Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Morphological Traits
2.4. Physiological Parameters
2.5. Biochemical Parameters
2.6. Fourier-Transform Infrared (FT-IR) Spectroscopy Assay
2.7. Statistical Analyses
3. Results
3.1. Morphological Traits
3.2. Physiological Parameters
3.3. Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ES | eroded soil |
| ES + SS | mixture of eroded soil (15%) + sewage sludge, (85%) |
References
- Duman, A.D.; Telci, I.; Dayisoylu, K.S.; Digrak, M.; Demirtas, I.; Alma, M.H. Evaluation of bioactivity of linalool-rich essential oils from Ocimum basilicum and Coriandrum sativum varieties. Nat. Prod. Commun. 2010, 5, 969–974. [Google Scholar] [PubMed]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Evans, W.B.; Ebelhar, M.W. Yield and composition of Ocimum basilicum L. and Ocimum sanctum L. grown at four locations. Hortscience 2008, 43, 737–741. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Tekwani, B.; Khan, S.I. Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J. Agric. Food Chem. 2008, 56, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Sgherri, C.; Baâtour, O.; Izzo, R.; Lachaâ, M.; Navari-Izzo, F.; Ouerghi, Z. Effects of oxidative stress caused by NaCl or Na2SO4 excess on lipoic acid and tocopherols in Genovese and Fine basil (Ocimum basilicum). Ann. Appl. Biol. 2013, 163, 23–32. [Google Scholar] [CrossRef]
- Castronuovo, D.; Russo, D.; Libonati, R.; Faraone, I.; Candido, V.; Picuno, P.; Andrade, P.; Valentao, P.; Milella, L. Influence of shading treatment on yield, morphological traits and phenolicprofile of sweet basil (Ocinum basilicum L.). Sci. Hortic. 2019, 254, 91–98. [Google Scholar] [CrossRef]
- Kliebenstein, D.K. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Teliban, G.C.; Burducea, M.; Lobiuc, A.; Stoleru, V.; Hamburdă, S.B.; Galea (Deleanu), F.M.; Onofrei, V.; Zamfirache, M.M.; Munteanu, N. Yield, morphological and physiological comparative aspects in Ocimum basilicum L. under different fertilization types. Sci. Papers Hortic. 2016, 59, 69–74. [Google Scholar]
- Stoleru, V.; Munteanu, N.; Sellitto, V.M. New Approach of Organic Vegetable Systems; Aracne Editrice: Rome, Italy, 2014. [Google Scholar]
- Onofrei, V.; Benchennouf, A.; Jancheva, M.; Loupassaki, S.; Ouaret, W.; Burducea, M.; Lobiuc, A.; Teliban, G.C.; Robu, T. Ecological foliar fertilization effects on essential oil composition of sweet basil (Ocimum basilicum L.) cultivated in a field system. Sci. Hortic. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Borrell, P.; Meusburger, K.; Klik, A.; Rousseva, S.; Tadić, M.P.; Michaelides, S.; Hrabalíková, M.; Olsen, P.; et al. Rainfall erosivity in Europe. Sci. Total Environ. 2015, 511, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagos, P.; Meusburger, K.; Ballabio, C.; Borrelli, P.; Alewell, C. Soil erodibility in Europe: A high-resolution dataset based on LUCAS. Sci. Total Environ. 2014, 480, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, P.; Farto, M.; Mourinha, C.; Palma, P. Beneficial use of dewatered and composted sewage sludge as soil amendments: Behaviour of metals in soils and their uptake by plants. Waste Biomass Valoriz. 2016, 7, 1189–1201. [Google Scholar] [CrossRef]
