Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum Turgidum ssp Durum) Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Measurements
2.2. Relative Water Content (RWC)
2.3. Measurement of Water-Soluble Carbohydrates (WSC)
2.4. Enzymatic Assays
2.5. Proteins Determination and Immunoblotting
2.6. RNA-Extraction and Real Time Q-RT-PCR
2.7. Bioinformatics Analyses
2.8. Statistical Analysis
3. Results
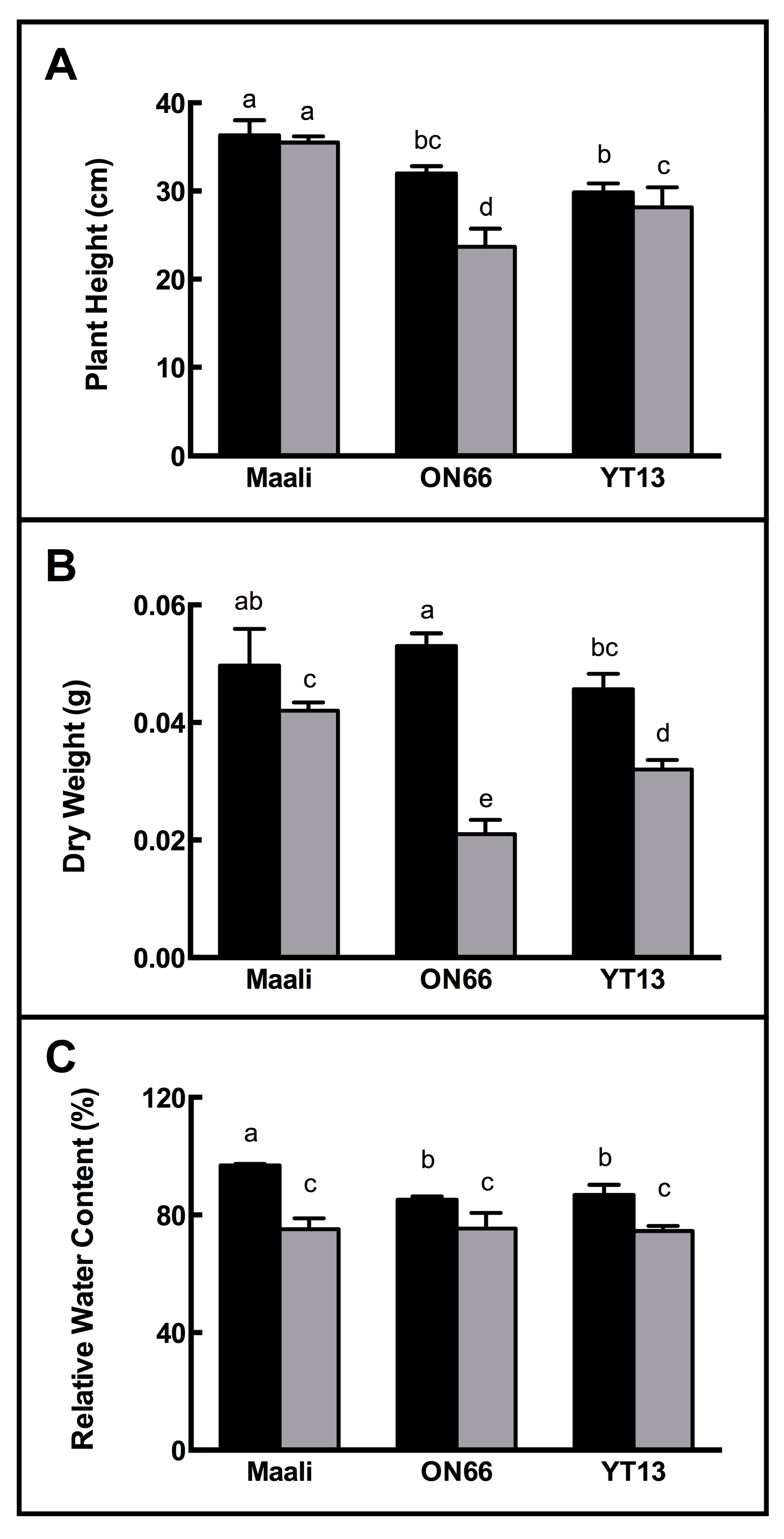
3.1. Impact of Osmotic Stress on Growth, RWC and WSC
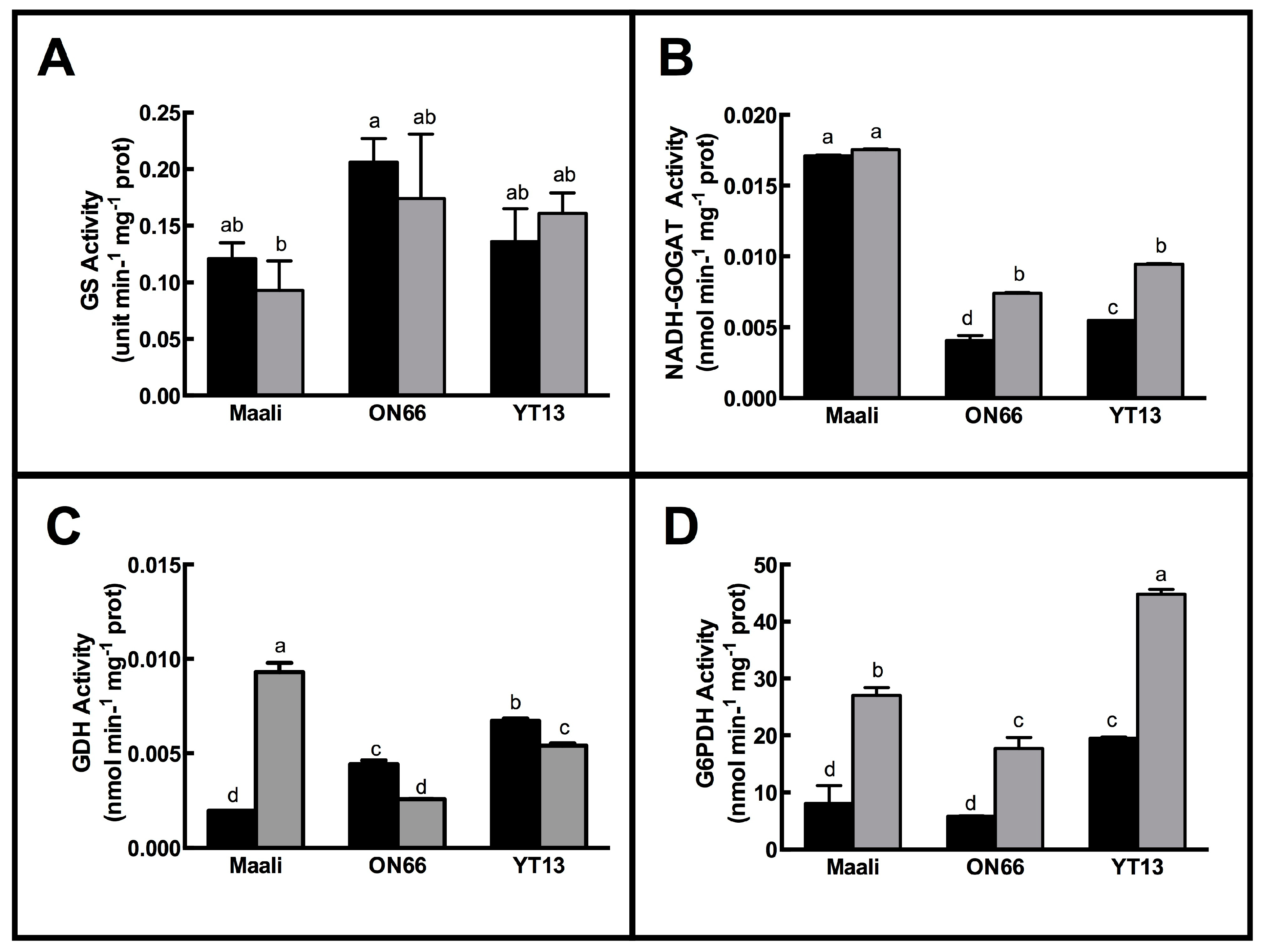
3.2. Impact of Osmotic Stress on Enzymes Involved in Nitrogen Metabolism
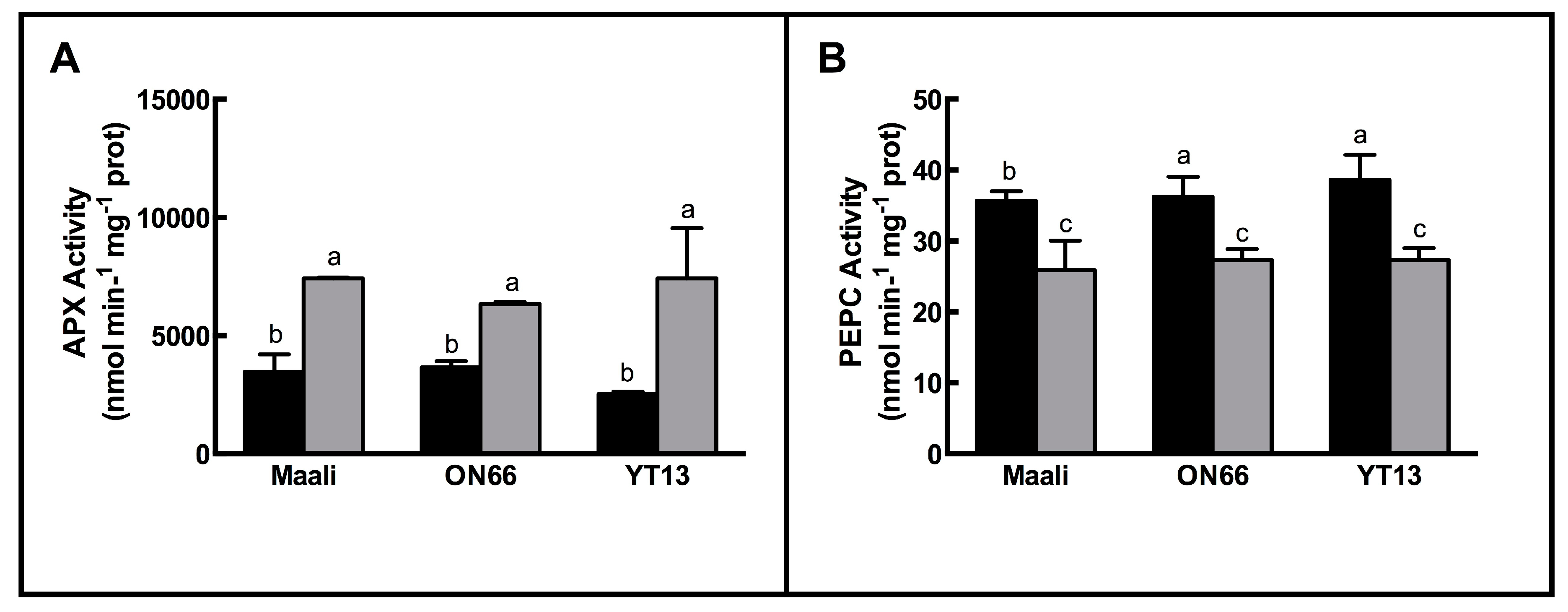
3.3. Changes in Antioxidant Enzymes
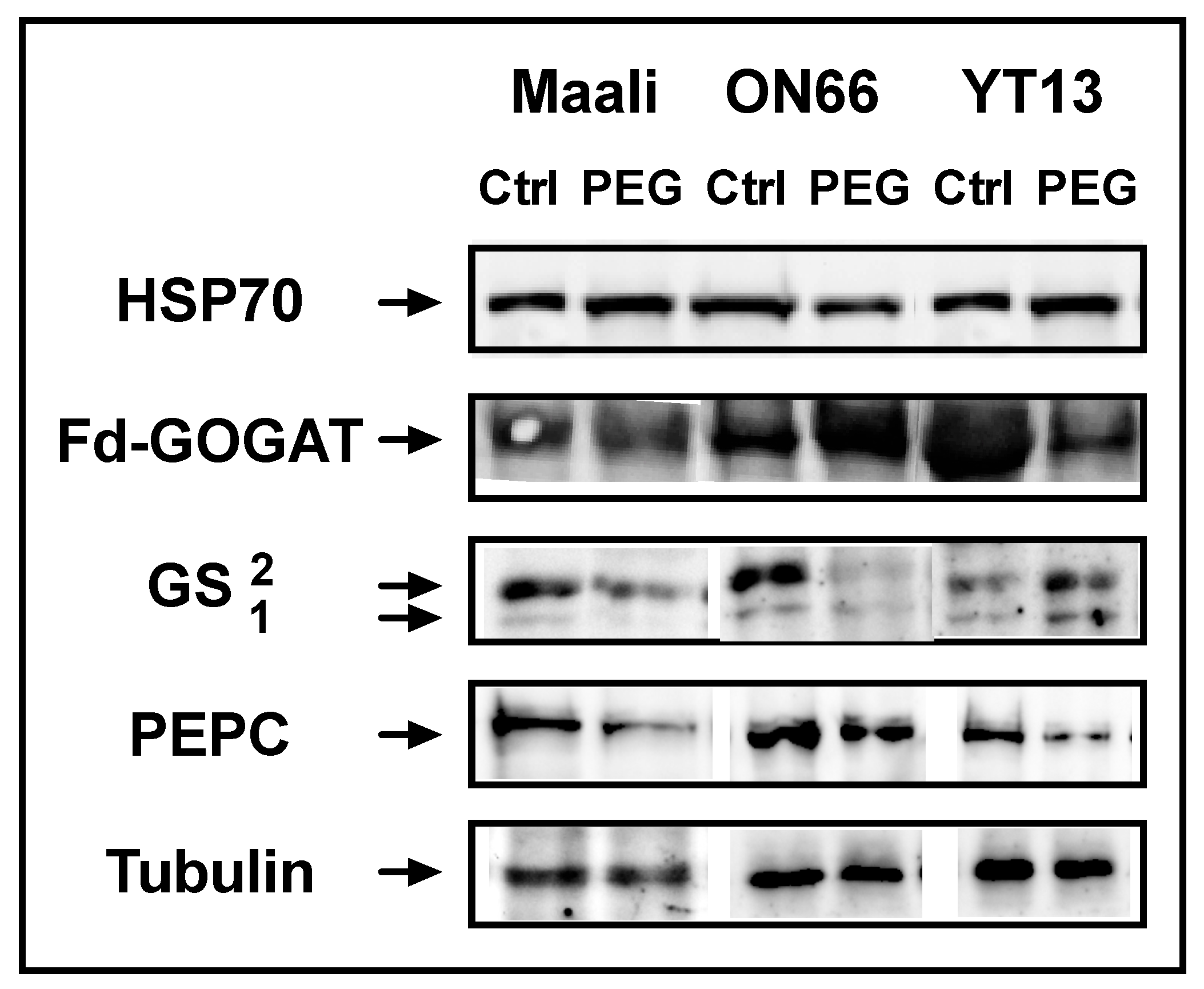
3.4. Immunoblots of HSP70, PEPC, GS and Fd-GOGAT
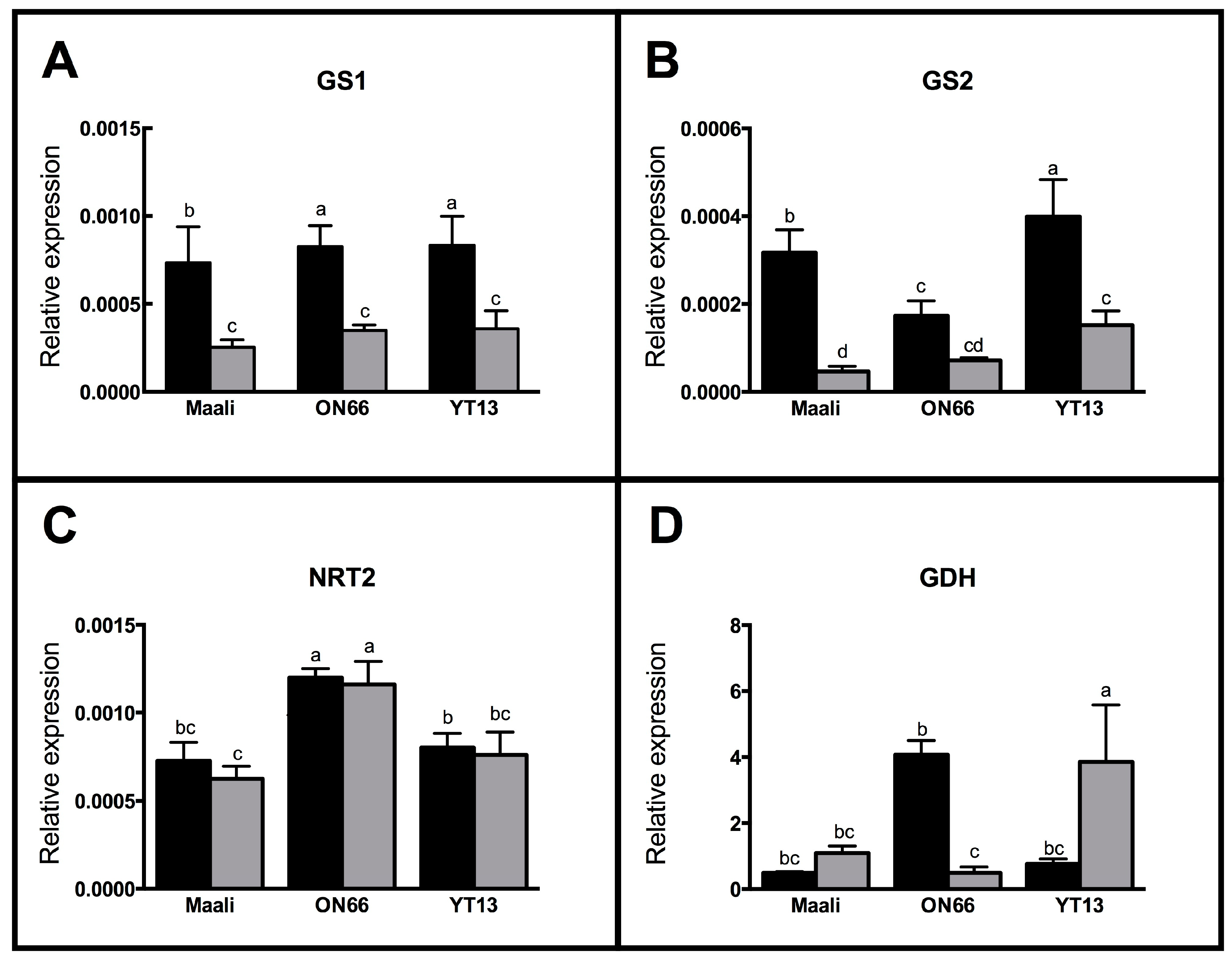
3.5. Differential Gene Expression in Osmotic Stressed Genotypes
4. Discussion
4.1. Differential Growth Performance of Durum Wheat Genotypes under Osmotic Stress
4.2. The GS-GOGAT Pathway Is Impacted by the Osmotic Stress
4.3. Antioxidant Enzymes Activities Increased upon Osmotic Stress
4.4. Differential Biochemical Behaviors of the Three Genotypes GDH Activity and Transcript Abundance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abis, S. Wheat in the Mediterranean Region: Societies, Trade and Strategies. Econ. Territ. 2012, 1, 218–224. [Google Scholar]
- Molnar, I.; Gaspar, L.; Sarvari, E.; Dulai, S.; Hoffmann, B.; Molnar-Lang, M.; Galiba, G. Physiological and morphological responses to water stress in Aegilops biuncialis and Triticum aestivum genotypes with differing tolerance to drought. Funct. Plant Biol. 2004, 31, 1149–1159. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Marquez, A.J.; Voltas, J.; Araus, J.L. Combined use of △13C, △18O and △15N tracks nitrogen metabolism and genotypic adaptation of durum wheat to salinity and water deficit. N. Phytol. 