Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials and Field Experiments
2.2. Yield and Fiber Trait Evaluation
2.3. Statistical Analysis
3. Results
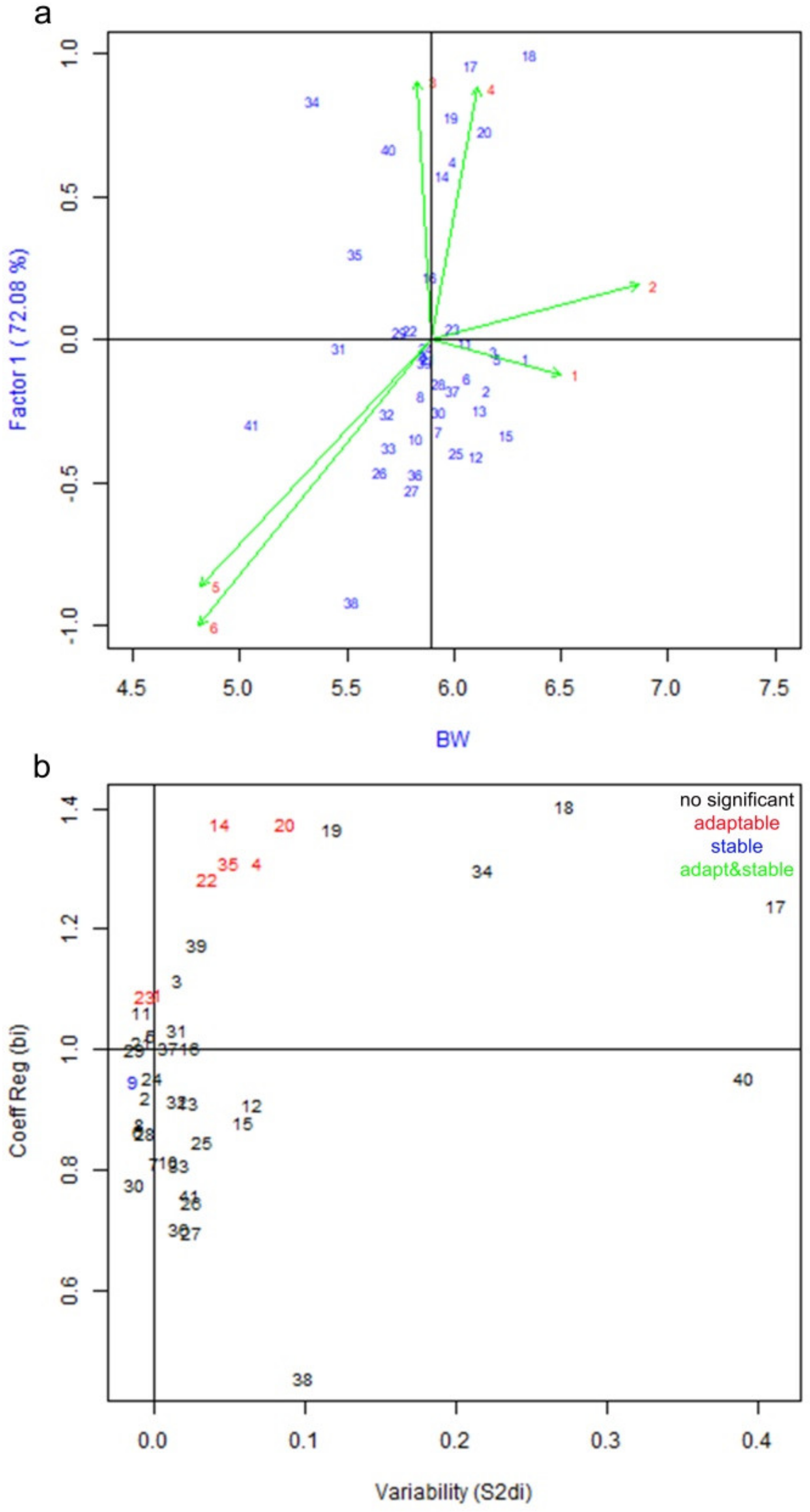
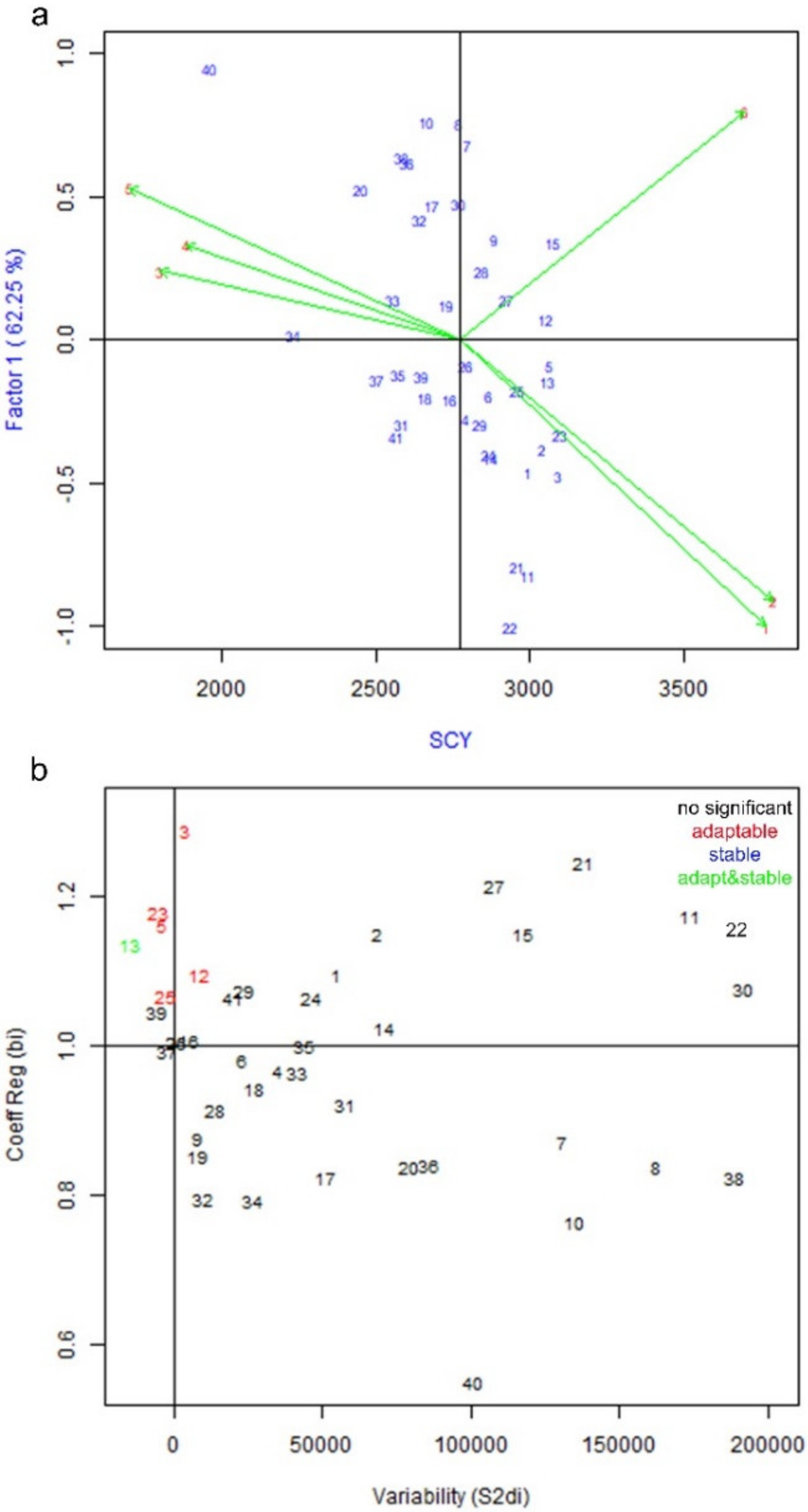
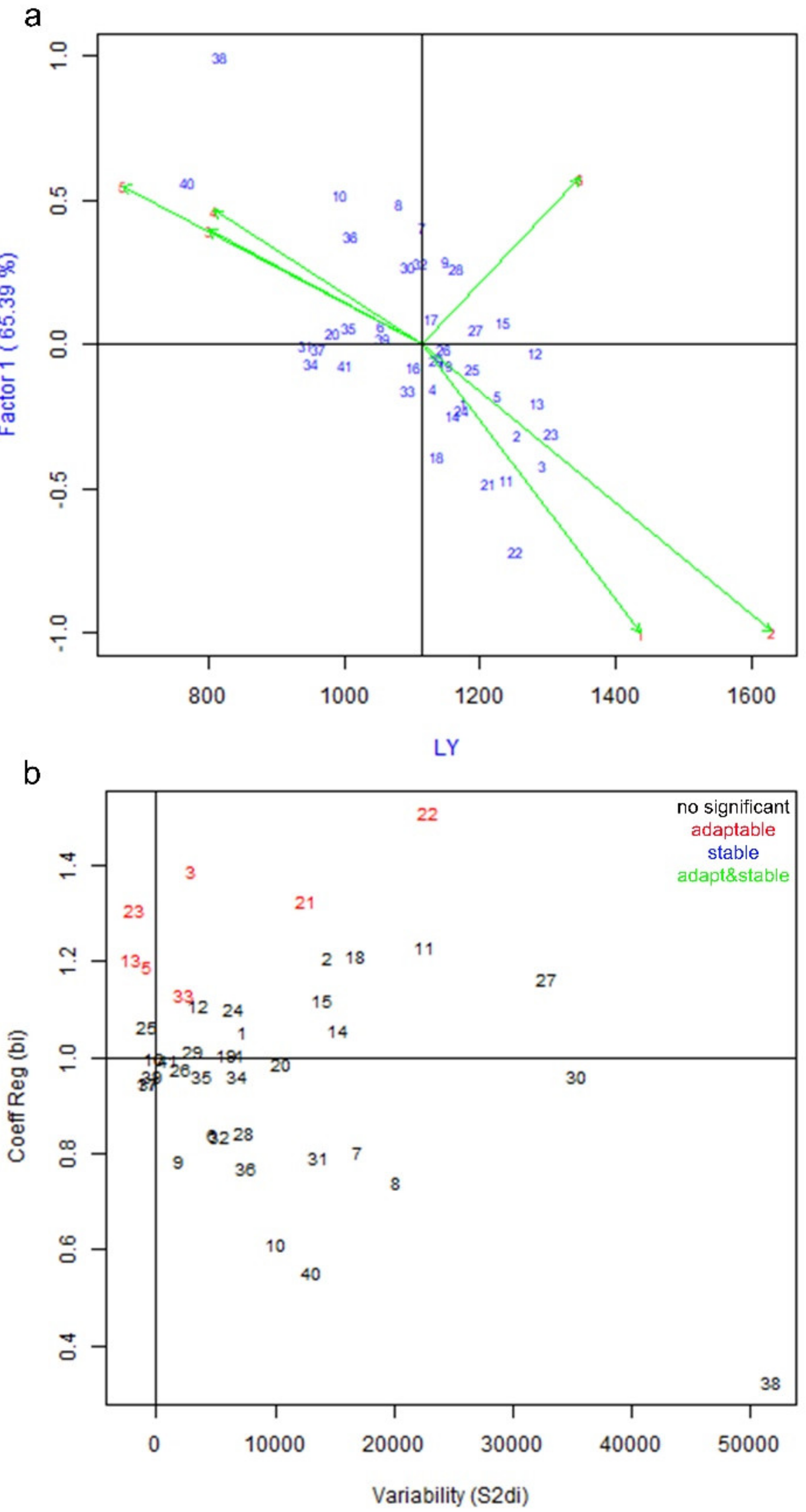
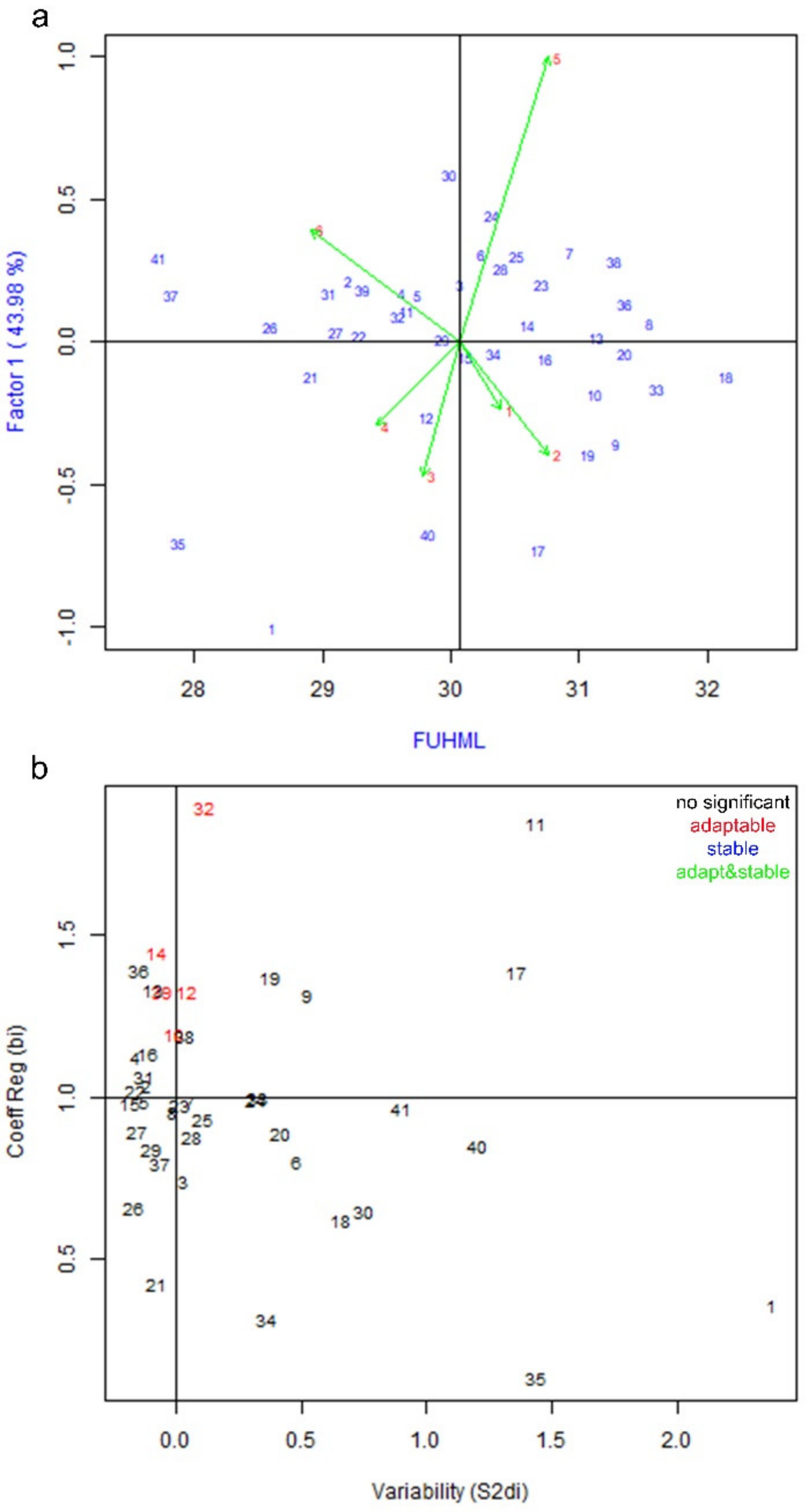
3.1. Genotype by Environment Interaction and Stability Analysis of Yield Parameters
3.2. Genotype by Environment Interaction and Stability Analysis of Fiber Quality Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Shahzad, K.; Guo, L.; Qi, T.; Zhang, X.; Wang, H.; Tang, H.; Qiao, X.; Zhang, J.; Wu, J. Using yield quantitative trait locus targeted SSR markers to study the relationship between genetic distance and yield heterosis in upland cotton (Gossypium hirsutum). Plant Breed. 2019, 138, 105–113. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef]
- Duvick, D.N. Biotechnology in the 1930s: The development of hybrid maize. Nat. Rev. Genet. 2001, 2, 69. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Yu, J.; Li, X.; Pei, W. Breeding potential of introgression lines developed from interspecific crossing between upland cotton (Gossypium hirsutum) and Gossypium barbadense: Heterosis, combining ability and genetic effects. PLoS ONE 2016, 11, e0143646. [Google Scholar] [CrossRef]
- Xing, C.; Jing, S.; Xing, Y. Review and Prospect on Cotton Heterosis Utilization and Study in China. Cotton Sci. 2007, 5, 337–345. [Google Scholar]
- Dong, H.; Li, W.; Tang, W.; Zhang, D. Development of hybrid Bt cotton in China–a successful integration of transgenic technology and conventional techniques. Curr. Sci. 2004, 86, 778–782. [Google Scholar]
- Kang, M.S. Using genotype-by-environment interaction for crop cultivar development. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1997; Volume 62, pp. 199–252. [Google Scholar]
- Yue, G.; Roozeboom, K.; Schapaugh, W., Jr.; Liang, G. Evaluation of soybean cultivars using parametric and nonparametric stability estimates. Plant Breed. 1997, 116, 271–275. [Google Scholar] [CrossRef]
