Planting Density Induced Changes in Cotton Biomass Yield, Fiber Quality, and Phosphorus Distribution under Beta Growth Model
Abstract
:1. Introduction
2. Materials and Methods
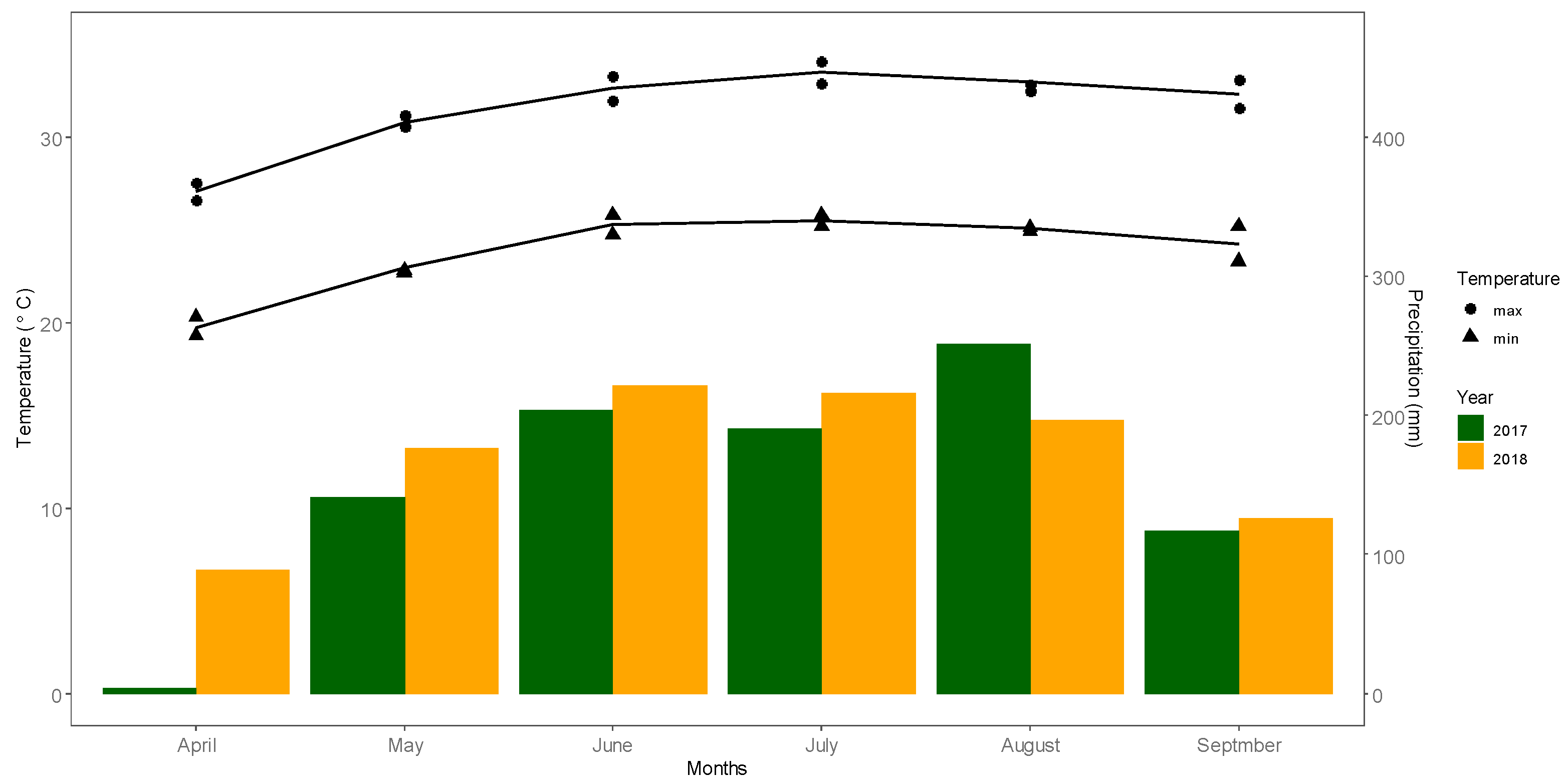
2.1. Summary of Experimental Site and Design
2.2. Agronomic Management
2.3. Data Collection
2.4. Cotton Plant Growth Characteristics
2.5. Biomass Partitioning
2.6. Measurements of Fiber Quality Parameters
2.7. Phosphorus Acquisition
2.8. Statistical Analysis
3. Results
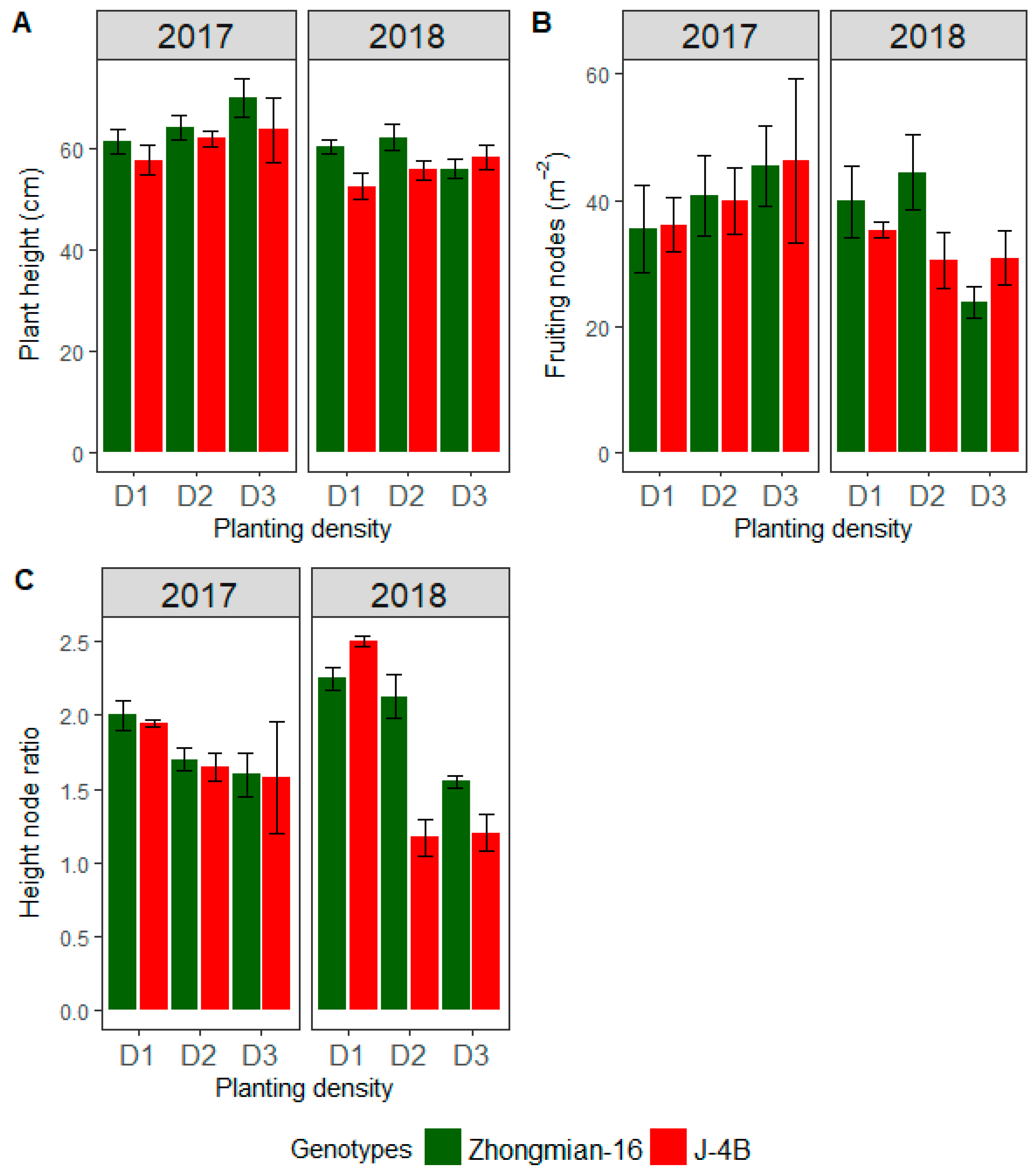
3.1. Cotton Plant Growth Attributes
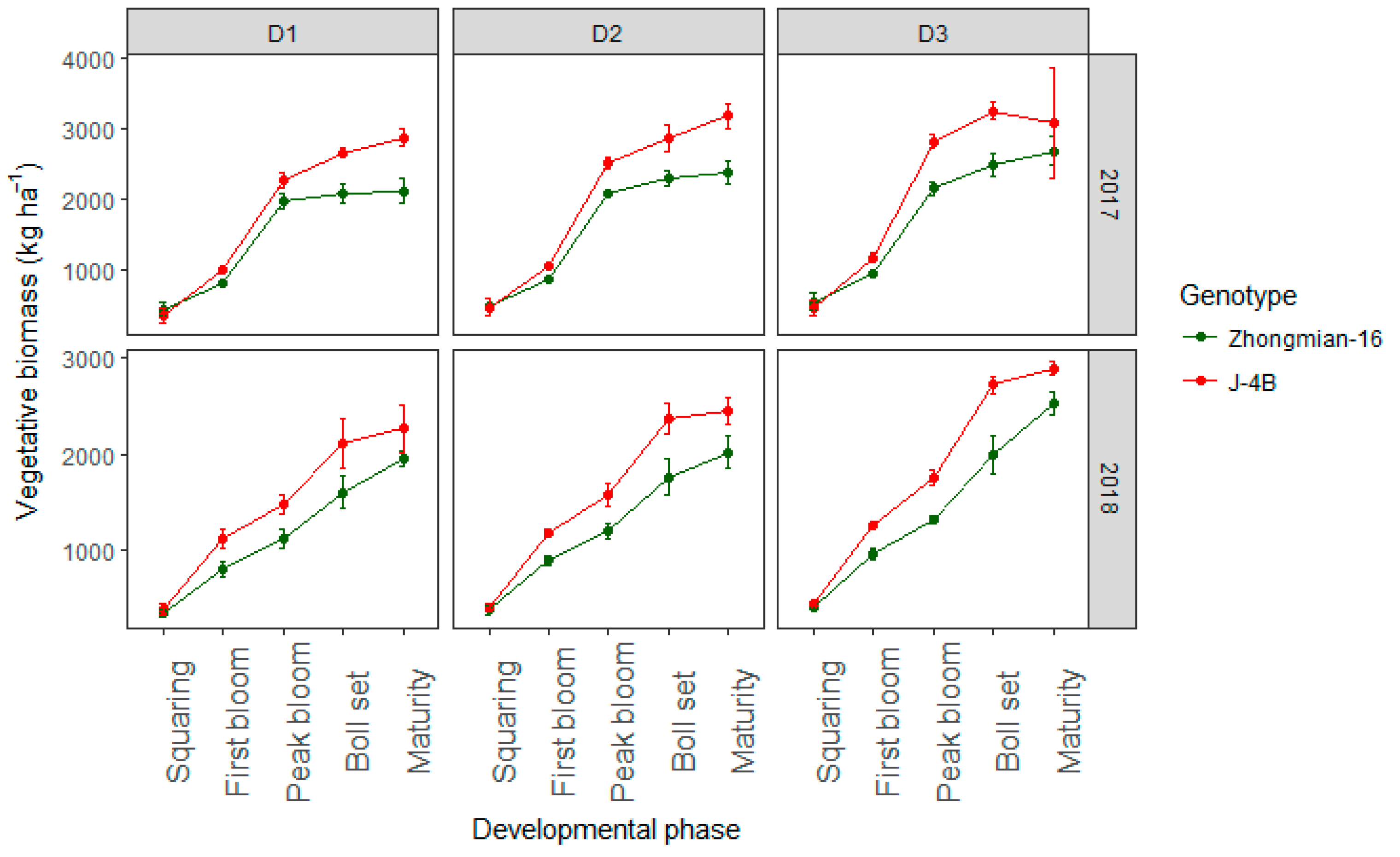
3.2. Biomass Yield
3.3. Fiber Quality
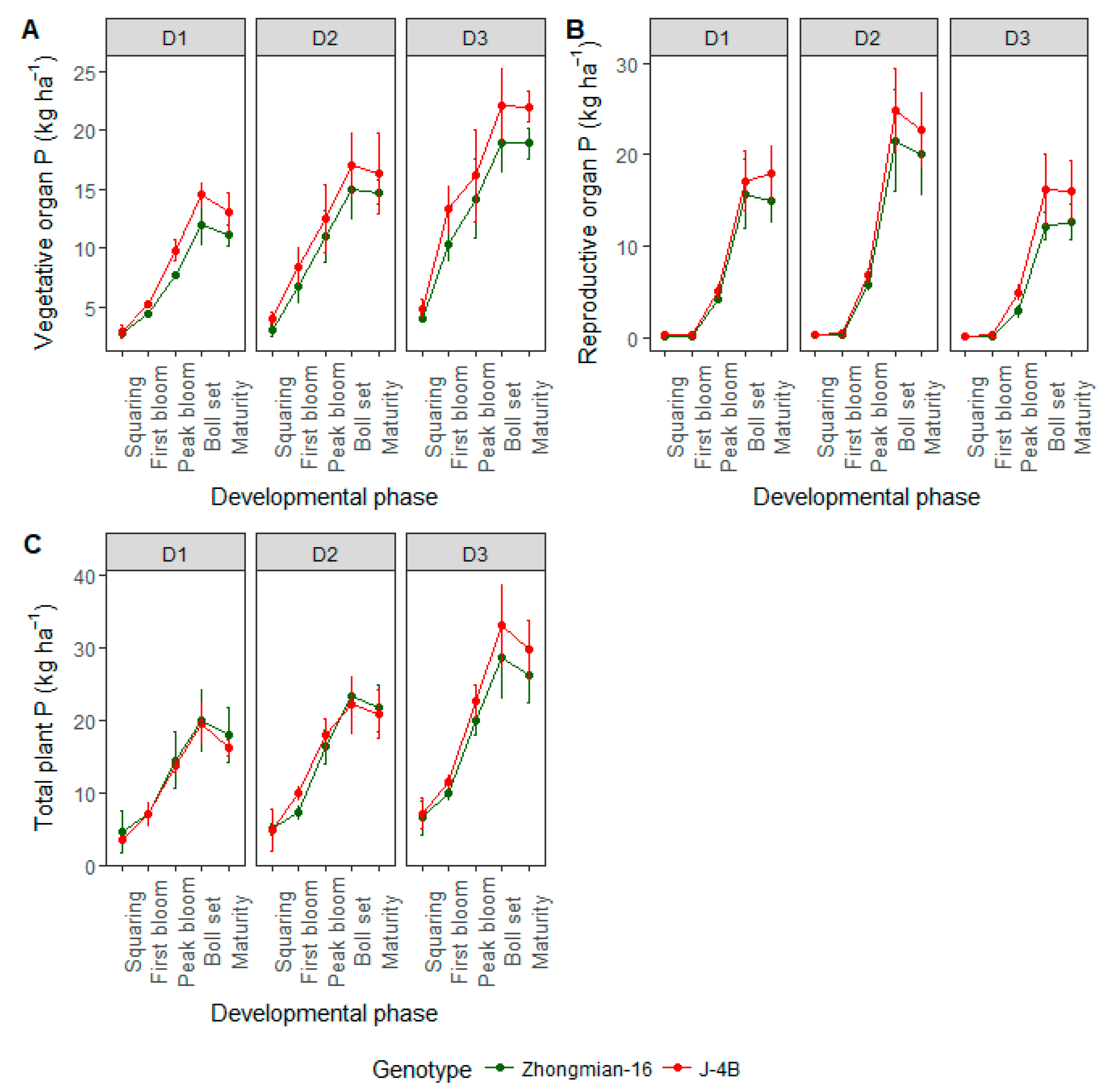
3.4. Phosphorus Accumulation
3.5. Simulation of Phosphorus Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, H.Y.; Deng, F.J.; Li, B.C.; Yang, B.Y.; Yang, L.Y.; Lin, H.; Wang, X.T. Effect of plant density on cotton yield and quality in Xinjiang. Jiangsu J. Agric. Sci. 2009, 4, 98–100. [Google Scholar]
- Dong, H.Z.; Li, W.J.; Eneji, A.E.; Zhang, D.M. Nitrogen rate and plant density effects on yield and late-season leaf senescence of cotton raised on a saline field. Field Crops Res. 2012, 126, 137–144. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, Y.; Zhao, R.; Han, C.; Han, H.; Luo, H. The combination of limited irrigation and high plant density optimizes canopy structure and improves the water use efficiency of cotton. Agric. Water Manag. 2019, 218, 139–148. [Google Scholar] [CrossRef]
- Yang, G.Z.; Jiao, L.X.; Chun, N.Y.; Long, Z.X. Effects of Plant Density on Yield and canopy micro environment in hybrid cotton. J. Integr. Agric. 2014, 13, 2154–2163. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Khan, A.; Souliyanonh, B.; Yang, G. Mepiquat chloride effects on cotton yield and biomass accumulation under late sowing and high density. Field Crops Res. 2018, 215, 59–65. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, X.; Khan, A.; Tan, D.K.Y.; Luo, H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 173. [Google Scholar] [CrossRef]
- Ali, H.; Afzal, M.N.; Ahmad, S.; Muhammad, D. Effect of cultivars and sowing dates on yield and quality of (Gossypium hirsutum L.). Crop Food Agric. Environ. 2009, 7, 244–247. [Google Scholar]
- Khan, A.; Najeeb, U.; Wang, L. Planting density and sowing date strongly influence growth and lint yield of cotton crops. Field Crops Res. 2017, 209, 129–135. [Google Scholar] [CrossRef]
