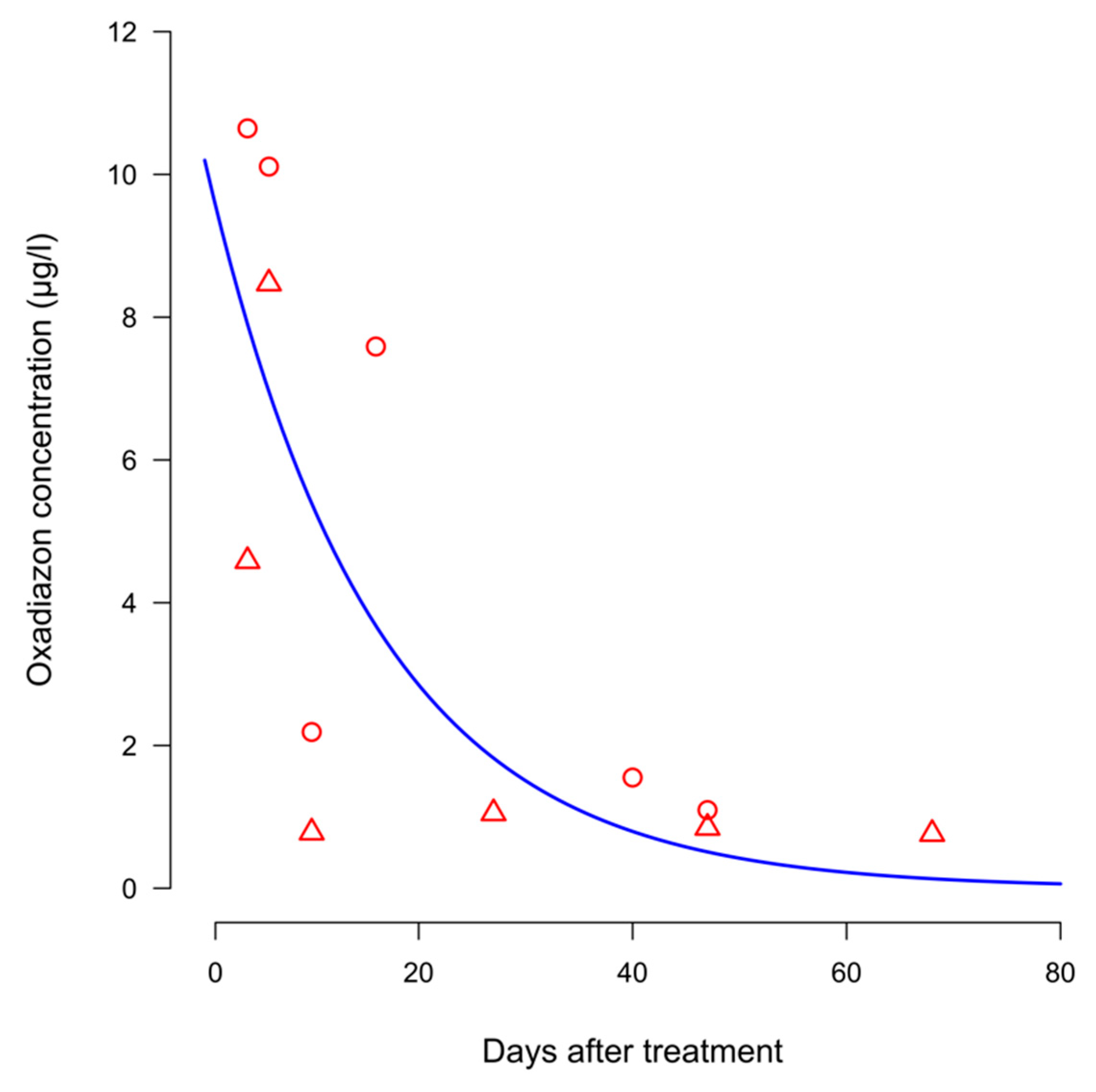
In system CON the ANCOVA did not reveal an effect of year on oxadiazon concentration in paddy water. Hence the data of the two years were pooled together and fitted with a single exponential decay model (
Figure 3). In general, concentrations found in water were much lower than that found in soil. Even in the case of the highest values, recorded in the sampling carried out at 4 DAT, these were about 100-fold lower than those recorded in soil.