- Scapim, C.A.; Oliveira, V.R.; Cruz, C.D.; Andrade, C.A.d.B.; Vidigal, M.C.G. Yield stability in maize (Zea mays L.) and correlations among the parameters of the Eberhart and Russell, Lin and Binns and Huehn models. Genet. Mol. Biol. 200, 23, 387–393. [Google Scholar] [CrossRef]
- Tai, G.C. Genotypic stability analysis and its application to potato regional trials. Crop Sci. 1971, 11, 184–190. [Google Scholar] [CrossRef]
- Eberhart, S.T.; Russell, W. Stability parameters for comparing varieties 1. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Becker, H.; Leon, J. Stability analysis in plant breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Hristov, N.; Mladenov, N.; Djuric, V.; Kondic-Spika, A.; Marjanovic-Jeromela, A.; Simic, D. Genotype by environment interactions in wheat quality breeding programs in southeast Europe. Euphytica 2010, 174, 315–324. [Google Scholar] [CrossRef]
- Hoeck, J.A.; Fehr, W.R.; Murphy, P.A.; Welke, G.A. Influence of genotype and environment on isoflavone contents of soybean. Crop Sci. 2000, 40, 48–51. [Google Scholar] [CrossRef]
- Anley, W.; Zeleke, H.; Dessalegn, Y. Genotype × environment interaction of maize (Zea mays L.) across North Western Ethiopia. J. Plant Breed. Crop Sci. 2013, 5, 171–181. [Google Scholar] [CrossRef]
- Akter, A.; Hasan, M.J.; Kulsum, M.; Rahman, M.; Paul, A.; Lipi, L.; Akter, S. Genotype× environment interaction and yield stability analysis in hybrid rice (Oryza sativa L.) by AMMI biplot. Bangladesh Rice J. 2015, 19, 83–90. [Google Scholar] [CrossRef]
- Miller, P.; Williams, J.; Robinson, H.; Comstock, R. Estimates of Genotypic and Environmental Variances and Covariances in Upland Cotton and Their Implications in Selection 1. Agron. J. 1958, 50, 126–131. [Google Scholar] [CrossRef]
- Miller, P.; Robinson, H.; Pope, O. Cotton Variety Testing: Additional Information on Variety × Environment Interactions 1. Crop Sci. 1962, 2, 349–352. [Google Scholar] [CrossRef]
- Pope, O.A.; Ware, J.O. Effect of Variety, Location, and Season on Oil, Protein, and Fuzz of Cottonseed and on Fiber Properties of Lint; US Department of Agriculture: Washington, DC, USA, 1945.