- Tariq, M.; Afzal, M.N.; Muhammad, D.; Ahmad, S.; Shahzad, A.N.; Kiran, A.; Wakeel, A. Relationship of tissue potassium content with yield and fiber quality components of Bt cotton as influenced by potassium application methods. Field Crops Res. 2018, 229, 37–43. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Hofman-Eijer, L.R.B.; Bakker, J.D.; Xue, L.B. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 2004, 55, 2261–2268. [Google Scholar] [CrossRef] [Green Version]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Yang, G.; Tanveer, M.; Iqbal, J. Leaf gas exchange, source—Sink relationship, and growth response of cotton to the interactive effects of nitrogen rate and planting density. Acta Physiol. Plant. 2017, 39, 119. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, J.H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhang, L.; Sun, X.; van der Werf, W.; Evers, J.B.; Zhao, X.; Li, Z. Use of the beta growth function to quantitatively characterize the effects of plant density and a growth regulator on growth and biomass partitioning in cotton. Field Crops Res. 2018, 224, 28–36. [Google Scholar] [CrossRef]
- Baytar, A.A.; Peynircioğlu, C.; Sezener, V.; Basal, H.; Frary, A.; Frary, A.; Doğanlar, S. Identification of stable QTLs for fiber quality and plant structure in Upland cotton (G. hirsutum L.) under drought stress. Ind. Crops Prod. 2018, 124, 776–786. [Google Scholar] [CrossRef]
- Bradow, J.M.; Bauer, P.J.; Hinojosa, O.; Sassenrath-Cole, G. Quantitation of cotton fibre-quality variations arising from boll and plant growth environments. Eur. J. Agron. 1997, 6, 191–204. [Google Scholar] [CrossRef]
- Yeates, S.J.; Constable, G.A.; McCumstie, T. Irrigated cotton in the tropical dry season. III: Impact of temperature, cultivar and sowing date on fiber quality. Field Crops Res. 2010, 116, 300–307. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Z.; Liu, S.; Li, W.; Dong, H. Effects of deficit irrigation and plant density on the growth, yield and fiber quality of irrigated cotton. Field Crops Res. 2016, 197, 1–9. [Google Scholar] [CrossRef]
- Shu, H.M.; Wang, Y.H.; Zhang, W.J.; Zhou, Z.G. Activity changes of enzymes associated with fiber development and relationship with fiber specific strength in two cotton cultivars. Acta Agron. Sin. 2008, 34, 437–446. [Google Scholar] [CrossRef]