In 2012 the first sampling of paddy water was conducted in system CON at four days after herbicide application and the oxadiazon concentration was 4.58 µg/L. Two days later (6 DAT) the concentration peaked at 8.47 µg/L. At the following samplings, the oxadiazon residues in paddy water declined, never exceeding 1.05 µg/L. More than two months after herbicide spraying, oxadiazon residues in paddy water were less than 0.8 µg/L. Even if the ANCOVA did not highlight differences attributable to years in system CON, the concentrations in the water were generally higher in 2013. In 2013, the concentration of oxadiazon at the time of the first water sampling was 10.65 µg/L and decreased almost steadily, following a quasi-linear trend (with the exception of the sampling carried out at 10 DAT) until the last sampling, carried out at 47 DAT. At this sampling date oxadiazon residues were still present at 1.09 µg/L.
3.3. Oxadiazon Dissipation in Inlet and Outlet Water
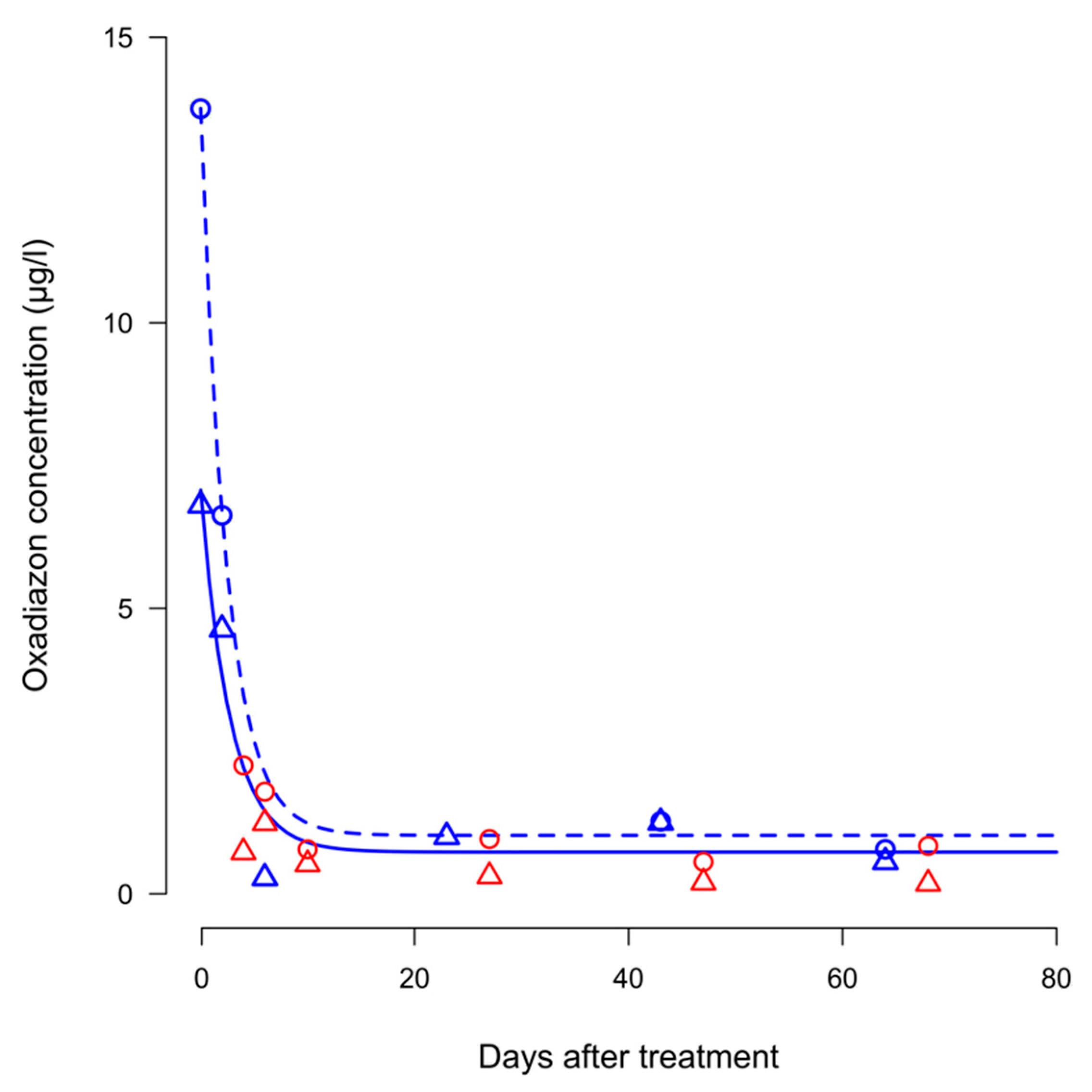
Concentration of oxadiazon in inlet water ranged from 0.18 µg/L (at 68 DAT) to 1.24 µg/L (6 DAT) in 2012 and from 0.56 µg/L (at 40 DAT) to 2.25 µg/L (4 DAT) in 2013. Regression analysis carried out on inlet data did not show a significant curve steepness in both years, indicating that the concentrations did not vary over time (
Figure 4, red symbols).