- Meredith, W., Jr.; Boykin, D.L.; Bourland, F.M.; Caldwell, W.D.; Campbell, B.T.; Gannaway, J.; Glass, K.; Jones, A.P.; May, L.M.; Smith, C. Genotype × environment interactions over seven years for yield, yield components, fiber quality, and gossypol traits in the regional high quality tests. J. Cotton Sci. 2012, 16, 160–169. [Google Scholar]
- Meredith, W.R., Jr. Thirty-six years of regional high quality variety tests. In Proceedings of the Beltwide Cotton Conferences, New Orleans, LA, USA, 7–9 January 2003; pp. 2561–2566. [Google Scholar]
- Campbell, B.; Jones, M. Assessment of genotype × environment interactions for yield and fiber quality in cotton performance trials. Euphytica 2005, 144, 69–78. [Google Scholar] [CrossRef]
- Campbell, B.; Chee, P.; Lubbers, E.; Bowman, D.; Meredith, W.; Johnson, J.; Fraser, D.; Bridges, W.; Jones, D. Dissecting genotype× environment interactions and trait correlations present in the Pee Dee cotton germplasm collection following seventy years of plant breeding. Crop Sci. 2012, 52, 690–699. [Google Scholar] [CrossRef]
- Bauer, P.J.; McAlister, D.D., III; Frederick, J.R. A comparison of Bollgard/Glyphosate tolerant cotton cultivars to their conventional parents for open end yarn processing performance. J. Cotton Sci. 2006, 10, 168–179. [Google Scholar]
- Kerby, T.; Burgess, J.; Bates, M.; Alberts, D.; Lege, K. Partitioning variety and environment contribution to variation in yield, plant growth, and fiber quality. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 4–8 January 2000; pp. 528–532. [Google Scholar]
- Lin, C.-S.; Binns, M.R. A superiority measure of cultivar performance for cultivar× location data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- Kang, M.; Pham, H. Simultaneous selection for high yielding and stable crop genotypes. Agron. J. 1991, 83, 161–165. [Google Scholar] [CrossRef]
- Farshadfar, E.; Sabaghpour, S.H.; Zali, H. Comparison of parametric and non-parametric stability statistics for selecting stable chickpea (‘Cicer arietinum’L.) genotypes under diverse environments. Aust. J. Crop Sci. 2012, 6, 54. [Google Scholar]