- Cao, T.-V.; Oumarou, P.; Gawrysiak, G.; Klassou, C.; Hau, B. Short-season cotton (Gossypium hirsutum) may be a suitable response to late planting in sub-Saharan regions. Field Crops Res. 2011, 120, 9–20. [Google Scholar] [CrossRef]
- Liu, J.R.; Meng, Y.L.; Chen, B.L.; Zhou, Z.G.; Ma, Y.N.; Lv, F.J.; Chen, J.; Wang, Y.H. Photosynthetic characteristics of the subtending leaf and the relationships with lint yield and fiber quality in the late-planted cotton. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Wang, P.; Li, Y.S.; Wang, G.; Liu, P.; Khan, A. Leaf gas exchange, phosphorus uptake, growth and yield responses of cotton cultivars to different phosphorus rates. Photosynthetica 2018, 56, 1414–1421. [Google Scholar] [CrossRef]
- Wang, J.; Ping, L.; Liu, Z.; Wu, Z.; Li, Y.; Guan, X. Dry matter accumulation and phosphorus efficiency response of cotton cultivars to phosphorus and drought. J. Plant Nutr. 2017, 40, 2349–2357. [Google Scholar]
- Luo, H.H.; Merope, T.M.; Zhang, Y.L. Combining gas exchange and chlorophylla lfuorescence measurements to analyze the photosynthetic activity of drip-irrigated cotton under different soil water deifcits. J. Agric. Sci. 2016, 15, 1256–1266. [Google Scholar]
- Singh, S.K.; Badgujar, G.B.; Reddy, V.R. Effect of phosphorus nutrition on growth and physiology of cotton under ambient and elevated carbon dioxide. J. Agron. Crop Sci. 2013, 199, 436–448. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, C.S.; Li, P.C. Effects of soil olsen-P levels on the dry matter accumulation and carbon and nitrogen metabolism of cotton plant at seedling stage. Cotton Sci. 2016, 6, 609–618. [Google Scholar]
- Khan, A.; Wang, L.; Ali, S.; Tung, S.A.; Hafeez, A.; Yang, G. Optimal planting density and sowing date can improve cotton yield by maintaining reproductive organ biomass and enhancing potassium uptake. Field Crops Res. 2017, 214, 164–174. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, H.; Li, W.; Zhao, Q.; Dai, J.; Tian, L.; Dong, H. Effects of reduced nitrogen rate on cotton yield and nitrogen use efficiency as mediated by application mode or plant density. Field Crops Res. 2018, 218, 150–157. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Yi, X.; Zuo, W.; Lei, Z.; Sui, L.; Zhang, W. Characters in light-response curves of canopy photosynthetic use efficiency of light and N in responses to plant density in field-grown cotton. Field Crops Res. 2017, 203, 192–200. [Google Scholar] [CrossRef]