In system CON, in both years, the highest concentrations of oxadiazon in outlet water were detected in the sampling carried out at 4 DAT, as soon as flooding conditions and water circulation in the field were restored after the treatment. Concentrations then decreased following an exponential-decay pattern. The data were better explained by two curves fitting separately 2012 and 2013, likely because of the higher concentrations recorded in the first sampling of 2013.
In system DRY, only few samples of outlet water were collected. In the first year, samples were collected at 47 and 68 DAT and the concentrations were 0.69 and 0.63 µg/L, respectively. In the second year, a single sample was collected at 47 DAT, with an oxadiazon concentration of 3.63 µg/L.
4. Discussion
This study was aimed at evaluating the dissipation over two growing seasons of oxadiazon in paddy field water and soil under two rice growing systems. Outcomes of this study may be considered of interest, as the dissipation of the herbicide oxadiazon in the paddy rice environment was studied under real field conditions. Oxadiazon dissipation in soil follows first order kinetics [
20]. Some studies conducted worldwide, both in laboratory and field conditions, showed that oxadiazon is persistent in the soil [
3,
4,
21] and degradation occurs slowly, due to the low water solubility (0.57 mg/L at 20 °C) and the absence of metabolizable groups in the molecule [
4]. Persistence of oxadiazon appears to be highly correlated to its chemical and physical properties. In general, the lower the solubility of a certain compound, the higher its adsorption [
22]. It has been found that soil organic content was the primary factor in oxadiazon adsorption [
22,
23]. According to Wehtje et al. [
6], adsorption of oxadiazon in different substrate types was at least 96%. The EFSA document made on this pesticide [
24] reported that the DT
50 of oxadiazon in soil may vary from 262–330 days in Northern Europe, to 90–95 days in Southern Europe. Chakraborty et al. [
21] found a soil half-life of 44 days and 45 days with application of 1 kg/ha and 2 kg/ha of oxadiazon, respectively. In a study conducted on soya bean and lettuce by Conte et al. [
25], the soil half-life of oxadiazon showed an important variation in time from 53 days to 192 over two years. The authors postulated that these differences were due to the agronomic practices adopted in the second year on lettuce, and in particular, to the frequent irrigations carried out over the season [
25]. Despite the low degradation, this herbicide is generally considered with a reduced risk of leaching [
4,
23,
24] and the losses by volatilization are negligible due to its low vapor pressure [
2,
4,
24].
In our study, the oxadiazon topsoil half-life was of about 3 days, without significant differences between systems and years. Other studies pointed out that oxadiazon dissipation was not different in both moist and flooded soils [
5] and similar results were found also both in greenhouse and laboratory experiments [
26]. In these studies, oxadiazon dissipation was rapid in the first weeks after herbicide spraying, with a sharp decrease in the rate of dissipation in the following weeks. These findings are in keeping with the results obtained in the current study.
Compared to the studies mentioned above, the soil half-life of oxadiazon found in our study was significantly lower. We hypothesize that the reduced depth of sampling adopted has an influence on the residue concentration found. After spraying most of the pesticide remain in top-soil layers [
27]. Pesticide dissipation is generally more pronounced in the upper soil layers because of the effect of different degradation biotic and abiotic phenomena, hence the resulting soil half-life could be lower compared to that observed at higher depths. Nonetheless, these data are of interest because they may help to understand the behavior of this herbicide in the soil layer that mostly interact with the paddy water. In paddies during submersion the water entering within the field gradually dissolve chemicals present in soil surface. These phenomena are likely more pronounced in case of pesticides applied in pre-emergence on bare soil and when flooding occurs early after the spraying. Previous studies carried out on rice pesticides confirmed the strict interaction between flooding water and the top soil layer [
28,
29], as they highlighted the formation of a gradient of concentration in paddy water from the upper part of the fields (close to inlet floodgate) to the bottom part of them (close to the outlet floodgate). The data of this study agree with the results of Das et al. [
30], which determined for oxadiazon an average soil half-life in the rice rhizosphere of 8.8 days. The same authors found that oxadiazon persists in soil for more than 60 days. A rapid dissipation of oxadiazon was also observed in the first ten days after herbicide application by Barret et al. [
26], linked to the presence of the herbicide in the surface soil layer. In addition, they observed a much slower dissipation pattern in the period ranging from 10 to 40 days after the herbicide spraying [
26]. A similar trend in oxadiazon dissipation was also observed in this study. A rapid degradation of oxadiazon in the top layers was also found by Ying et al. [
31], which observed a reduced mobility of oxadiazon in the soil with most of the oxadiazon trace amounts present at 0–5 cm soil depth.