2012, 194, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Hausman, J.F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Navakode, S.; Scheibal, V.; Baum, M.; Nachit, M.; Roder, M.S.; Borner, A. The genetic basis of durum wheat germination and seedling growth under osmotic stress. Biol. Plant. 2014, 58, 681–688. [Google Scholar] [CrossRef]
- Bajji, M.; Lutts, S.; Kinet, J.M. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 2001, 160, 669–681. [Google Scholar] [CrossRef]
- Yousfi, S.; Marquez, A.J.; Betti, M.; Araus, J.L.; Serret, M.D. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J. Integr. Plant Biol. 2016, 58, 48–66. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat: A Review. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- Kerepesi, I.; Galiba, G. Osmotic and Salt Stress-Induced Alteration in Soluble Carbohydrate Content in Wheat Seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Kichey, T.; Heumez, E.; Pocholle, D.; Pageau, K.; Vanacker, H.; Dubois, F.; Le Gouis, J.; Hirel, B. Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. N. Phytol. 2006, 169, 265–278. [Google Scholar] [CrossRef]
- Bernard, S.M.; Blom Moller, A.L.; Dionisio, G.; Kichey, T.; Jahn, T.P.; Dubois, F.; Baudo, M.; Lopes, M.S.; Terce-Laforgue, T.; Foyer, C.H.; et al. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 67, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. N. Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Goel, P.; Singh, A.K. Abiotic Stresses Downregulate Key Genes Involved in Nitrogen Uptake and Assimilation in Brassica juncea L. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S. Nitrogen Assimilation, Abiotic Stress and Glucose 6-Phosphate Dehydrogenase: The Full Circle of Reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Guerriero, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S.; Rigano, C. Glutamate synthase activities and protein changes in relation to nitrogen nutrition in barley: The dependence on different plastidic glucose-6P dehydrogenase isoforms. J. Exp. Bot. 2005, 56, 55–64. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Hucklesby, D.P.; Emes, M.J. Nitrite reduction and carbohydrate metabolism in plastids purified from roots of Pisum sativum L. Planta 1989, 177, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Massaro, G.; Di Martino Rigano, V.; Carfagna, S. Glutamate synthesis in barley roots: The role of the plastidic glucose-6-phosphate dehydrogenase. Planta 2003, 216, 639–647. [Google Scholar]
- Esposito, S.; Carillo, P.; Carfagna, S. Ammonium metabolism stimulation of glucose-6P dehydrogenase and phosphoenol-pyruvate carboxylase in young barley roots. J. Plant Physiol. 1998, 153, 61–66. [Google Scholar] [CrossRef]
- Fercha, A.; Gherroucha, H. The role of osmoprotectants and antioxidant enzymes in the differential response of durum wheat genotypes to salinity. J. Appl. Bot. Food Qual. 2014, 87, 74–79. [Google Scholar]
- Michel, B.E.; Kaufman, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, A.A.; Boyce, A.N.; Barakbah, S.S. Study on Osmotic Stress Tolerance in Promising Durum Wheat GenotypesUsing Drought Stress Indices. Res. J. Agric. Biol. Sci. 2009, 5, 603–607. [Google Scholar]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Alaei, M.; Zaefizadeh, M.; Khayatnezhad, M.; Alaei, Z.; Alaei, Y. Evaluation of Germination Properties of Different Durum Wheat Genotypes under Osmotic Stress. Middle-East J. Sci. Res. 2010, 6, 642–646. [Google Scholar]