- Bremer, J.M.; Mulvaney, C.S. Nitrogen Total. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; American Society of Agronomy Inc.; Soil Science Society of America Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Yang, G.; Tang, H.; Nie, Y.; Zhang, X. Response of cotton growth, yield, and biomass to nitrogen split application ratio. Eur. J. Agron. 2011, 35, 164–170. [Google Scholar] [CrossRef]
- Israel, D.W.; Rufty, T.W. Influence of phosphorus nutrition on phosporus and nitrogen utilization efficiencies and associated physiological responses in soybean. Crop Sci. 1988, 28, 954–960. [Google Scholar] [CrossRef]
- Dai, J.; Li, W.; Tang, W.; Zhang, D.; Li, Z.; Lu, H.; Eneji, A.E.; Dong, H. Manipulation of dry matter accumulation and partitioning with plant density in relation to yield stability of cotton under intensive management. Field Crops Res. 2015, 180, 207–215. [Google Scholar] [CrossRef]
- Brodrick, R.; Bange, M.P.; Milroy, S.P.; Hammer, G.L. Physiological determinants of high yielding ultra-narrow row cotton: Biomass accumulation and partitioning. Field Crops Res. 2015, 134, 122–129. [Google Scholar] [CrossRef]
- Darawsheh, M.K.; Chachalis, D.; Aivalakis, G.; Khah, E.M. Cotton row spacing and plant density cropping systems II. Effects on seed cotton yield, boll components and lint quality. J. Food Agric. Environ. 2009, 7, 262–265. [Google Scholar]
- Xue, J.; Li, M.Y.; Ning, S.; Wei-shao, S.; Zhou, Z. Effects of planting density on uptake and utilization of NPK of transgenic cotton. Plant Nutr. Fert. 2013, 19, 179–186. [Google Scholar]
- Singh, S.K.; Reddy, V.R. Combined effects of phosphorus nutrition and elevated carbon dioxide concentration on chlorophyll fluorescence, photosynthesis, and nutrient efficiency of cotton. J. Plant Nutr. Soil Sci. 2014, 177, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.L.; Sheng, J.D.; Jiang, P.A.; Liu, Y.G. Effect of applying different forms and rates of phosphoric fertilizer on phosphorus efficiency and cotton yield. Cotton Sci. 2010, 22, 49–56. [Google Scholar]
- Dannowski, M.; Block, A. Fractal geometry and root system structures of heterogeneous plant communities. Plant Soil 2005, 272, 61–76. [Google Scholar] [CrossRef]
