Physical Location of New Stripe Rust Resistance Gene(s) and PCR-Based Markers on Rye (Secale cereale L.) Chromosome 5 Using 5R Dissection Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Non-Denaturing Fluorescence in situ Hybridization (ND-FISH) Analysis
2.3. Developing PCR-Based Markers
2.4. PCR Assay, 5RKu-Specific Markers Testing and Physical Location
2.5. Similarity Searches of the Pair-End Reads Against S. cereale L. Lo7 Scaffolds
2.6. Stripe Rust Resistance Testing
3. Results
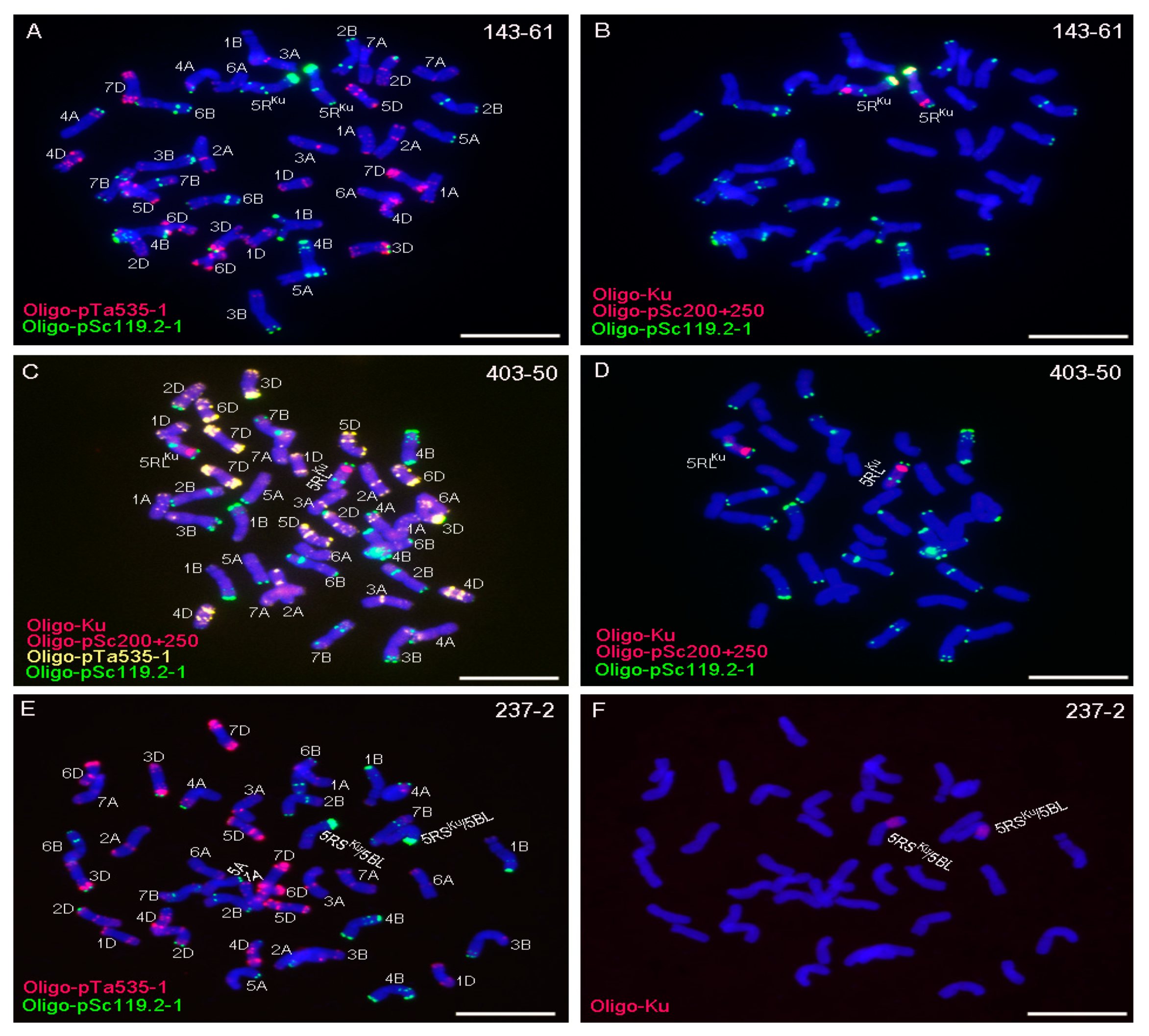
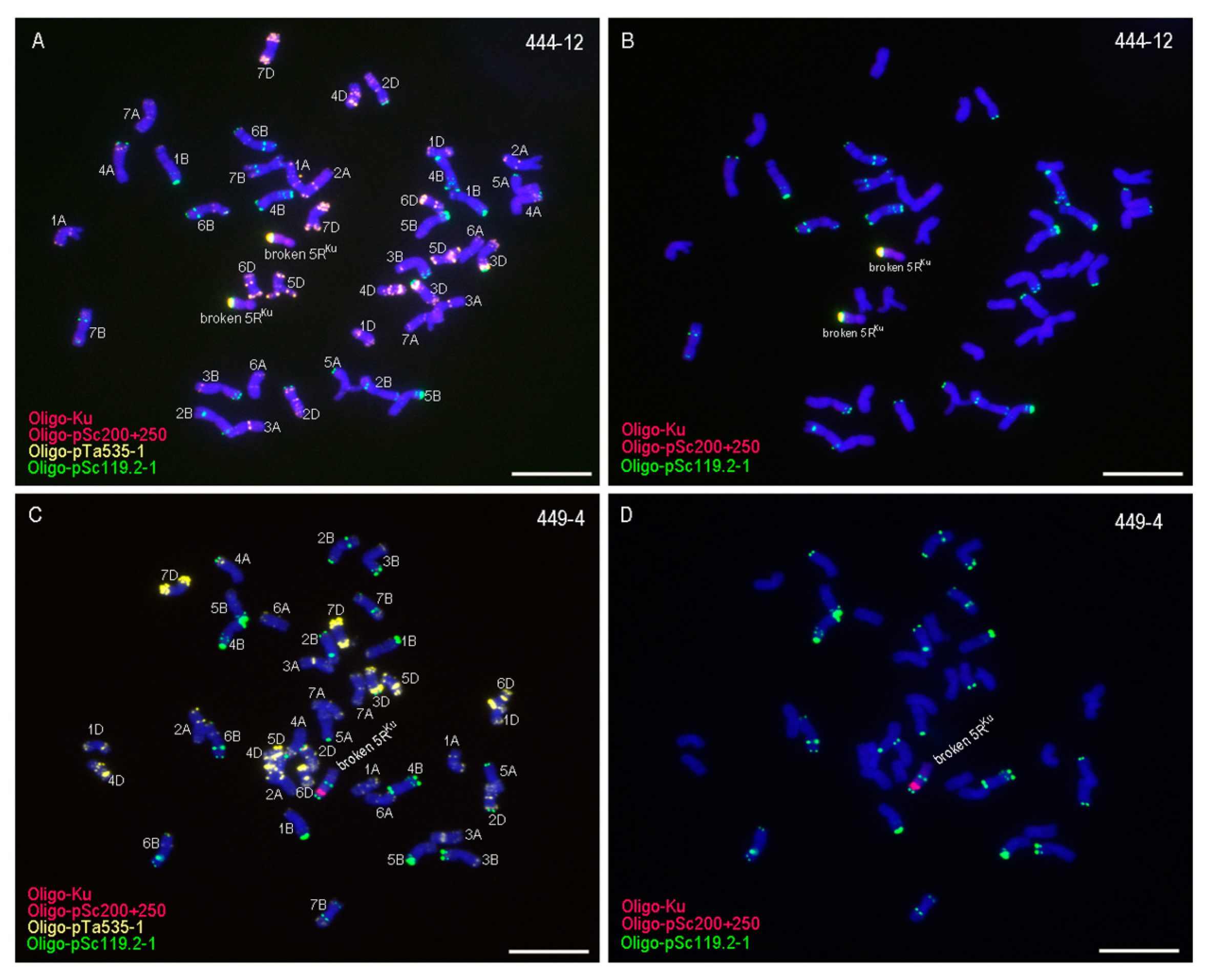
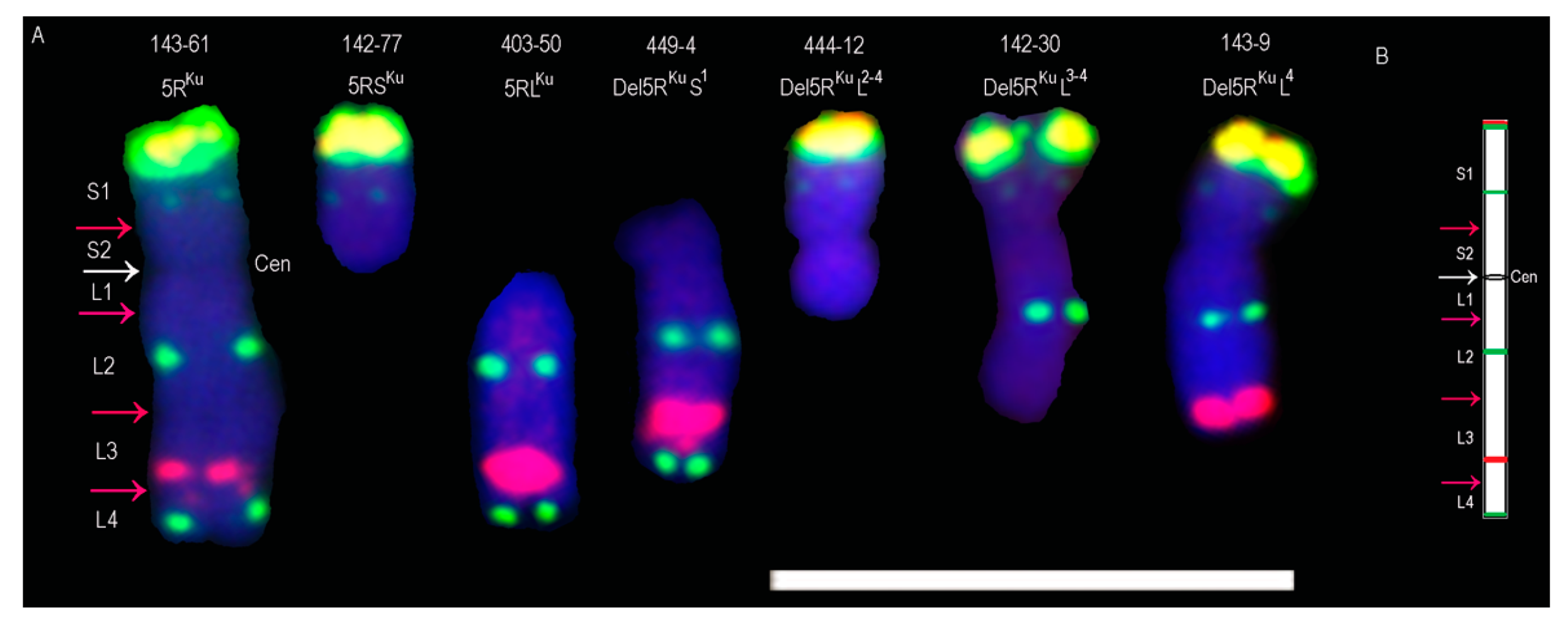
3.1. Obtaining 5RKu Dissection Lines
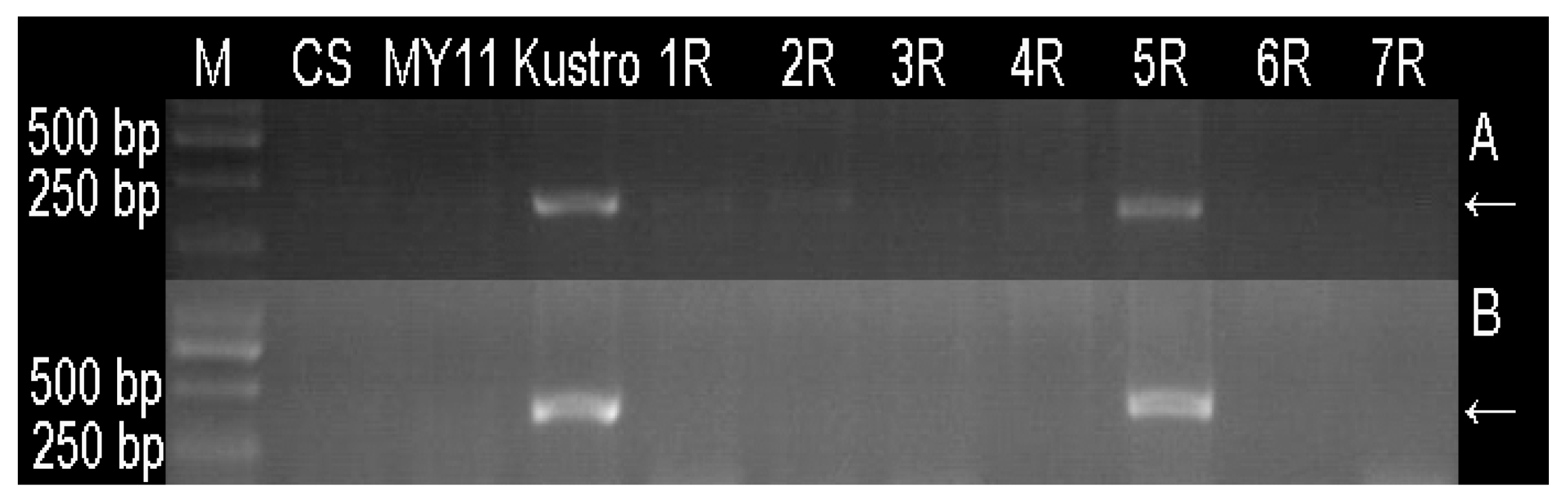
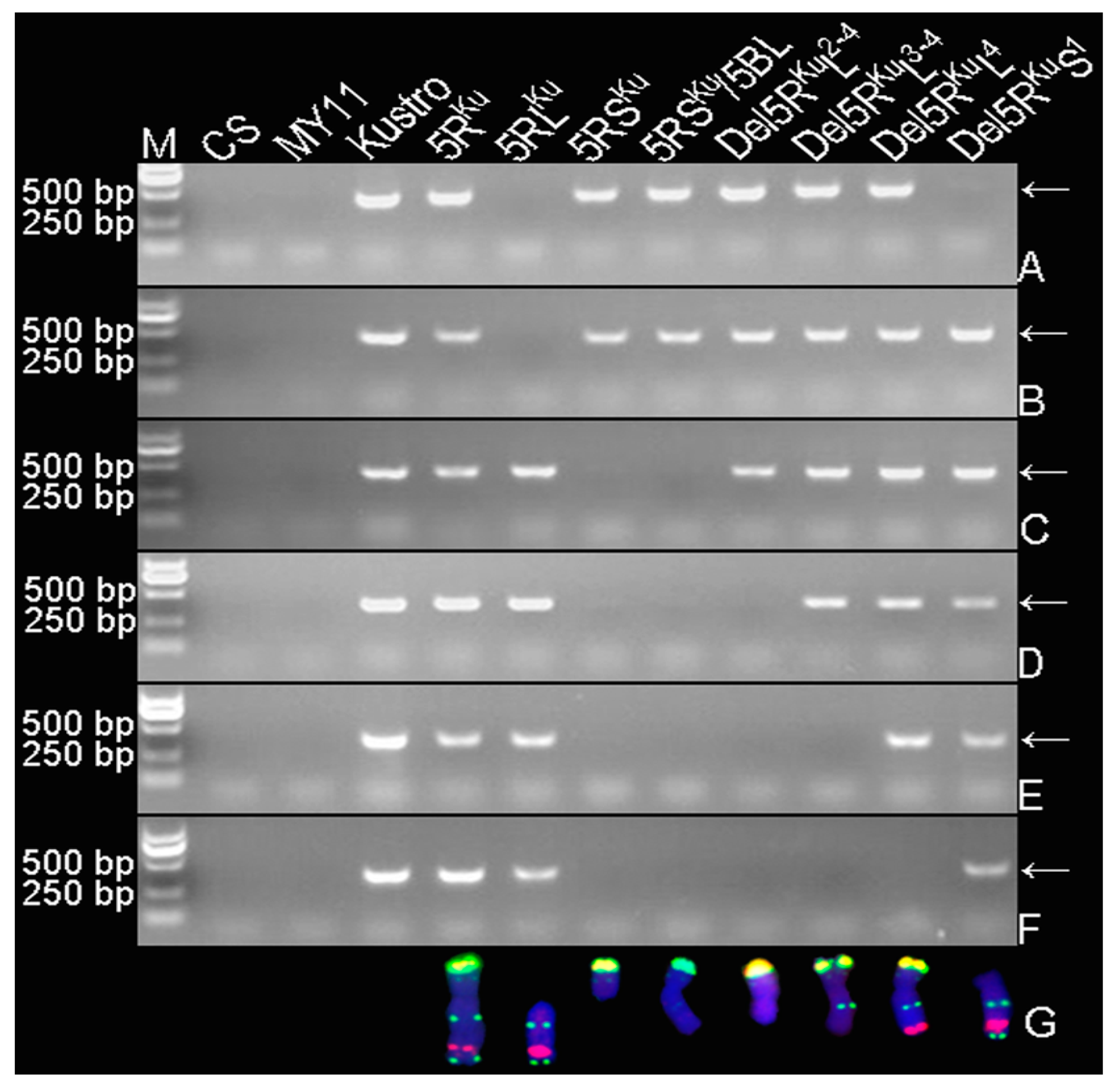
3.2. Developing 5RKu-Specific Markers and Physical Mapping
3.3. Similarity between S. cereale Lo7 Scaffolds and Pair-End Reads Used for 5RKu-Specific Primers Design
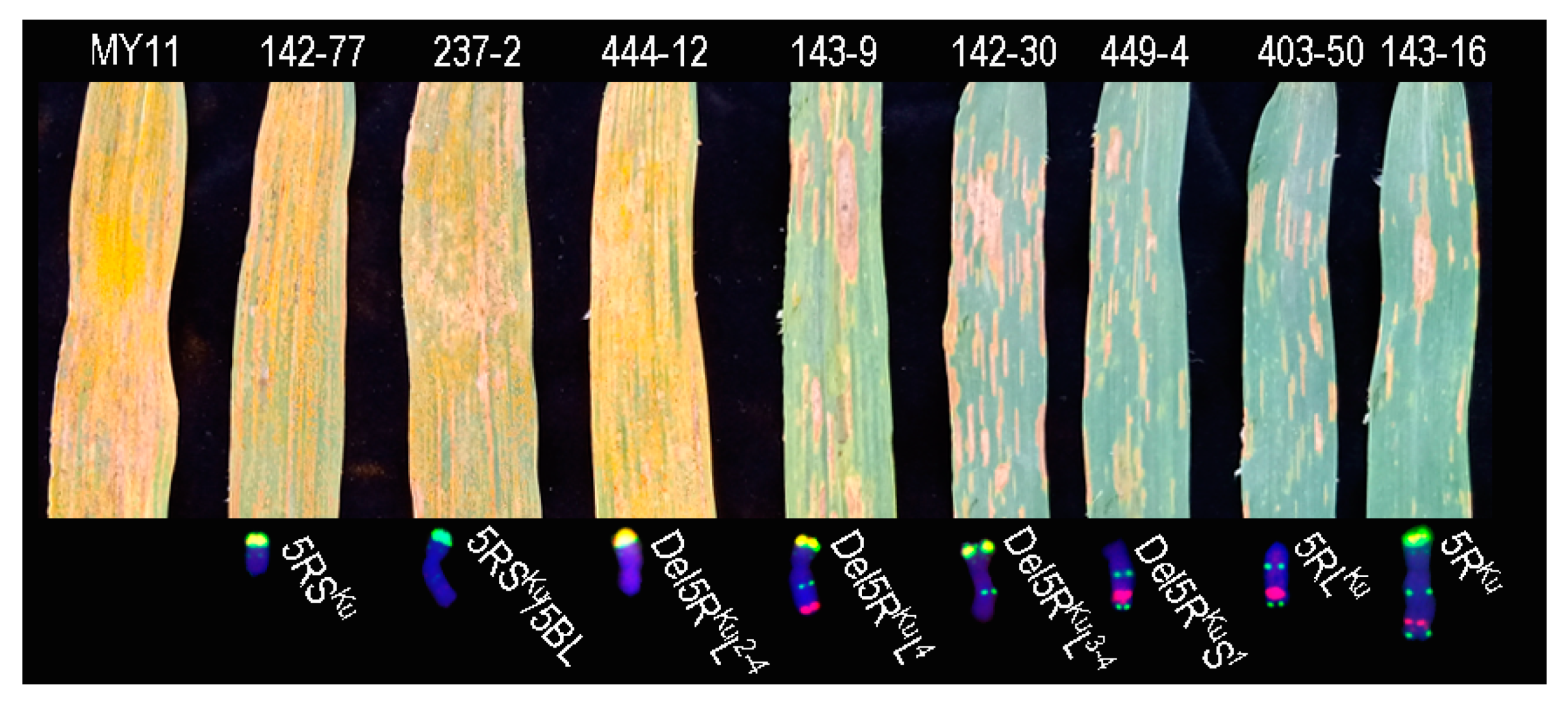
3.4. Location of Stripe Rust Gene(s) on 5RKu Chromosome
4. Discussion
4.1. 5R-Specific Markers
4.2. Stripe Rust Resistance Gene(s) on the 5RL Arm Might be New One
4.3. Variations of Wheat Chromosomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dundas, I.S.; Frappell, D.E.; Crack, D.M.; Fisher, J.M. Deletion mapping of a nematode resistance gene on rye chromosome 6R in wheat. Crop Sci. 2001, 41, 1771–1778. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. Isolation and characterisation of two MATE genes in rye. Funct. Plant Biol. 2010, 37, 296–303. [Google Scholar] [CrossRef]