- El Siddig, M.A.; Baenziger, S.; Dweikat, I.; El Hussein, A.A. Preliminary screening for water stress tolerance and genetic diversity in wheat (Triticum aestivum L.) cultivars from Sudan. J. Genet. Eng. Biotechnol. 2013, 11, 87–94. [Google Scholar] [CrossRef]
- Kacem, N.S.; Delporte, F.; Yordan, Y.; Abdelhamid, D.; Bernard, W. In vitro screening of durum wheat against water-stress mediated through polyethylene glycol. J. Genet. Eng. Biotechnol. 2017, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Agomz, K. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; IRRI: Los Baños, Philippines, 1976. [Google Scholar]
- Sade, N.; Galkin, E.; Moshelion, M. Measuring Arabidopsis, Tomato and Barley Leaf Relative Water Content (RWC). Bio-Protocol 2015, 5, 1451. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Castiglia, D.; Cardi, M.; Landi, S.; Cafasso, D.; Esposito, S. Expression and characterization of a cytosolic glucose-6-phosphate dehydrogenase isoform from barley (Hordeum vulgare) roots. Prot. Expr. Purif. 2015, 112, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Carfagna, S.; Massaro, G.; Vona, V.; Di Martino Rigano, V. Glucose-6-phosphate dehydrogenase in barley roots: Kinetic properties and localization of the isoforms. Planta 2001, 212, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.F.; Louie, M.; Lerner, A. Glutamine Synthetase from Rat Liver. J. Biol. Chem. 1978, 253, 6111–6118. [Google Scholar] [PubMed]
- Groat, R.G.; Vance, C.P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.)—Developmental patterns and response to applied nitrogen. Plant Physiol. 1981, 67, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.D.; Srivastava, H.S. Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Cardi, M.; Zaffagnini, M.; De Lillo, A.; Castiglia, D.; Chibani, K.; Gualberto, J.M.; Rouhier, N.; Jacquot, J.P.; Esposito, S. Plastidic P2 glucose-6P dehydrogenase from poplar is modulated by thioredoxin m-type: Distinct roles of cysteine residues in redox regulation and NADPH inhibition. Plant Sci. 2016, 252, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A. Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant Soil 2003, 253, 201–218. [Google Scholar] [CrossRef]
- Turkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2014, 209, 2–9. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Shahbazi, M.; Shariati, M.; Mulo, P. Effects of Mild and Severe Drought Stress on Photosynthetic Efficiency in Tolerant and Susceptible Barley (Hordeum vulgare L.) Genotypes. J. Crop Sci. 2014, 200, 403–415. [Google Scholar] [CrossRef]
- Johnová, P.; Skalák, J.; Saiz-Fernández, I.; Brzobohatý, B. Plant responses to ambient temperature fluctuations and water-limiting conditions: A proteome-wide perspective. Biochimica et Biophysica Acta 2016, 1864, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Németh, E.; Guoth, A.; Bona, L.; Wodala, B.; Pecsvaradi, A. Metabolic indicators of drought stress tolerance in wheat: Glutamine synthetase isoenzymes and Rubisco. Plant Physiol. Biochem. 2013, 67, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Ghosh, S. Regulation of glutamine synthetase isoforms in two differentially drought-tolerant rice (Oryza sativa L.) cultivars under water deficit conditions. Plant Cell Rep. 2013, 32, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.; Costa, A.; Punzo, P.; Landi, S.; Ruggiero, A.; Batelli, G.; Grillo, S. Genetics of drought stress tolerance in crop plants. In Drought stress tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattachajee, S., Burrit, D., Phan tran, L., Eds.; Springer: Cham, Switzerland, 2016; Volume 2, pp. 39–70. [Google Scholar] [CrossRef]