- Yan, B.; Wu, B.; Gao, Y.; Wu, J.; Niu, J.; Xie, Y.; Zhang, Z. Effects of nitrogen and phosphorus on the regulation of nonstructural carbohydrate accumulation, translocation and the yield formation of oilseed flax. Field Crops Res. 2018, 219, 229–241. [Google Scholar] [CrossRef]
- Liu, R.X.; Shi, W.; Xu, L.H.; Yang, C.Q.; Guo, W.Q.; Zhang, P.T. Effects of planting density on dry matter and nitrogen accumulation and distribution of cotton. Jiangsu J. Agric. Sci. 2011, 2, 106–113. [Google Scholar]
- Kaggwa-Asiimwe, R.; Andrade-Sanchez, P.; Wang, G. Plant architecture influences growth and yield response of upland cotton to population density. Field Crops Res. 2013, 145, 52–59. [Google Scholar] [CrossRef]
- Sawan, Z.M. Plant density; plant growth retardants: Its direct and residual effects on cotton yield and fiber properties. Cogent Biol. 2016, 2, 1234959. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Yi, X. Plant density alters nitrogen partitioning among photosynthetic components, leaf photosynthetic capacity and photosynthetic nitrogen use efficiency in field-grown cotton. Field Crops Res. 2015, 184, 39–49. [Google Scholar] [CrossRef]
- Read, J.J.; Reddy, K.R.; Jenkins, J.N. Yield and fiber quality of upland cotton as influenced by nitrogen and potassium nutrition. Eur. J. Agron. 2006, 24, 282–290. [Google Scholar] [CrossRef]
- Jones, M.A.; Wells, R. Fiber yield and quality of cotton grown at two divergent population densities. Crop Sci. 1998, 38, 1190–1195. [Google Scholar] [CrossRef]
- Bednarz, C.W.; Nichols, R.L.; Brown, S.M. Plant density modifications of cotton within-boll yield components. Crop Sci. 2006, 46, 2076–2080. [Google Scholar] [CrossRef]


| Source of Variance | Year | Density | Variety | Density × Variety | Year × Density | Year × Variety | Year × Density × Variety |
|---|---|---|---|---|---|---|---|
| Total biomass (kg ha−1) | |||||||
| Squaring | 34,416.6 * | 16,238 * | 6620.1 ns | 4056.5 ns | 2134.1 ns | 2396.8 ns | 1337.5 ns |
| First bloom | 279,334 ** | 36,703 * | 150,047 ** | 6056.5 ns | 8571 ns | 80,182 ns | 25,960 ns |
| Peak bloom | 3,677,454 ** | 329,578 * | 367,679 * | 41,735 ns | 110,000 ns | 286,066 ns | 86,448 ns |
| Boll set | 1,434,828 * | 2,739,528 ** | 391,809 * | 138,145 ns | 541,390 ns | 286,994 ns | 163,932 ns |