- Holz, S.C.; Ingelo, F.; Canet, R. Long term effect of the application of sewage sludge and vegetal cover on some physical and physiochemical properties of a degraded soil. Agrochimica 2000, 44, 132–139. [Google Scholar]
- Ros, M.; Hernandez, M.T.; García, C. Bioremediation of soil degraded by sewage sludge: Effects on soil properties and erosion losses. Environ. Manag. 2003, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Bai, Y.; Tao, T.; Chen, G.; Shan, Y. Effect of sewage sludge amendment on heavy metal uptake and yield of ryegrass seedling in a mudflat soil. J. Environ. Qual. 2013, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, M.; Ilie, L.; Madjar, R. Translocation of heavy metals from sewage sludge amended soil to plant. Rev. Roum. Chim. 2014, 59, 81–89. [Google Scholar]
- Kelessidis, A.; Stasinakis, A.S. Review Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Cornfield, A.H.; Beckett, P.H.T.; Davis, R.D. Effect of sewage sludge on mineralisation of organic carbon in soil. Nature 1976, 260, 518–520. [Google Scholar] [CrossRef]
- Vaca, R.; Lugo, J.; Martínez, R.; Esteller, M.V.; Zavaleta, H. Effects of sewage sludge and sewage sludge compost amendment on soil properties and Zea mays L. plants (heavy metals, quality and productivity). Rev. Int. Contam. Ambient. 2011, 27, 303–311. [Google Scholar]
- Özyazıcı, M.A. Effects of sewage sludge on the yield of plants in the rotation system of wheat-white head cabbage-tomato. Eurasian J. Soil Sci. 2013, 2, 35–44. [Google Scholar]
- Chrysargyris, A.; Tzortzakis, N. Municipal solid wastes and mineral fertilizer as an eggplant transplant medium. J. Soil Sci. Plant Nutr. 2015, 15, 11–23. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Mazen, A.; Faheed, F.A.; Ahmed, A.F. Study of potential impacts of using sewage sludge in the amendment of desert reclaimed soil on wheat and jews mallow plants. Braz. Arch. Biol. Technol. 2010, 53, 917–930. [Google Scholar] [CrossRef] [Green Version]
- Vaitkutė, D.; Baltrėnaitė, E.; Booth, C.A.; Fullen, M.A. Does sewage sludge amendment to soil enhance the development of Silver birch and Scots pine? Hung. Geogr. Bull. 2010, 59, 393–410. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Padovani, S.; Frascarolo, M.; Sciunnach, D.; Turconi, M.; Orlando, M. Recovery of sewage sludge on agricultural land in Lombardy: Current issues and regulatory scenarios. Environ. Eng. Manag. J. 2015, 14, 1477–1486. [Google Scholar]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Smith, B.R. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Phil. Trans. R. Soc. A 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [Green Version]
- Burducea, M.; Trofin, O.; Stoleru, T.; Lobiuc, A.; Teliban, G.C.; Onofrei, V.; Zamfirache, M.M. On the agricultural use of sewage sludge in Romania. Agron. Ser. Sci. Res. 2016, 59, 147–150. [Google Scholar]
- Zheljazkov, V.D.; Warman, P.R. Source-separated municipal solid waste compost application to Swiss chard and basil. J. Environ. Qual. 2004, 33, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirachea, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Badulescu, B.; Mircea, S.; Chitu, Z. Assessment of soil erosion risk using gis techniques, in Romanian Subcarpathian Mountains. Int. Multidiscip. Sci. GeoConference SGEM 2017, 32, 39–42. [Google Scholar]
- Eurostat. Available online: http://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 18 August 2018).