- Kulsum, M.U.; Hasan, M.J.; Akter, A.; Rahman, H.; Biswas, P. Genotype-environment interaction and stability analysis in hybrid rice: An application of additive main effects and multiplicative interaction. Bangladesh J. Bot. 2013, 42, 73–82. [Google Scholar] [CrossRef]
- Elbasyoni, I. Performance and stability of commercial wheat cultivars under terminal heat stress. Agronomy 2018, 8, 37. [Google Scholar] [CrossRef]
- Crossa, J.; Gauch, H.; Zobel, R.W. Additive main effects and multiplicative interaction analysis of two international maize cultivar trials. Crop Sci. 1990, 30, 493–500. [Google Scholar] [CrossRef]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Gauch, H.; Zobel, R.W. Identifying mega-environments and targeting genotypes. Crop Sci. 1997, 37, 311–326. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Chen, C.; McPhee, K.; Miller, P.; McVay, K.; Eckhoff, J.; Lamb, P.; Miller, J.; Khan, Q.; Bohannon, B. Yield performance and stability of dry pea and lentil genotypes in semi-arid cereal dominated cropping systems. Field Crop. Res. 2016, 188, 31–40. [Google Scholar] [CrossRef]
- Pinthus, M.J. Estimate of genotypic value: A proposed method. Euphytica 1973, 22, 121–123. [Google Scholar] [CrossRef]
- Feng, L.; Dai, J.; Tian, L.; Zhang, H.; Li, W.; Dong, H. Review of the technology for high-yielding and efficient cotton cultivation in the northwest inland cotton-growing region of China. Field Crop. Res. 2017, 208, 18–26. [Google Scholar] [CrossRef]
- Shuxun, Y. Present situation and development trend of cotton production in China. Eng. Sci. 2013, 4, 9–13. [Google Scholar]
- Gollob, H.F. A statistical model which combines features of factor analytic and analysis of variance techniques. Psychometrika 1968, 33, 73–115. [Google Scholar] [CrossRef]