- Weng, M.; Cui, L.; Liu, F.; Zhang, M.; Shan, L.; Yang, S.; Geng, X. Effects of Drought Stress on Antioxidant Enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Nurcato, R.; De Lillo, A.; Lentini, M.; Grillo, S.; Esposito, S. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol. Biochem. 2016, 105, 79–89. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- Miyao, M.; Fukayama, H. Metabolic consequences of overproduction of phosphoenolpyruvate carboxylase in C3 plants. Arch. Biochem. Biophys. 2003, 414, 197–203. [Google Scholar] [CrossRef]
- Bouthour, D.; Kalai, T.; Haouari, C.; Chaffei, C.; Gouia, H.; Corpas, F.J. Differential response of NADP-dehydrogenases and carbon metabolism in leaves and roots of two durum wheat (Triticum durum Desf.) cultivars (Karim and Azizi) with different sensitivities to salt stress. J. Plant Physiol. 2015, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Dong, S.; Zhao, B.; Liu, P.; Zhang, J. Responses of Nitrogen Metabolism, Uptake and Translocation of Maize to Waterlogging at Different Growth Stages. Front. Plant Sci. 2017, 8, 1216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthin, B.; Bains, N.S. Modulation of proline metabolism under drought and salt stress conditions in wheat seedling. Indian J. Biochem. Biophys. 2018, 55, 114–124. [Google Scholar]
- Lee, T.E. Selecting drought and salt tolerant wheat cultivar. Increasing Wheat Production in Central Asia through Science and International Cooperation. In Proceedings of the First Central Asian Wheat Conference, Almaty, Kazakhstan, 10–13 June 2003; pp. 109–113. [Google Scholar]