- Howell, T.; Hale, I.; Jankuloski, L.; Bonafede, M.; Gilbert, M.; Dubcovsky, J. Mapping a region within the 1RS.1BL translocation in common wheat affecting grain yield and canopy water status. Theor. Appl. Genet. 2014, 127, 2695–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Q.; Wang, Y.Y.; Qiu, L.; Ren, T.H.; Li, Z.; Fu, S.L.; Tang, Z.X. Physical location of new PCR-based markers and powdery mildew resistance gene(s) on rye (Secale cereale L.) chromosome 4 using 4R dissection lines. Front. Plant Sci. 2017, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Tang, Z.; Duan, Q.; Tang, S.; Fu, S. Using the 6RLKu minichromosome of rye (Secale cereale L.) to create wheat-rye 6D/6RLKu small segment translocation lines with powdery mildew resistance. Int. J. Mol. Sci. 2018, 19, 3933. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Ma, P.; Zheng, Q.; Fu, S.; Li, L.; Han, F.; Han, G.; Wang, J.; Xu, Y.; Jin, Y.; et al. Development and molecular cytogenetic identification of a new wheat-rye 4R chromosome disomic addition line with resistances to powdery mildew, stripe rust and sharp eyespot. Theor. Appl. Genet. 2019, 132, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, R.; Werner, T.; Hülgenhof, E. Confirmation of a 4BL/5RL wheat-rye chromosome translocation line in the wheat cultivar ‘Viking’ showing high copper efficiency. Plant Breed. 1991, 107, 226–234. [Google Scholar] [CrossRef]