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Phys. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Shaltouta, A.A.; Allama, M.A.; Moharram, M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta Part. A 2011, 83, 56–60. [Google Scholar] [CrossRef]
- Anuradha, G.; Syama, S.B.; Sreekanth, K.J.; Ramana, M.V. Synthesis and characterization of silver nanoparticles from Ocimum basilicum L. var. thyrsiflorum. Eur. J. Acad. Essay. 2014, 1, 5–9. [Google Scholar]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Zhoua, W.; Yanga, K.; Bai, Z.; Chengc, H.; Liu, F. The development of topsoil properties under different reclaimed land uses in the Pingshuo opencast coalmine of Loess Plateau of China. Ecol. Eng. 2017, 100, 237–245. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, S.; Yang, J.; Zhao, X.; Cao, J. Changes in soil microbial community structure following amendment of biosolids for seven years. Environ. Pollut. Bioavailab. 2019, 31, 24–31. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K. Using an Environment-Friendly Fertiliser from Sewage Sludge Ash with the Addition of Bacillus megaterium. Minerals 2019, 9, 423. [Google Scholar] [CrossRef]
- Vieira, R.F.; Pazianotto, R.A.A. Microbial activities in soil cultivated with corn and amended with sewage sludge. SpringerPlus 2016, 5, 1844. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintrau, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Costa, M.; Beltrao, J.; Carrasco De Brito, J.; Neves, M.A.; Guerrero, C. The sludge and manure effects on the growth of citrus trees. WSEAS Trans. Environ. Dev. 2010, 6, 721–730. [Google Scholar]
- Kashani, A.; Pirdashti, H.; Kashani, K. Effects of four year sewage sludge application on some morphological traits and chlorophyll content in basil (Ocimum basilicum L.). J. Agric. Technol. 2013, 9, 451–460. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- Oleszczuk, P.; Malara, A.; Jośko, I.; Lesiuk, A. The phytotoxicity changes of sewage sludge-amended soils. Water Air Soil Pollut. 2012, 223, 4937–4948. [Google Scholar] [CrossRef]
- Przewrocki, P.; Kulczycka, J.; Wzorek, Z.; Kowalski, Z.; Gorazda, K.; Jodko, M. Risk analysis of sewage sludge—Poland and EU comparative approach. Polish J. Environ. Stud. 2004, 13, 237–244. [Google Scholar]
- Shipley, B.; Vu, T.T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002, 153, 359–364. [Google Scholar] [CrossRef]