- Pacheo, A.; Vargas, M.; Alvarado, G.; Rodriguez, F.; Lopez, M.; Crossa, J.; Burgueno, J. GEA-R (Genotype × Environment Analysis with R for Windows); Version 2.0; International Maize and Wheat Improvment Center: Mexico City, Mexico, 2015. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Blanche, S.B.; Myers, G.O.; Zumba, J.Z.; Caldwell, D.; Hayes, J. Stability comparisons between conventional and near-isogenic transgenic cotton cultivars. J. Cotton Sci. 2006, 10, 59–63. [Google Scholar]
- Abou-El-Fittouh, H.; Rawlings, J.O.; Miller, P. Genotype by Environment Interactions in Cotton—Their Nature and Related Environmental Variables 1. Crop Sci. 1969, 9, 377–381. [Google Scholar] [CrossRef]
- Zeng, L.; Meredith, J.; Campbell, B.T.; Dever, J.K.; Zhang, J.; Glass, K.M.; Jones, A.S.; Myers, G.O.; Bourland, F.M. Genotype-by-environment interaction effects on lint yield of cotton cultivars across major regions in the US cotton belt. J. Cotton Sci. 2014, 18, 75–84. [Google Scholar]
- Laghari, S.; Kandhro, M.M.; Ahmed, H.M.; Sial, M.A.; Shad, M. Genotype X Environment (GxE) interactions in Cotton (Gossypium hirsutum L.) Genotypes. Asian J. Plant Sci. 2003, 2, 480–482. [Google Scholar]
- Li, W.; Yan, Z.H.; Wei, Y.M.; Lan, X.J.; Zheng, Y.L. Evaluation of genotype× environment interactions in Chinese spring wheat by the AMMI model, correlation and path analysis. J. Agron. Crop Sci. 2006, 192, 221–227. [Google Scholar] [CrossRef]
- Fan, X.-M.; Kang, M.S.; Chen, H.; Zhang, Y.; Tan, J.; Xu, C. Yield stability of maize hybrids evaluated in multi-environment trials in Yunnan, China. Agron. J. 2007, 99, 220–228. [Google Scholar] [CrossRef]
- Nzuve, F.; Githiri, S.; Mukunya, D.; Gethi, J. Analysis of genotype x environment interaction for grain yield in maize hybrids. J. Agric. Sci. 2013, 5, 75. [Google Scholar] [CrossRef]
- Dwivedi, S.; Nigam, S.; Jambunathan, R.; Sahrawat, K.; Nagabhushanam, G.; Raghunath, K. Effect of genotypes and environments on oil content and oil quality parameters and their correlation in peanut (Arachis hypogaea L.). Peanut Sci. 1993, 20, 84–89. [Google Scholar] [CrossRef]
- Gauch, H.G. A simple protocol for AMMI analysis of yield trials. Crop Sci. 2013, 53, 1860–1869. [Google Scholar] [CrossRef]