- Sibikeev, S.N.; Sibikeeva, Y.E.; Krupnov, V.A. Transmission of 5R chromosomes via gametes and its influence on spring bread wheat somatic embryoidogenesis in vitro. Russ. J. Genet. 2005, 41, 1650–1655. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, X.P.; Zhong, L.; Xu, X.L. Study on homoeologous chromosome pairing and translocation induced by 5A/5R × 6A/6R wheat-rye substitution lines. Acta Genet. Sin. 2006, 33, 244–250. [Google Scholar] [CrossRef]
- Chumanova, E.V.; Efremova, T.T.; Trubacheeva, N.V.; Arbuzova, V.S.; Rosseeva, L.P. Chromosome composition of wheat-rye lines and the influence of rye chromosomes on disease resistance and agronomic traits. Russ. J. Genet. 2014, 50, 1169–1178. [Google Scholar] [CrossRef]
- Andersson, S.C.; Johansson, E.; Baum, M.; Rihawi, F.; Bouhssini, M.E.L. New resistance sources to Russian wheat aphid (Diuraphis noxia) in Swedish wheat substitution and translocation lines with rye (Secale cereale) and Leymus mollis. Czech J. Genet. Plant Breed. 2015, 51, 162–165. [Google Scholar] [CrossRef]
- Li, G.; Gao, D.; La, S.; Wang, H.; Li, J.; He, W.; Yang, E.; Yang, Z. Characterization of wheat-Secale africanum chromosome 5Ra derivatives carrying Secale specific genes for grain hardness. Planta 2016, 243, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.M.; Tsvetkova, N.; Rotter, B.; Siekmann, D.; Schwefel, K.; Krezdorn, N.; Plieske, J.; Winter, P.; Melz, G.; Voylokov, A.V.; et al. Gene expression profiling and fine mapping identifies