| Plant removal | 1,210,120 * | 2,619,483 ** | 621,994 * | 81,055 ns | 855,670 ns | 490,292 ns | 20,179 ns |
| Vegetative organ biomass (kg ha−1) | |||||||
| Squaring | 30,180.3 * | 20,965.9 ns | 881.3 ns | 2932.3 ns | 9923.4 ns | 2751.0 ns | 7885.8 ns |
| First bloom | 20,184 * | 45,759 ** | 168,638 ** | 2384 ns | 3071 ns | 16,537 ns | 1437 ns |
| Peak bloom | 5,285,010 ** | 174,087 ** | 437,049 ** | 16,561 ns | 2420 ns | 6712 ns | 6392 ns |
| Boll set | 2,023,067 ** | 401,322 ** | 694,473 ** | 16,459 ns | 4089 ns | 9826 ns | 2527 ns |
| Plant removal | 1,060,410 ** | 597,758 ** | 906,263 ** | 63,456 ns | 29,126 ns | 131,645 ns | 19,288 ns |
| Reproductive organ biomass (kg ha−1) | |||||||
| Squaring | 19.7633 ** | 31.9648 ** | 2.7603 ns | 2.3961 ns | 11.48 ns | 6.348 ns | 4.732 ns |
| First bloom | 35.56 ns | 2538.8 * | 37,974.5 ** | 4518.2 * | 11.7 ns | 1634.7 ns | 9.941 ns |
| Peak bloom | 5283.6 ns | 237,90.8 * | 69,389.1 ** | 2500.3 ns | 5193.5 ns | 23,665.8 ns | 7504.7 ns |
| Boll set | 510,263 * | 376,954 * | 2,620,967 ** | 15,678 ns | 105,955 ns | 23,324 ns | 41,685 ns |
| Plant removal | 550,263 * | 428,504 * | 924,797 * | 82,879 ns | 4546 ns | 502.0 ns | 1093 ns |
| Plant height (cm) | 145.6 ** | 11.22 ** | 127.9 ** | 4.981 * | 32.84 ns | 0.525 ns | 1.4210 ns |
| Fruiting nodes number (m−2) | 1356.2 ** | 32.3 7 ns | 62.03 * | 52.45 * | 1.80 ns | 0.97 ns | 19.57 ns |
| Height to node ratio | 2.219 ** | 0.42389 ** | 1.021 ** | 3.739 ** | 0.023 ns | 0.005 ns | 0.022 ns |
| Treatment | Length | Strength | Micronaire | Elongation | Uniformity Index |
|---|---|---|---|---|---|
| (mm) | (cN/tex) | value | (%) | (%) | |
| Year 2017 | |||||
| Density (D) | |||||
| D1 (low) | 30.7a | 31.9a | 4.1a | 6.5a | 81.8a |
| D2 (moderate) | 28.5b | 29.4b | 3.4b | 6.5a | 82.8a |
| D3 (high) | 25.0c | 27.8c | 3.1b | 6.5a | 82.2a |
| Variety (V) | |||||
| V1 (Zhongmian-16) | 28.2a | 30.2a | 3.6a | 6.5a | 82.0a |
| V2 (J-4B) | 28.0a | 29.2b | 3.4a | 6.5a | 82.5a |
| Interaction | |||||
| D1V1 | 32.5a | 33.9a | 4.5a | 6.5ab | 81.5a |
| D1V2 | 28.9b | 30.0b | 3.7b | 6.4b | 82.1a |
| D2V1 | 28.3b | 28.4b | 3.2bc | 6.5ab | 82.4a |
| D2V2 | 28.7b | 30.4ab | 3.5bc | 6.6a | 83.1a |
| D3V1 | 23.7c | 28.5b | 3.0c | 6.5ab | 82.1a |
| D3V2 | 26.4b | 27.1b | 3.3c | 6.5ab | 82.2a |
| Year 2018 | |||||
| D1 (low) | 29.2a | 28.0a | 3.8a | 6.5a | 82.1c |