- Zhou, Y.; Zhang, C.; Lin, J.; Yang, Y.; Peng, Y.; Tang, D.; Zhao, X.; Zhu, Y.; Liu, X. Over-expression of a glutamate dehydrogenase gene, MgGDH, from Magnaporthe grisea confers tolerance to dehydration stress in transgenic rice. Planta 2015, 241, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, D.A.; Mungur, R.; Ameziane, R.; Ameziane, R.; Nolste, S.; Long, L.; Bernhard, K.; Colter, A.; Jones, K.; Iqbal, M.J.; et al. Improved drought tolerance of transgenic Zea mays plants that express the glutamate dehydrogenase gene (gdhA) of E. coli. Euphytica 2007, 156, 103–116. [Google Scholar] [CrossRef]
| Height(cm) | FW (g) | DW (g) | RWC (%) | WSC (mg g−1 FW) | ||
|---|---|---|---|---|---|---|
| Genotypes (G) | ||||||
| YT13 | 27.833 ± 1.93 | 0.361 ± 0.18 | 0.037 ± 0.007 | 80.260 ± 6.66 | 0.305 ± 0.08 | |
| ON66 | 35.916 ± 4.45 | 0.418 ± 0.24 | 0.045 ± 0.01 | 85.971 ± 6.17 | 0.343 ± 0.15 | |
| MAALI | 30.916 ± 1.36 | 0.372 ± 0.18 | 0.040 ± 0.006 | 82.296 ± 11.13 | 0.286 ± 0.05 | |
| Treatment (T) | ||||||
| Control | 32.722 ± 2.97 | 0.574 ± 0.05 | 0.049 ± 0.005 | 89.547 ± 5.57 | 0.214 ± 0.11 | |
| Osmotic stress | 29.111 ± 5.2 | 0.17 ± 0.06 | 0.031 ± 0.008 | 75.046 ± 3.85 | 0.340 ± 0.1 | |
| Mean | 30.916 ± 4.6 | 0.372 ± 0.21 | 0.040 ± 0.01 | 82.296 ± 8.7 | 0.286 ± 0.12 | |
| ANOVA | ||||||
| df | ||||||
| G | 2 | 114.54 *** | 0.0105 | 0.000131 ** | 61.0 * | 0.0176 |
| T | 1 | 58.68 ** | 0.7361 *** | 0.001422 *** | 946.3 *** | 0.0605 * |
| G × T | 2 | 25.35 ** | 0.0070 | 0.000241 *** | 59.4 * | 0.0269 |
| Error | 12 | 3.60 | 0.0029 | 0.000015 | 14.4 | 0.718 |
| GS1 | GS2 | GDH | NRT2 | PEPc Activity | APX Activity | GS Activity | G6PDH | |
|---|---|---|---|---|---|---|---|---|
| Genotypes | ||||||||
| Maali | 4.50 × 10−4 | 1.82 × 10−4 | 1.06 | 6.99 × 10−4 | 2.96 | 12.7 × 104 | 0.099 | 2.21 |
| YT13 | 6.63 × 10−4 | 2.91 × 10−4 | 3.59 | 7.82 × 10−4 | 3.14 | 52.1 × 104 | 0.147 | 2.74 |