a gibberellin 2-Oxidase gene co-segregating with the dominant dwarfing gene Ddw1 in rye (Secale cereale L.). Front. Plant Sci. 2019, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, A.A.; Moiseeva, E.A.; Goncharov, N.P.; Kondratenko, E.Y. The order of the bs, Skdh, and Aadh1 genes in chromosome 5R of rye secale cereal L. Russ. J. Genet. 2010, 46, 666–671. [Google Scholar] [CrossRef]
- Lukaszewski, A.J.; Porter, D.R.; Baker, C.A.; Rybka, K.; Lapinski, B. Attempts to transfer Russion wheat aphid resistance from a rye chromosome in Russian triticales to wheat. Crop Sci. 2001, 41, 1743–1749. [Google Scholar] [CrossRef]
- Lukaszewski, A.J. Alien Introgression in Wheat, 1st ed.; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer: Cham, Switzerland, 2015; Chapter 7; pp. 163–189. [Google Scholar]
- Silkova, O.G.; Leonova, I.N.; Krasilova, N.M.; Dubovets, N.I. Preferential elimination of chromosome 5R of rye in the progeny of 5R5D dimonosomics. Russ. J. Genet. 2011, 47, 942–950. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Qiu, L.; Wang, Y.; Tang, S.; Fu, S. Identification and physical mapping of new PCR-Based markers specific for the long arm of rye (Secale cereale L.) chromosome 6. J. Genet. Genom. 2016, 43, 209–216. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, S.; Qiu, L.; Tang, Z.; Fu, S. Oligonucleotides and ND-FISH displaying different arrangements of tandem repeats and identification of Dasypyrum villosum chromosomes in wheat backgrounds. Molecules 2017, 22, 973. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Fu, S.; Chen, L.; Wang, Y.; Li, M.; Yang, Z.; Qiu, L.; Yan, B.; Ren, Z.; Tang, Z. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015, 5, 10552. [Google Scholar] [CrossRef]
- Han, F.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. Available online: http://bioinfo.ut.ee/primer3 (accessed on 11 May 2016). [CrossRef] [PubMed]
- Qiu, L.; Tang, Z.X.; Li, M.; Fu, S.L. Development of new PCR-based markers specific for chromosome arms of rye (Secale cereale L.). Genome 2016, 59, 159–165. [Google Scholar] [CrossRef]
- Blake, V.C.; Woodhouse, M.R.; Lazo, G.R.; Odell, S.G.; Wight, C.P.; Tinker, N.A.; Wang, Y.; Gu, Y.Q.; Birkett, C.L.; Jannink, J.L.; et al. GrainGenes: Centralized Small Grain Resources and Digital Platform for Geneticists and Breeders. Database (Oxford) 2019, 2019, baz065. Available online: https://wheat.pw.usda.gov/cgi-bin/seqserve/blast_rye.cgi (accessed on 16 August 2019). [PubMed]
- Bauer, E.; Schmutzer, T.; Barilar, I.; Mascher, M.; Gundlach, H.; Martis, M.M.; Twardziok, S.O.; Hackauf, B.; Gordillo, A.; Wilde, P.; et al. Towards a whole-genome sequence for rye (Secale cereale L.). Plant J. 2017, 89, 853–869. [Google Scholar] [CrossRef]
- Wan, A.; Wang, X.; Kang, Z.; Chen, X. Variability of the Stripe Rust Pathogen. In Stripe Rust; Chen, X., Kang, Z., Eds.; Springer: Dordrecht, The Netherlands, 2017; Chapter 2; pp. 35–154. [Google Scholar]
