Bastard Cabbage (Rapistrum rugosum L.) Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium in Spain and Alternative Herbicides for Its Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Verification of Herbicide Resistance (Trial 1) and Quantification of the Resistance (Trial 2)
2.3. Alternative Herbicides to Tribenuron-Methyl and to Iodosulfuron-Methyl (Trial 3)
2.4. Proline 197 for Resistance Mechanism Identification; Process of ALS Gene Amplification and Sequencing
2.5. Data Processing
3. Results and Discussion
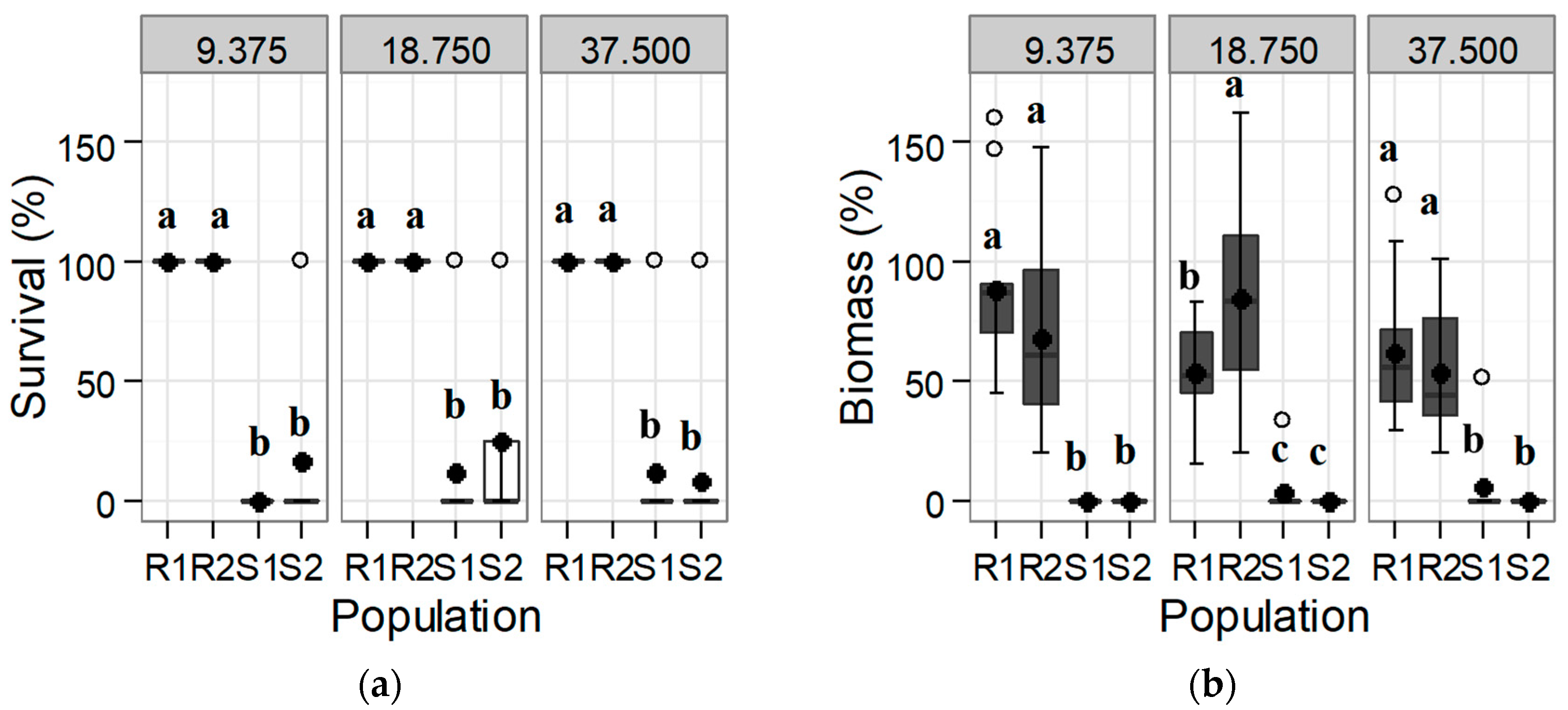
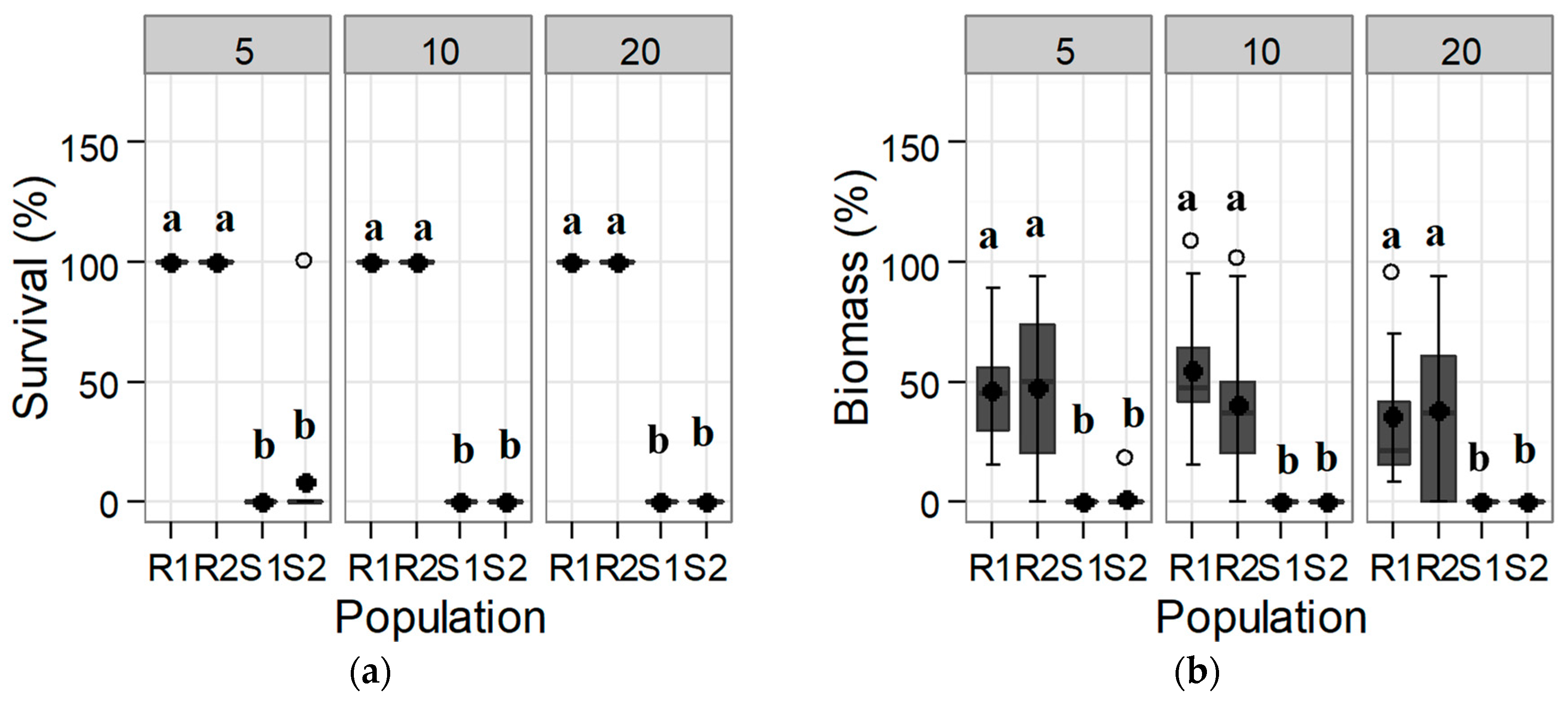
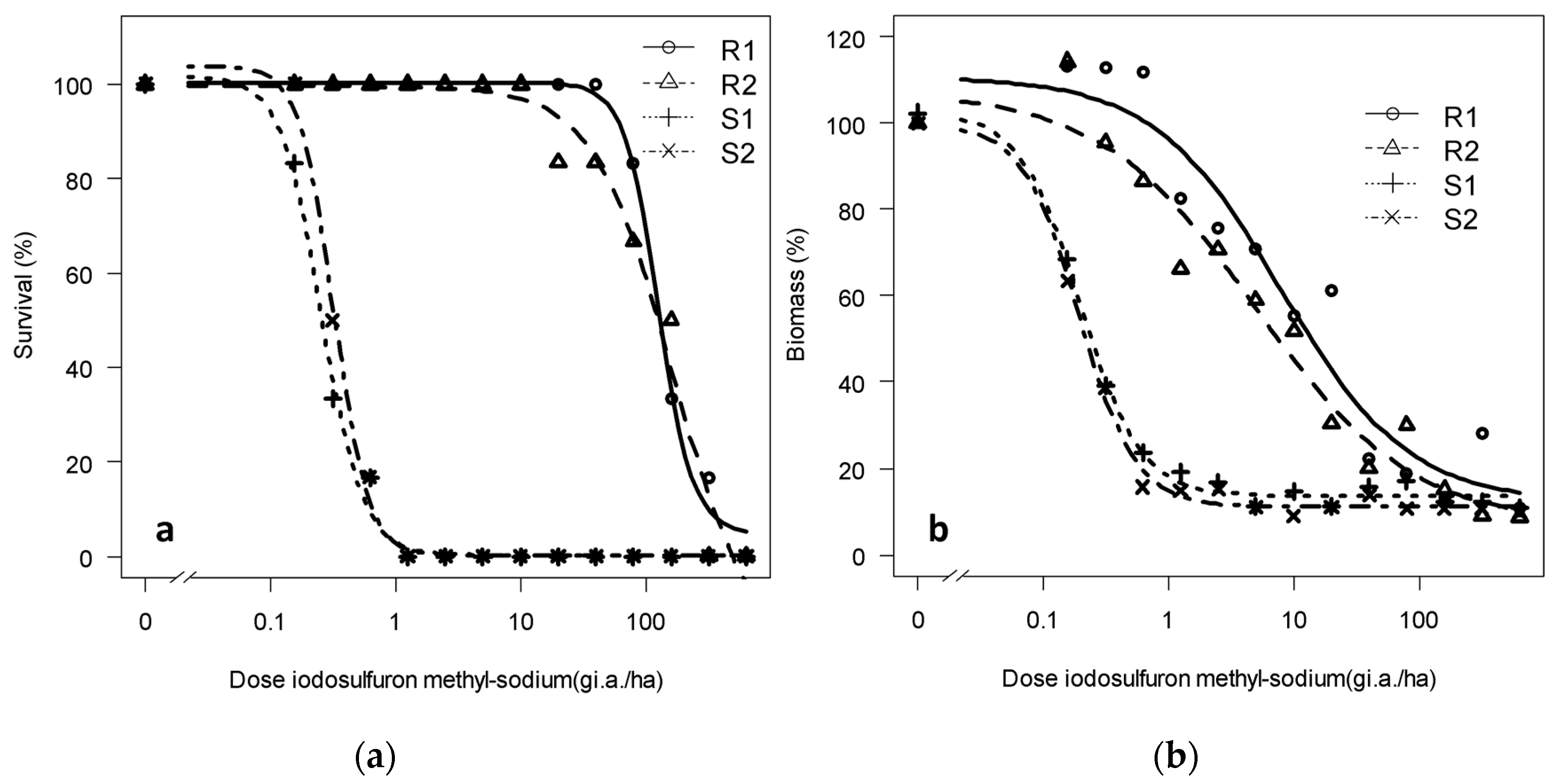
3.1. Confirmation and Quantification of the Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium
3.1.1. Response of the Initial Population: Trial 1
3.1.2. Dose-Response of the Progeny to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium: Trial 2
3.2. Alternative Herbicides to Tribenuron-Methyl and to Iodosulfuron-Methyl: Trial 3
3.2.1. ALS Inhibitors
3.2.2. Auxinic Herbicides
3.2.3. Other Post-Emergence Herbicides
3.3. Sequencing of Rapistrum Rugosum ALS Point Mutations Associated with Herbicide Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.H.; Buchanan, G.A. Crop manipulation in integrated weed management systems. Weed Sci. 1982, 30, 17–24. [Google Scholar] [CrossRef]
- Duke, S.O.; Heap, I. Evolution of weed resistance to herbicides: What have we learned after 70 years? In Biology, Physiology and Molecular Biology of Weeds; Jugulam, M., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 63–86. [Google Scholar]
- Ruegg, W.T.; Quadranti, M.; Zoschke, A. Herbicide research and development: Challenges and opportunities. Weed Res. 2007, 47, 271–275. [Google Scholar] [CrossRef]
- Heap, I.M. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- HRAC. Guideline to the Management of Herbicide Resistance. Available online: https://hracglobal.com/files/Management-of-Herbicide-Resistance.pdf (accessed on 24 January 2019).
- Heap, I. Criteria for Confirmation of Herbicide-Resistant Weeds—With Specific Emphasis on Confirming Low Level Resistance. Available online: http://www.weedscience.org/Documents/ResistanceCriterion.pdf (accessed on 15 December 2018).