| D2 (moderate) | 28.8a | 29.1a | 3.3a | 6.5a | 82.7b |
| D3 (high) | 25.6b | 26.7b | 3.0c | 6.6a | 83.5a |
| V1 | 27.7a | 29.3a | 3.4a | 6.5a | 82.6a |
| V2 | 28.1a | 27.2b | 3.4a | 6.5a | 82.9a |
| D1V1 | 29.4a | 29.4ab | 3.9a | 6.6ab | 81.7d |
| D1V2 | 29.1a | 26.6c | 3.7a | 6.4b | 82.6bc |
| D2V1 | 29.0a | 31.4a | 3.2b | 6.5ab | 83.2c |
| D2V2 | 28.6ab | 26.7c | 3.3a | 6.4ab | 82.7cd |
| D3V1 | 24.7c | 27.1bc | 3.0b | 6.5ab | 83.0b |
| D3V2 | 26.5bc | 28.2bc | 3.1b | 6.6ab | 83.9a |
| Source of variance | |||||
| Y | 8.218 ns | 10.17 ns | 0.100 ns | 0.004 ns | 3.300 ns |
| D | 207.7 ** | 243.6 ** | 4.361 ** | 0.009 ns | 2.507 ns |
| Y*D | 0.764 ns | 0.005 ns | 0.006 ns | 0.006 ns | 2.108 ns |
| V | 0.054 ns | 14.31 ** | 0.147 ns | 0.018 ns | 4.341 * |
| Y*V | 0.160 ns | 0.034 ns | 0.003 ns | 0.001 ns | 0.380 ns |
| D*V | 13.98 ** | 25.79 ** | 0.503 ** | 0.027 ns | 0.352 ns |
| Y*D*V | 0.653 ns | 0.202 ns | 0.076 ns | 0.017 ns | 1.173 ns |
| Items | Treatment | Regression Equation | p Value |
|---|---|---|---|
| Cotton plant | |||
| D1V1 | Y = 25.8592/(1 + 5.3998e−0.069361t) | 0.0067 | |
| D1V2 | Y = 28.8033/(1 + 7.2515e−0.090655t) | 0.0045 | |
| D2V1 | Y = 33.8419/(1 + 3.6664e−0.044682t) | 0.0091 | |
| D2V2 | Y = 38.0327/(1 + 5.1905e−0.069398t) | 0.0044 | |
| D3V1 | Y = 40.0233/(1 + 3.7972e−0.05563t) | 0.0078 | |
| D3V2 | Y = 49.3420/(1 + 6.4825e−0.095022t) | 0.0058 | |
| Vegetative organs | |||
| D1V1 | Y = 11.0623/(1 + 4.4306e−e0.055937t) | 0.0065 | |
| D1V2 | Y = 12.7236/(1 + 5.4970e−0.065756t) | 0.0044 | |
| D2V1 | Y = 16.0106/(1 + 5.2762e−0.068643t) | 0.0004 | |
| D2V2 | Y = 18.4841/(1 + 4.9136e−0.060650t) | 0.0045 | |
| D3V1 | Y = 24.2157/(1 + 3.3978e−0.040419t) | 0.0067 | |
| D3V2 | Y = 28.6854/(1 + 4.1888e−0.050052t) | 0.0006 | |
| Reproductive organs | |||
| D1V1 | Y = 15.5702/(1 + 17.8065e−0.194612t) | 0.0003 | |
| D1V2 | Y = 17.4225/(1 + 13.5270e−0.137846t) | 0.0009 | |
| D2V1 | Y = 18.1896/(1 + 40.9772e−0.431232t) | 0.0024 | |
| D2V2 | Y = 21.1415/(1 + 21.9906e−0.245461t) | 0.0071 | |
| D3V1 | Y = 12.0661/(1 + 12.9458e−0.128539t) | 0.0002 | |
| D3V2 | Y = 14.4129/(1 + 19.1064e−0.199205t) | 0.0003 |
| Treatment | Fast Accumulation Period | Fastest Accumulation Point | ||||
|---|---|---|---|---|---|---|
| t1 DAE | t2 DAE | T d | VT kg·ha−1·d−1 | VM kg·ha−1·d−1 | at DAE | |