- Hao, M.; Luo, J.; Fan, C.; Yi, Y.; Zhang, L.; Yuan, Z.; Ning, S.; Zheng, Y.; Liu, D. Introgression of powdery mildew resistance gene Pm56 on rye chromosome arm 6RS into wheat. Front. Plant Sci. 2018, 9, 1040. [Google Scholar] [CrossRef]
- Camacho, M.V.; Matos, M.; Gonzales, C.; Perez-Flores, V.; Pernauta, B.; Pinto-Carnida, O.; Benito, C. Secale cereale inter-microsatellites (SCIMs): Chromosomal location and genetic inheritance. Genetica 2005, 123, 303–311. [Google Scholar] [CrossRef]
- Tomita, M.; Okutani, A.; Beiles, A.; Nevo, E. Genomic, RNA, and ecological divergences of the Revolver transposon-like multi-gene family in Triticeae. BMC Evol. Biol. 2011, 11, 269. [Google Scholar] [CrossRef]
- Xu, H.; Yin, D.; Li, L.; Wang, Q.; Li, X.; Yang, X.; An, D. Development and application of EST-based markers specific for chromosome arms of rye (Secale cereale L.). Cytogenet. Genome Res. 2012, 136, 220–228. [Google Scholar] [CrossRef]
- Li, J.; Endo, T.R.; Saito, M.; Ishikawa, G.; Nakamura, T.; Nasuda, S. Homoeologous relationship of rye chromosome arms as detected with wheat PLUG markers. Chromosoma 2013, 122, 555–564. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Ren, T.H.; Yang, Z.J.; Yan, B.J.; Zhang, H.Q.; Fu, S.L.; Ren, Z.L. Development and characterization of a new 1BL. 1RS translocation line with resistance to stripe rust and powdery mildew of wheat. Euphytica 2009, 169, 207–213. [Google Scholar] [CrossRef]