| ON66 | 6.11 × 10−4 | 1.16 × 10−4 | 1.69 | 12.2 × 10−4 | 3.23 | 69.7 × 104 | 0.198 | 1.86 |
| Treatments | ||||||||
| Control | 8.13 × 10−4 | 3.03 × 10−4 | 1.72 | 9.26 × 10−4 | 3.69 | 12200 | 0.151 | 1.95 |
| Osmotic stress | 3.36 × 10−4 | 0.89 × 10−4 | 2.56 | 8.72 × 10−4 | 2.53 | 4410 | 0.144 | 2.59 |
| Means | 5.74 × 10−4 | 1.96 × 10−4 | 2.09 | 8.99 × 10−4 | 3.11 | 8280 | 0.148 | 2.27 |
| ANOVA (mean square) | ||||||||
| Genotype | 7.4 × 10−8 *** | 4.7 × 10−8 *** | 8.726 * | 4.6 × 10−7 *** | 11.6 | 6.07 × 107 | 1.2 × 10−2 * | 77.21 |
| Treatment | 1.0 × 10−6 *** | 2.04 × 10−7 *** | 3.259 | 1.30 × 10−8 | 599 *** | 1.80 × 108 | 4.11 × 10−4 | 125.29 |
| G × T | 1.9 × 10−8 * | 1.7 × 10−8 * | 23.571 ** | 1.18 × 10−8 | 14.6* | 5.86 × 107 | 9.32 × 10−4 | 5.84 |
| Error | 4.7 × 10−9 | 2.6 × 10−9 | 1.697 | 9.90 × 10−9 | 3.2 | 1.22 × 108 | 15.8 × 10−4 | 144.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jallouli, S.; Ayadi, S.; Landi, S.; Capasso, G.; Santini, G.; Chamekh, Z.; Zouari, I.; Ben Azaiez, F.E.; Trifa, Y.; Esposito, S. Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum Turgidum ssp Durum) Genotypes. Agronomy 2019, 9, 550. https://doi.org/10.3390/agronomy9090550
Jallouli S, Ayadi S, Landi S, Capasso G, Santini G, Chamekh Z, Zouari I, Ben Azaiez FE, Trifa Y, Esposito S. Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum Turgidum ssp Durum) Genotypes. Agronomy. 2019; 9(9):550. https://doi.org/10.3390/agronomy9090550
Chicago/Turabian StyleJallouli, Salma, Sawsen Ayadi, Simone Landi, Giorgia Capasso, Giorgia Santini, Zoubeir Chamekh, Inés Zouari, Fatma Ezzahra Ben Azaiez, Youssef Trifa, and Sergio Esposito. 2019. "Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum Turgidum ssp Durum) Genotypes" Agronomy 9, no. 9: 550. https://doi.org/10.3390/agronomy9090550