- Baxevanos, D.; Goulas, C.; Tzortzios, S.; Mavromatis, A. Interrelationship among and repeatability of seven stability indices estimated from commercial cotton (Gossypium hirsutum L.) variety evaluation trials in three Mediterranean countries. Euphytica 2008, 161, 371–382. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Bassi, F.; Sanchez-Garcia, M. Adaptation and Stability Analysis of ICARDA Durum Wheat Elites across 18 Countries. Crop Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
- Bach, S.; Yada, R.Y.; Bizimungu, B.; Sullivan, J.A. Genotype by environment interaction effects on fibre components in potato (Solanum tuberosum L.). Euphytica 2012, 187, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.; Said, A. Stability parameters for comparing bread wheat genotypes under combined heat and drought stress. Egypt. J. Agron. 2014, 36, 123–146. [Google Scholar]
- Mosavi, A.A.; Jelodar, N.B.; Kazemitabar, K. Environmental responses and stability analysis for grain yield of some rice genotypes. World Appl. Sci. J. 2013, 21, 105–108. [Google Scholar]
- Witcombe, J. Estimates of stability for comparing varieties. Euphytica 1988, 39, 11–18. [Google Scholar] [CrossRef]
- Bourland, F.M. Yield and fiber quality of transgenic vs. conventional cotton varieties in the Arkansas cotton variety tests, 1995–2004. In Proceedings of the Beltwide Cotton Conference, New Orleans, LA, USA, 4–7 January 2005; National Cotton Council of America: Memphis, TN, USA, 2005; pp. 1002–1006. [Google Scholar]
- Blanche, S.B.; Utomo, H.S.; Wenefrida, I.; Myers, G.O. Genotype× environment interactions of hybrid and varietal rice cultivars for grain yield and milling quality. Crop Sci. 2009, 49, 2011–2018. [Google Scholar] [CrossRef]
- Li, Z.; Coffey, L.; Garfin, J.; Miller, N.D.; White, M.R.; Spalding, E.P.; De Leon, N.; Kaeppler, S.M.; Schnable, P.S.; Springer, N.M. Genotype-by-environment interactions affecting heterosis in maize. PLoS ONE 2018, 13, e0191321. [Google Scholar] [CrossRef]
- Nyombayire, A.; Derera, J.; Sibiya, J.; Ngaboyisonga, C. Genotype × Environment Interaction and Stability Analysis for Gran Yield of Diallel Cross Maize Hybrids Across Tropical Medium and Highland Ecologies. J. Plant Sci. 2018, 6, 101–106. [Google Scholar]