Water resources contamination by herbicides is an environmental concern all over the world. Herbicides may reach water compartments through several phenomena of transport, as runoff, leaching, drift and soil erosion [
13]. Risk of contamination of water bodies is particularly important in paddy fields, where the presence of flooding water over the season may facilitate the transfer of herbicides out of the fields by irrigation drainage and runoff [
28,
32]. Despite oxadiazon being an herbicide with a long history of application—it was introduced on the market in 1969 by Rhône-Poulenc—only a few studies have investigated the fate of this chemical in paddy fields.
Occurrence of oxadiazon in surface waters is reported in several studies conducted worldwide in the last thirty years [
9,
15,
16,
33,
34]. Despite the low solubility in water and high adsorption of oxadiazon on the organic matrix of the soil [
21,
22], runoff of this herbicide may occur from paddy fields [
34]. In water, oxadiazon may undergo degradation by means of sunlight, while volatilization from water is not considered an important dissipation pathway [
23]. Oxadiazon is stable in water at pH values from 4 to 7, while degradation may occur at a pH above 9 [
2].
The presence of oxadiazon in inlet waters can be ascribed to discharges of paddy waters into the ditches, and to direct contamination of ditch water during herbicide application (drift). The residues of oxadiazon in inlet waters should confirm a certain transport of the herbicide from paddies to discharge ditches and/or a direct contamination during herbicide application. Similar findings were observed in our previous studies carried out on propanil and imazamox [
27]. Transfer of oxadiazon residues from the outlet floodgates occurred over the growing season even though the maximum concentrations were found in the first weeks after herbicide spraying.
In the studied conditions, oxadiazon dissipation in paddy water was rapid during the first week; afterwards a less pronounced drop in the concentration was measured. The highest concentrations were measured soon after herbicide application as already observed for other pesticides in similar agronomic conditions [
27]. Residues of oxadiazon in paddy waters where found even two months after the treatment. In system DRY the presence of oxadiazon in paddy water was monitored for a shorter period compared to system CON because of the absence of water in the field for the majority of the sampling period. As a consequence, less water samples were available for DRY, hampering the comparison between the two systems. However, because the field was not submerged for a longer period than CON, the risk of oxadiazon transport by water is reduced. The oxadiazon concentration found in the CON system decreased significantly over the time. Despite the fact that the concentrations recorded were generally higher in 2013, the data of the two years can be described by a single exponential-decay model.
The initial hypothesis that dissipation of oxadiazon is faster in conventional flooded system cannot be confirmed by this study, at least for topsoil. In the case of water, the limited number of samples collected from system DRY did not allow to have a full comparison of dissipation dynamics between the systems.
This study leads to the conclusion that oxadiazon could be transported into discharge ditches long after its application. Nevertheless, even though the concentrations observed across the season always exceeded the reference limit for surface waters (0.1 µg/L), the potential amount of oxadiazon that may reach the discharge ditches could be remarkably dropped by means of degradation phenomena and dilution.
A possible way to reduce the potential transfer of oxadiazon to water courses could be to increase the residence time of the herbicide in the paddies during the first week after spraying or limiting the release of paddy water for a period comparable to herbicide persistence [
35]. A similar measure has been proposed for certain rice pesticides classified as toxic to aquatic organisms in Japan and California [
33,
36]. Avoiding discharge of paddy water from rice fields in the first 7–10 days after spraying may represent a useful practice to reduce the transfer of oxadiazon to surface water [
37]. The same authors suggest a slow restoring of field submersion conditions after the treatment as well as a good leveling of it in order to ensure a uniform distribution of water within the field basin. These practices may help to significantly reduce the transfer of pesticides into surface waters. A water-friendly use of rice pesticides must be encouraged by national and local administrations. To this regard, the Italian Ministry of Health and the Regione Piemonte administration has already welcomed and promoted these set of measures. Specific prescriptions, based on these measures, have been recently stated on the labels of some important herbicides with a potential high risk of water contamination; they might be extended to other important pesticides.