- Fu, S.; Tang, Z.; Ren, Z.; Zhang, H. Transfer to wheat (Triticum aestivum) of small chromosome segments from rye (Secale cereale) carrying disease resistance genes. J. Appl. Genet. 2010, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.P.; Li, G.R.; Liu, C.; Yang, Z.J. Characterization of wheat-Secale africanum introgression lines reveals evolutionary aspects of chromosome 1R in rye. Genome 2012, 55, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, Z.; Tan, F.; Tang, Z.; Fu, S.; Yan, B.; Ren, T. Molecular cytogenetic characterization of New Wheat-Rye 1R(1B) Substitution and Translocation Lines from a Chinese Secale cereal L. Aigan with resistance to stripe rust. PLoS ONE 2016, 11, e0163642. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.P.; Li, G.R.; Zhou, L.; Li, C.H.; Liu, C.; Yang, Z.J. Identification of wheat-Secale africanum chromosome 2Rafr introgression lines with novel disease resistance and agronomic characteristics. Euphytica 2013, 194, 197–205. [Google Scholar] [CrossRef]
- Schneider, A.; Rakszegi, M.; Molnár-Láng, M.; Szakács, É. Production and cytomolecular identification of new wheat-perennial rye (Secale cereanum) disomic addition lines with yellow rust resistance (6R) and increased arabinoxylan and protein content (1R, 4R, 6R). Theor. Appl. Genet. 2016, 129, 1045–1059. [Google Scholar] [CrossRef]



| Name | Chromosome Constitution |
|---|---|
| 142-30 | one broken 5RKu, 39 intact wheat chromosomes and one 7BL arm |
| 142-77 | one 5RSKu arm, 40 intact wheat chromosomes |