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Bio. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Menne, H.; Köcher, H. HRAC classification of herbicides and resistance development. In Modern Crop Protection Compounds; Krämer, W., Schirmer, U., Jeschke, P., Witschel, M., Eds.; Wiley-VCH Publishing: Weingeim, Germany, 2007; pp. 5–26. [Google Scholar]
- Heap, I. International Survey of Herbicide Resistant Weeds. Annual Report Internet. Available online: http://www.weedscience.com (accessed on 15 December 2018).
- Drobny, H.G. Weed control in distress—Can all weeds still be controlled with herbicides in future? Julius Kühn Archiv 2016, 452, 19–23. [Google Scholar] [CrossRef]
- Claude, J.P.; Gabard, J.; De Prado, R.; Taberner, A. AnALS-resistantpopulation of Papaver rhoeas in Spain. In Proceedings of the 6th Mediterranean Symposium EWRS (EWRS 1998), Montpellier, France, 13–15 May 1998; pp. 181–187. [Google Scholar]
- Cirujeda, A. Integrated Management of Herbicide Resistant Papaver rhoeas L. Populations. Ph.D. Thesis, Universitat de Lleida, Lleida, Spain, 2001; p. 274. Available online: http://www.tdx.cat/TDX-0829103-105707 (accessed on 20 December 2019).
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, X.Q.; Hashem, A.; Walsh, M.J.; Powles, S.B. ALS gene proline (197) mutations confer ALS herbicide resistance in eight separated wild radish (Raphanus raphanistrum) populations. Weed Sci. 2003, 51, 831–838. [Google Scholar] [CrossRef]
- Rosario, J.M.; Cruz-Hipolito, H.; Smeda, R.; De Prado, R. White mustard (Sinapis alba) resistance to ALS-inhibiting herbicides and alternative herbicides for control in Spain. Eur. J. Agron. 2011, 35, 57–62. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Rosario, J.; Ioli, G.; Osuna, M.D.; Smeda, R.J.; González-Torralva, F.; De Prado, R. Resistance mechanism to tribenuron-methyl in white mustard (Sinapis alba) from Southern Spain. Weed Sci. 2013, 61, 341–347. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.Q.; Chen, S.L.; Fan, C.Q.; Li, B.H. Confirmation of flixweed (Descurainia sophia) resistance to tribenuron-methyl, using three different assay methods. Weed Sci. 2010, 58, 56–60. [Google Scholar] [CrossRef]
- Deng, W.; Liu, M.J.; Yang, Q.; Mei, Y.; Li, X.F.; Zheng, M.Q. Tribenuron-methyl resistance and mutation diversity of Pro197 in flixweed (Descurainia sophia L.) accessions from China. Pestic. Biochem. Physiol. 2015, 117, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, L.J.; Hall, L.M.; O’Donovan, J.T.; Dyer, W.; Hall, J.C. Metabolism-based resistance of a wild mustard (Sinapis arvensis L.) biotype to ethametsulfuron-methyl. J. Agric. Food Chem. 2000, 48, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- HRAC. Classification of Herbicides According to Site of Action. Available online: http://www.weedscience.org/Documents/ShowDocuments.aspx?DocumentID=1193 (accessed on 15 October 2018).
- Topuz, M.; Nemli, Y.; Fatima, T.; Mattoo, A.K. Seed dormancy is modulated in recently evolved chlorsulfuron-resistant Turkish biotypes of wild mustard (Sinapis arvensis). Front.Chem. 2015, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 20 August 2018).