- Pengcheng, G.; Xinbao, T.; Yanan, T.; Yingxu, C. Application of sewage sludge compost on highway embankments. Waste Manag. 2008, 28, 1630–1636. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant. Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Martinez, L.J.; Ramos, M.A. Estimation of chlorophyll concentration in maize using spectral reflectance. Int. Arch. Photogramm Remote Sens. Spat. Inf. Sci. 2015, 40, 65–71. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant. J. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Holert, H. Comparison of sewage sludge toxicity to plants and invertebrates in three different soils. Chemosphere 2011, 83, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Adamcová, D.; Vaverková, M.D.; Břoušková, E. The toxicity of two types of sewage sludge from wastewater treatment plant for plants. J. Ecol. Eng. 2016, 17, 33–37. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Review article applications of chlorophyll fluorescence an improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Augustynowicz, J.; Pietkiewicz, S.; Kalaji, M.H.; Russel, S. The effect of sludge fertilization on choosen parameters of chlorophyll fluorescence and biomass yield of Jerusalem artichoke (Helianthus tuberosus L.). Contemp. Probl. Manag. Environ. Prot. Sew. Wastes Mater. Environ. 2009, 4, 129–139. [Google Scholar]
- Han, S.H.; Lee, J.C.; Jang, S.S.; Kim, P.G. Composted sewage sludge can improve the physiological properties of Betula schmidtii grown in tailings. J. Plant. Biol. 2004, 47, 99–104. [Google Scholar] [CrossRef]
- Tan, L.P.; He, J.; Lee, S.K. Physiological responses of certain ornamental plants to sludge and artificial topsoils derived from flyash, sludge, and rengam series subsoil. J. Plant. Nutr. 1999, 22, 987–999. [Google Scholar] [CrossRef]
- Le Bot, L.; Bénard, C.; Robin, C.; Bourgaud, F.; Adamowicz, S. The ‘trade-off’ between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: Experimental evidence and model consistency. J. Exp. Bot. 2009, 60, 4301–4314. [Google Scholar] [CrossRef]
- Paneque, M.; Knicker, H.; Kern, J.; De la Rosa, J.M. Hydrothermal carbonization and pyrolysis of sewage sludge: Effects on Lolium perenne germination and growth. Agronomy 2019, 9, 363. [Google Scholar] [CrossRef]
| Parameter | Eroded Soil | Sewage Sludge | Eroded Soil + Sewage Sludge |
|---|---|---|---|
| Coarse sand % | 3.5 | - | - |
| Medium sand % | 45.4 | - | - |
| Fine sand % | 22.8 | - | - |
| Clay % | 28.3 | - | - |
| Physical clay % | 37.3 | - | - |
| pH | 8.05 | 6.96 | 7.5 |
| E.C. µS/cm (electrical conductivity) | 221.4 | 6280 | 5200 |
| O.M. % (organic matter) | 1.24 | 26.6 | 20.5 |
| Total N % | 0.068 | 4.83 | 3.7 |
| P2O5 % | 0.019 | 2.43 | 2.09 |
| K2O % | 0.023 | 0.51 | 0.35 |
| Heavy metals (mg/kg) | |||
| Co | 2.53 | 2.07 | 2.5 |
| Ni | 7.56 | 9.38 | 8.4 |