| Genotypes | Codes | Genotypes | Codes |
|---|---|---|---|
| Zhong901-19 × GC-8 | 1 | L28-2 × Z98-15 | 22 |
| L28-2 × GC-8 | 2 | SJ48-1 × Z98-15 | 23 |
| SJ48-1 × GC-8 | 3 | ZB-1 × Z98-15 | 24 |
| ZB-1 × GC-8 | 4 | K8-1 × Z98-15 | 25 |
| K8-1 × GC-8 | 5 | Zhong901-19 × RP24-10 | 26 |
| Zhong901-19 × 851-2 | 6 | L28-2 × RP24-10 | 27 |
| L28-2 × 851-2 | 7 | SJ48-1 × RP24-10 | 28 |
| SJ48-1 × 851-2 | 8 | ZB-1 × RP24-10 | 29 |
| ZB-1 × 851-2 | 9 | K8-1 × RP24-10 | 30 |
| K8-1 × 851-2 | 10 | Zhong901-19 | 31 |
| Zhong901-19 × A2-10 | 11 | L28-2 | 32 |
| L28-2 × A2-10 | 12 | SJ48-1 | 33 |
| SJ48-1 × A2-10 | 13 | ZB-1 | 34 |
| ZB-1 × A2-10 | 14 | K8-1 | 35 |
| K8-1 × A2-10 | 15 | GC-8 | 36 |
| Zhong901-19 × DT-8 | 16 | 851-2 | 37 |
| L28-2 × DT-8 | 17 | A2-10 | 38 |
| SJ48-1 × DT-8 | 18 | DT-8 | 39 |
| ZB-1 × DT-8 | 19 | Z98-15 | 40 |
| K8-1 × DT-8 | 20 | RP24-10 | 41 |
| Zhong901-19 × Z98-15 | 21 |
| Trial Codes | Field Locations | MT (°C) | MMT (°C) | MmT (°C) | TP (mm) | TSS (hours) |
|---|---|---|---|---|---|---|
| 1 | 2016Anyang | 23.16 | 29.27 | 17.94 | 504.7 | 1130.9 |
| 2 | 2017Anyang | 23.49 | 29.76 | 18.01 | 290.5 | 1201.4 |
| 3 | 2016Alar | 21 | 28.96 | 13.92 | 97.4 | 1609.2 |
| 4 | 2017Alar | 20.46 | 28.98 | 13.07 | 75.9 | 1563.1 |
| 5 | 2016Wuwei | 24.1 | 28.04 | 20.92 | 1426.2 | 882.3 |
| 6 | 2017Wuwei | 24.31 | 28.34 | 21.02 | 734.8 | 929.4 |
| Source | DF | BN | BW | SCY | LY | LP |
|---|---|---|---|---|---|---|
| E | 5 | 184,544 *** (97.6%) | 448.7 *** (81.5%) | 705,895,298 *** (88%) | 99,554,257 *** (81.9%) | 5946.1 *** (67.5%) |
| G | 40 | 1737 *** (0.91%) | 49.8 *** (9%) | 41,447,190 *** (5.2%) | 10,953,851 *** (9%) | 1754.6 *** (19.9%) |
| G × E | 200 | 2810 *** (1.48) | 54.4 *** (9.8%) | 54,981,190 *** (6.9%) | 11,053,169 *** (9.1%) | 1108.1 *** (12.6%) |
| IPC1 | 44 | 2202 *** (78.4%) | 39.2 *** (72%) | 34,223,281 *** (62.2%) | 7,228,074 *** (65.4) | 852.6 *** (76.5%) |