- Ritz, C.; Streibig, J.C. Bioassay Analysis using R. J. Stat. Soft. 2005, 12, 1–22. Available online: http://bioassay.dk/ (accessed on 15 December 2016). [CrossRef]
- Warwick, S.I.; Sauder, C.; Beckie, H. Resistance in Canadian biotypes of wild mustard (Sinapis arvensis) to acetolactate synthase inhibiting herbicides. Weed Sci. 2005, 53, 631–639. [Google Scholar] [CrossRef]
- Christoffers, J.M.; Nandula, V.K.; Howatt, K.A.; Wehking, T.R. Target-site resistance to acetolactate synthase inhibitors in wild mustard (Sinapis arvensis). Weed Sci. 2006, 54, 191–197. [Google Scholar] [CrossRef]
- Ntoanidou, S.; Madesis, P.; Diamantidis, G.; Eleftherohorinos, I. Trp574 substitution in the acetolactate synthase of Sinapis arvensis confers cross-resistance to tribenuron and imazamox. Pestic. Bioch. Phys. 2017, 142, 9–14. [Google Scholar] [CrossRef]
- Tan, M.K.; Medd, R.W. Characterization of the acetolactate synthase (ALS) gene of Raphanus raphanistrum L. and the molecular assay of mutations associated with herbicide resistance. Plant Sci. 2002, 163, 195–200. [Google Scholar] [CrossRef]
- Boutsalis, P.; Karotam, J.; Powles, S.B. Molecular basis of resistance to acetolactate synthase–inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic. Sci. 1999, 55, 507–516. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.X.; Wei, S.H.; Zhang, H.J.; Li, X.J.; Zhang, Y.Q.; Wang, G.Q. Acetolactate synthase gene proline (197) mutations confer tribenuron-methyl resistance in flixweed (Descurainia sophia) populations from China. Weed Sci. 2011, 59, 376–379. [Google Scholar] [CrossRef]

| Active Ingredient/ Commercial Name | Company | Application Time | Mode of Action | HRAC Group 1 | Dose x (g a.i. ha−1) |
|---|---|---|---|---|---|
| 2,4-D acid U-46D Complet® | Nufarm Gmbh & Co. KG (Linz, Austria) | Post-emergence | Action comparable to that of indole acetic acid (synthetic auxins) | O | 840 |
| bentazone Basagran L® | Basf SE (Ludwigshafen (Rhein), Germany) | Post-emergence | Inhibition of photosynthesis at photosystem II | C3 | 960 |
| bromoxynil Bromotril 25 SC® | Adama Agan Ltd. (Ashdod, Israel) | Post-emergence | Inhibition of photosynthesis at photosystem II | C3 | 225 |
| carfentrazone-ethyl Platform 40 WG® | FMC Chemical SPRL (Brussels, Belgium) | Post-emergence | Inhibition of protoporphyrinogen oxidase (PPO) (photosynthetic pigments) | E | 20 |
| florasulam Nikos® | Dow Agrosciences LTD. (La Rinconada, Seville, Spain) | Post-emergence | Inhibition of acetolactate synthase (ALS) (acetohydroxy acid synthase, AHAS) | B | 7.5 |
| fluroxypyr Flurostar 200® | Globachem N.V. (Sint-Truiden, Belgium) | Post-emergence | Action comparable to that of indole acetic acid (synthetic auxins) | O | 200 |
| MCPA 2 U-46 SP Fluid® | Nufarm B.V (Capelle aan den Ijssel, Netherlands) | Post-emergence | Action comparable to that of indole acetic acid (synthetic auxins) | O | 1200 |
| Iodosulfuron-methyl-sodium 3 Hussar® | Bayer AG (Leverkusen, Germany) | Post-emergence | Inhibition of ALS (AHAS) | B | 10 |
| Tribenuron-methyl 4 Granstar 50 SX® | FMC International Switzerland Sàrl (Genève, Switzerland) | Post-emergence | Inhibition of acetolactate synthase ALS (acetohydroxy acid synthase AHAS) | B | 18.75 |
| Primer Sequence 5′–3′ | |
|---|---|
| RaprugALSF1deg | TTCRTCTCCCGMTACGCTCCC [23] |
| RaprugALSR1deg | CAARCTGYTGCTGAATATC [23] |
| RaprugALSF1 | GAAACCGTMTTCGCTTACC |
| RaprugALSR1 | CCACCACCAACATACAA |
| Survival/Biomass | Population | Lower Limit | Upper Limit | Slope | LD50/WG50 | RF |
|---|---|---|---|---|---|---|
| Survival | R1 | −0.11 (3.3) | 100.91 (3.13) | 1.71 (0.56) | 945.03 (359) | 511 |
| R2 | −0.14 (4.3) | 99.39 (3.17) | 2.54 (0.66) | 833.12 (224) | 450 | |
| S1 | −0.13 (5.02) | 104.75 (9.04) | 0.92 (0.19) | 1.85 (0.55) | - | |