| Cu | 4.67 | 32.07 | 20 |
| Zn | 13.6 | 952.1 | 502 |
| As | 6.17 | 7.09 | 6.2 |
| Cd | 0.06 | 0.55 | 0.12 |
| Hg | - | 0.18 | - |
| Pb | 0.32 | 1.5 | 0.65 |
| Al | 246 | 271 | 255 |
| Cr | 20.3 | 27.0 | 20.3 |
| Parameter | Eroded Soil | Eroded Soil + Sewage Sludge | Sewage Sludge |
|---|---|---|---|
| Height (cm) | 28.56 ± 0.53 a | 38.64 ± 0.55 b | 28.64 ± 0.96 a |
| Fresh weight (g) | 8.75 ± 0.18 a | 11 ± 0.1 b | 8.03 ± 0.53 a |
| Root length (cm) | 5.83 ± 1.09 a | 7.33 ± 0.33 a | 4.33 ± 0.6 a |
| Parameter | Eroded Soil | Eroded Soil + Sewage Sludge | Sewage Sludge |
|---|---|---|---|
| ΦPS2 | 0.83 ± 0 a | 0.82 ± 0 a | 0.84 ± 0 a |
| Fv/Fm | 0.80 ± 0 a | 0.83 ± 0 b | 0.82 ± 0 a |
| Parameter | Eroded Soil | Eroded Soil + Sewage Sludge | Sewage Sludge |
|---|---|---|---|
| chlorophyll a | 0.84 ± 0.06 a | 0.91 ± 0.05 a | 1.02 ± 0.04 a |
| chlorophyll b | 0.44 ± 0.01 a | 0.51 ± 0.02 b | 0.61 ± 0.01 c |
| carotenoids | 0.18 ± 0.02 a | 0.19 ± 0.01 ab | 0.27 ± 0.02 c |
| Parameter | Eroded Soil | Eroded Soil + Sewage Sludge | Sewage Sludge | Control ASCORBIC Acid (1mM) |
|---|---|---|---|---|
| Total phenols (GAE/g f.w.) | 7.34 ± 0.03 b | 7.18 ± 0.11 b | 5.17 ± 0.16 a | - |
| Total flavonoids (mg quercetin eq./g f.w.) | 14.41 ± 0.23 c | 7.04 ± 0.11 b | 5.07 ± 0.25 a | - |
| DPPH radical Scavenging activity (%) | 44.37 ± 0.47 b | 44.02 ± 0.51 b | 25.98 ± 1.22 a | 91.97 ± 0.16 |
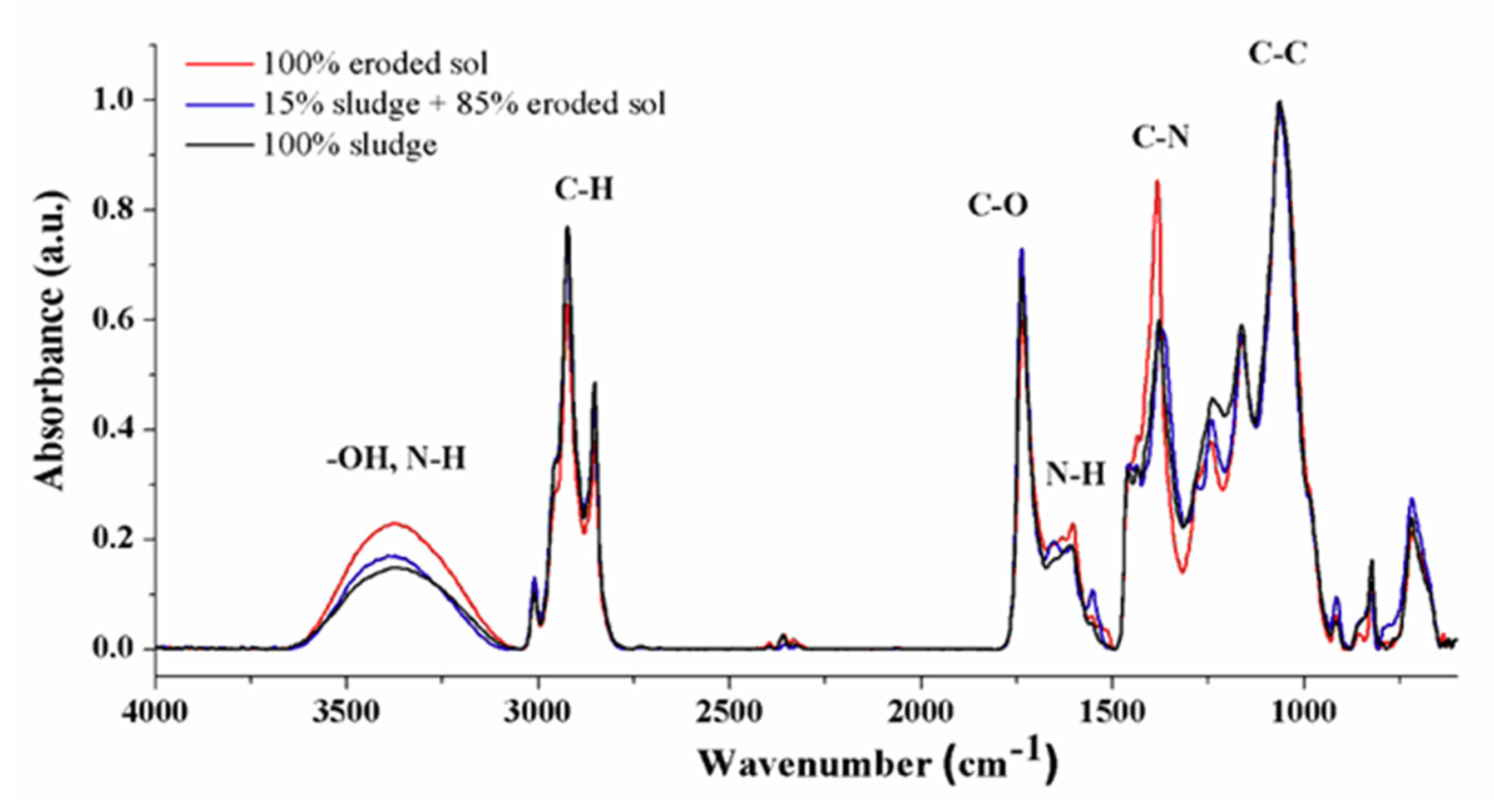
| Wavenumber (cm−1) | Chemical Group |
|---|---|
| 3620–3080 | -OH stretching vibrations from alcohols and phenols, N-H bond of amino type |
| 2918, 2853 | To aliphatic ν(C-H) from methyl and methylene functional groups |
| 1737 | Stretching vibrations of carbonyl C=O |
| 1610 | N-H bending of the primary amine |
| 1379 | C-N stretching of aromatic amine |
| 1063 | C-C stretching of alcohols |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burducea, M.; Lobiuc, A.; Asandulesa, M.; Zaltariov, M.-F.; Burducea, I.; Popescu, S.M.; Zheljazkov, V.D. Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil. Agronomy 2019, 9, 548. https://doi.org/10.3390/agronomy9090548
Burducea M, Lobiuc A, Asandulesa M, Zaltariov M-F, Burducea I, Popescu SM, Zheljazkov VD. Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil. Agronomy. 2019; 9(9):548. https://doi.org/10.3390/agronomy9090548
Chicago/Turabian StyleBurducea, Marian, Andrei Lobiuc, Mihai Asandulesa, Mirela-Fernanda Zaltariov, Ion Burducea, Simona Mariana Popescu, and Valtcho D. Zheljazkov. 2019. "Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil" Agronomy 9, no. 9: 548. https://doi.org/10.3390/agronomy9090548