| Cotton plant | ||||||
| D1V1 | 55.6 | 98.5 | 43.0 | 0.5 | 0.6 | 82.1 |
| D1V2 | 69.3 | 88.8 | 19.5 | 0.7 | 1.0 | 76.1 |
| D2V1 | 58.9 | 96.8 | 38.0 | 0.9 | 1.0 | 77.9 |
| D2V2 | 44.6 | 91.9 | 47.3 | 1.2 | 1.4 | 68.3 |
| D3V1 | 71.2 | 95.0 | 23.7 | 1.5 | 1.6 | 89.6 |
| D3V2 | 78.0 | 98.3 | 20.4 | 1.9 | 2.2 | 95.0 |
| Average | 62.9 | 94.8 | 31.8 | 1.1 | 1.3 | 81.5 |
| Vegetative organs | ||||||
| D1V1 | 55.7 | 102.8 | 47.1 | 0.1 | 0.2 | 79.2 |
| D1V2 | 63.6 | 103.6 | 40.1 | 0.3 | 0.4 | 83.6 |
| D2V1 | 59.3 | 102.7 | 43.4 | 0.4 | 0.5 | 81.0 |
| D2V2 | 57.7 | 96.0 | 38.4 | 0.5 | 0.4 | 76.9 |
| D3V1 | 60.5 | 103.3 | 43.6 | 0.6 | 0.6 | 84.1 |
| D3V2 | 57.4 | 98.5 | 41.2 | 0.8 | 1.0 | 83.7 |
| Average | 59.0 | 101.1 | 42.3 | 0.4 | 0.5 | 81.4 |
| Reproductive organs | ||||||
| D1V1 | 82.3 | 104.5 | 22.2 | 0.4 | 0.7 | 95.9 |
| D1V2 | 55.8 | 93.8 | 38.0 | 0.6 | 0.6 | 74.8 |
| D2V1 | 65.5 | 94.5 | 29.1 | 0.6 | 0.7 | 80.0 |
| D2V2 | 54.4 | 82.1 | 27.7 | 0.8 | 1.0 | 68.2 |
| D3V1 | 90.5 | 111.0 | 20.5 | 0.3 | 0.4 | 100.7 |
| D3V2 | 88.6 | 107.7 | 19.1 | 0.5 | 0.6 | 98.1 |
| Average | 72.8 | 98.9 | 26.1 | 0.5 | 0.7 | 86.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Kong, X.; Najeeb, U.; Zheng, J.; Tan, D.K.Y.; Akhtar, K.; Munsif, F.; Zhou, R. Planting Density Induced Changes in Cotton Biomass Yield, Fiber Quality, and Phosphorus Distribution under Beta Growth Model. Agronomy 2019, 9, 500. https://doi.org/10.3390/agronomy9090500
Khan A, Kong X, Najeeb U, Zheng J, Tan DKY, Akhtar K, Munsif F, Zhou R. Planting Density Induced Changes in Cotton Biomass Yield, Fiber Quality, and Phosphorus Distribution under Beta Growth Model. Agronomy. 2019; 9(9):500. https://doi.org/10.3390/agronomy9090500
Chicago/Turabian StyleKhan, Aziz, Xiangjun Kong, Ullah Najeeb, Jie Zheng, Daniel Kean Yuen Tan, Kashif Akhtar, Fazal Munsif, and Ruiyang Zhou. 2019. "Planting Density Induced Changes in Cotton Biomass Yield, Fiber Quality, and Phosphorus Distribution under Beta Growth Model" Agronomy 9, no. 9: 500. https://doi.org/10.3390/agronomy9090500
APA StyleKhan, A., Kong, X., Najeeb, U., Zheng, J., Tan, D. K. Y., Akhtar, K., Munsif, F., & Zhou, R. (2019). Planting Density Induced Changes in Cotton Biomass Yield, Fiber Quality, and Phosphorus Distribution under Beta Growth Model. Agronomy, 9(9), 500. https://doi.org/10.3390/agronomy9090500