| 143-9 | two broken 5RKu chromosomes, 39 intact wheat chromosomes |
| 143-61 | two intact 5RKu chromosomes, 40 intact wheat chromosomes |
| 237-2 | two 5RSKu/5BL translocation chromosomes, 40 intact wheat chromosomes |
| 403-50 | two 5RLKu arms, 40 intact wheat chromosomes |
| 444-12 | two broken 5RKu chromosomes, 42 intact wheat chromosomes |
| 449-4 | one broken 5RKu chromosome, 41 intact wheat chromosomes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, W.; Tang, Z.; Luo, J.; Fu, S. Physical Location of New Stripe Rust Resistance Gene(s) and PCR-Based Markers on Rye (Secale cereale L.) Chromosome 5 Using 5R Dissection Lines. Agronomy 2019, 9, 498. https://doi.org/10.3390/agronomy9090498
Xi W, Tang Z, Luo J, Fu S. Physical Location of New Stripe Rust Resistance Gene(s) and PCR-Based Markers on Rye (Secale cereale L.) Chromosome 5 Using 5R Dissection Lines. Agronomy. 2019; 9(9):498. https://doi.org/10.3390/agronomy9090498
Chicago/Turabian StyleXi, Wei, Zongxiang Tang, Jie Luo, and Shulan Fu. 2019. "Physical Location of New Stripe Rust Resistance Gene(s) and PCR-Based Markers on Rye (Secale cereale L.) Chromosome 5 Using 5R Dissection Lines" Agronomy 9, no. 9: 498. https://doi.org/10.3390/agronomy9090498
APA StyleXi, W., Tang, Z., Luo, J., & Fu, S. (2019). Physical Location of New Stripe Rust Resistance Gene(s) and PCR-Based Markers on Rye (Secale cereale L.) Chromosome 5 Using 5R Dissection Lines. Agronomy, 9(9), 498. https://doi.org/10.3390/agronomy9090498