| IPC2 | 42 | 335 *** (11.9%) | 9.3 *** (17%) | 12,359,426 *** (22.5%) | 2,305,268 *** (20.9%) | 154.9 *** (13.9) |
| IPC3 | 40 | 239 *** (8.5%) | 3.5 ** (6.4%) | 5,653,926 *** (10.3%) | 941,370.6 *** (8.5%) | 45.1 ns (4.1%) |
| Residual | 492 | 1211.5 | 24.2 | 23,658,785 | 3,981,410 | 429.14933 |
| Source | DF | FUHML | FE | FU | FS | MIC |
|---|---|---|---|---|---|---|
| E | 5 | 353.7 *** (23.6%) | 2.11 *** (37.3%) | 639.9 *** (45.7%) | 1178.6 *** (40.6%) | 62.1 *** (47.4%) |
| G | 40 | 844.3 *** (56.3%) | 1.87 *** (33.2%) | 395.8 *** (28.2%) | 1314.4 *** (45.2%) | 36 *** (27.5%) |
| G × E | 200 | 301.7 *** (20.1%) | 1.67 ns (29.5%) | 365.8 *** (26%) | 412.3 *** (14.2%) | 32.9 *** (25%) |
| IPC1 | 44 | 132.7 *** (44%) | 0.78 *** (47%) | 185.2 *** (50.6%) | 140.1 *** (34%) | 19.6 *** (59.6%) |
| IPC2 | 42 | 66 *** (22%) | 0.48 * (29%) | 76.1 ** (20.8% | 116.4 *** (28.2%) | 5.5 *** (16.6%) |
| IPC3 | 40 | 48 ** (16%) | 0.17 ns (10%) | 59.8 * (16.3%) | 79.7 ns (19.3%) | 5 *** (15%) |
| Residual | 492 | 357.3 | 3.94 | 510.40 | 712.8 | 23.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, K.; Qi, T.; Guo, L.; Tang, H.; Zhang, X.; Wang, H.; Qiao, X.; Zhang, M.; Zhang, B.; Feng, J.; et al. Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits. Agronomy 2019, 9, 516. https://doi.org/10.3390/agronomy9090516
Shahzad K, Qi T, Guo L, Tang H, Zhang X, Wang H, Qiao X, Zhang M, Zhang B, Feng J, et al. Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits. Agronomy. 2019; 9(9):516. https://doi.org/10.3390/agronomy9090516
Chicago/Turabian StyleShahzad, Kashif, Tingxiang Qi, Liping Guo, Huini Tang, Xuexian Zhang, Hailin Wang, Xiuqin Qiao, Meng Zhang, Bingbing Zhang, Juanjuan Feng, and et al. 2019. "Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits" Agronomy 9, no. 9: 516. https://doi.org/10.3390/agronomy9090516
APA StyleShahzad, K., Qi, T., Guo, L., Tang, H., Zhang, X., Wang, H., Qiao, X., Zhang, M., Zhang, B., Feng, J., Shahid Iqbal, M., Wu, J., & Xing, C. (2019). Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits. Agronomy, 9(9), 516. https://doi.org/10.3390/agronomy9090516