| S2 | −0.12 (4.46) | 103 (8.95) | 1.12 (0.25) | 1.89 (0.66) | 1.02 | |
| Biomass | R1 | −1.15 (3.4) | 99.57 (7.39) | 0.47 (0.19) | 82.69 (68.23) | 188 |
| R2 | 28.97 (1.9) | 103.67 (4.45) | 0.84 (0.39) | 111.33 (90.33) | 253 | |
| S1 | 11.94 (2.62) | 99.72 (8.17) | 2.40 (0.8) | 0.44 (0.07) | - | |
| S2 | 9.29 (2.51) | 100.42 (8.10) | 4.03 (1.43) | 0.46 (0.07) | 1.04 |
| Survival/Biomass | Population | Lower Limit | Upper Limit | Slope | LD50/WG50 | RF |
|---|---|---|---|---|---|---|
| Survival | R1 | 4.73 (0.8) | 100.31 (2.70) | 3.15 (0.31) | 164.77 (54.44) | 633 |
| R2 | −12.39 (5.84) | 99.51 (2.28) | 1.37 (0.44) | 126.84 (16.83) | 487 | |
| S1 | 0.27 (0.27) | 101.67 (8.65) | 2.63 (0.80) | 0.31 (0.36) | 1.19 | |
| S2 | 0.21 (0.26) | 103.75 (7.16) | 3.19 (0.98) | 0.26 (0.34) | - | |
| Biomass | R1 | 12.23 (5.66) | 110.62 (4.77) | 0.88 (0.14) | 7.89 (2.25) | 41.56 |
| R2 | 7.04 (6.60) | 107.27 (5.72) | 0.69 (0.13) | 4.95 (1.78) | 26.05 | |
| S1 | 13.66 (2.39) | 102.51 (7.67) | 1.81 (0.57) | 0.20 (0.03) | 1.05 | |
| S2 | 11.12 (2.32) | 99.77 (7.06) | 1.92 (0.56) | 0.19 (0.03) | - |
| Group | Active Ingredient | Dose (g a.i. ha−1) | Survival (%) | Dry Biomass (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Population | |||||||||
| R1 | R2 | S1 | S2 | R1 | R2 | S1 | S2 | |||
| ALS Inhibitors | florasulam | 7.5 | 75 a | 50 a | 0 b | 0 b | 8.2 a | 12.4 a | 4.6 a | 5.7 a |
| 15 | 25 ab | 50 a | 0 b | 0 b | 3.3 a | 4.36 a | 4.6 a | 5.0 a | ||
| iodosulfuron-methyl-sodium | 10 | 100 a | 100 a | 0 b | 0 b | 60.6 a | 46.7 a | 6.8 b | 6.9 b | |
| 20 | 100 a | 100 a | 0 b | 0 b | 35.0 a | 59.0 a | 5.7 b | 4.3 b | ||
| tribenuron-methyl | 18.75 | 100 a | 100 a | 0 b | 0 b | 74.6 a | 56.0 a | 5.6 b | 6.1 b | |
| 37.5 | 100 a | 100 a | 0 b | 0 b | 55.8 a | 67.9 a | 8.0 b | 6.5 b | ||
| Auxinics | 2,4-D acid | 840 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| 1680 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
| MCPA | 1200 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | |
| 2400 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
| fluroxypyr | 200 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | |
| 400 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
| Others | bentazone | 960 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| 1920 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
| bromoxynil | 225 | 0 a | 25 a | 25 a | 0 a | 0 | 6.6 a | 0.9 a | 0 a | |
| 450 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
| carfentrazone-ethyl | 20 | 0 a | 0 a | 0 a | 25 a | 0 a | 0 a | 0 a | 7.2 a | |
| 40 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, G.; Marí, A.I.; Aibar, J.; Vilaplana, L.; Cirujeda, A. Bastard Cabbage (Rapistrum rugosum L.) Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium in Spain and Alternative Herbicides for Its Control. Agronomy 2019, 9, 492. https://doi.org/10.3390/agronomy9090492
Pardo G, Marí AI, Aibar J, Vilaplana L, Cirujeda A. Bastard Cabbage (Rapistrum rugosum L.) Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium in Spain and Alternative Herbicides for Its Control. Agronomy. 2019; 9(9):492. https://doi.org/10.3390/agronomy9090492
Chicago/Turabian StylePardo, Gabriel, Ana I. Marí, Joaquín Aibar, Lluïsa Vilaplana, and Alicia Cirujeda. 2019. "Bastard Cabbage (Rapistrum rugosum L.) Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium in Spain and Alternative Herbicides for Its Control" Agronomy 9, no. 9: 492. https://doi.org/10.3390/agronomy9090492
APA StylePardo, G., Marí, A. I., Aibar, J., Vilaplana, L., & Cirujeda, A. (2019). Bastard Cabbage (Rapistrum rugosum L.) Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium in Spain and Alternative Herbicides for Its Control. Agronomy, 9(9), 492. https://doi.org/10.3390/agronomy9090492





