1. Introduction
In Europe there are currently increasing concerns about herbicide resistance and the promotion of new regulations for limiting the dependence on synthetic herbicides, which can be dangerous to human health and the environment, and thus other methods for controlling weeds must be found. Thermal weed control technology plays an important role in managing weeds in synthetic herbicide-free systems, particularly in organic agriculture [
1,
2,
3,
4,
5,
6]. Thermal weed control involves heat being transferred to plant material (leaves, stems, flowers, propagules, etc.) in order to destroy cell structures and cause the denaturation of proteins [
1,
2]. Several factors influence the heat injury of the plants, including temperature, energy input, exposure period, and weed species [
7]. Most thermal methods affect the aboveground portion of the plants, but some weeds (i.e., perennial weeds) may regrow from their belowground components and thus repeated application of thermal control is required [
7,
8]. Flaming, hot water and steam are the primary heat sources for weed control purposes in agriculture and on hard surfaces in urban areas and have the advantage of not inducing plant resistance [
1,
3,
4,
5,
6].
Hot foam represents an evolution of the hot water weed control method, modified by the addition of biodegradable foaming agents, and was first patented in 1995 [
2,
9]. Hot foam weed control is a technique with limited risks to the environment and human health and is applicable for numerous weed species [
10]. The foam insulates the weeds from the surrounding air and increases the energy transfer to the plants, thus lowering the dose of hot water required and increasing efficiency [
2]. Compared to hot water alone, the addition of foam has the advantages of less water being required, less susceptibility to weather changes, a high application accuracy and speed, more heat transfer time, and a low cost [
7,
10].
To control above-ground vegetation with hot foam, as for other thermal weed control methods the heat requirement depends on the weed species, their growth stages, their water status and the presence of moisture on the leaf surface [
1]. The dose applied is a critical parameter that can determine the efficacy of weed control, and an appropriate dose can increase the overall efficiency [
2].
Hot foam has been used to control weeds in cotton fields [
8], but the application of this high-energy demand weed control method (due to the high thermal capacity of water [
11]) is more realistic in urban area contexts (e.g., on pavements) [
12]. The growth of weeds on road pavements is different from that in a field, because the characteristics of the pavements affect the weed growth (i.e., fewer appear on frequently used roads with small joints than in infrequently used pavements with medium or wide joints) [
12,
13]. In Sweden hot foam was used to control weeds along railways [
2].
The aim of this study was to test the weeding effect of five different hot foam doses in two different weed composition fields by evaluating the weed devitalisation, regrowth, and dry biomass at the end of the experiment, and the temperature of hot foam as affected by different foam doses.
4. Discussion
One day after the hot foam application, the plant structures were not completely denatured, and the green colour persisted in the damaged leaves in all the experiments. Two days after the treatment, some green pigments had not yet brown in both the
Festuca arundinacea (Schreb.) and
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) infested field experiments, although the majority of the leaf structures appeared damaged. The effect of hot foam on weeds was fully visible three days after the application of treatments. The green pigments contained in the damaged weeds took a range from one to three days to turn brown, with a higher number of days at lower foam doses (
Table 4 and
Table 5). This delay in the desiccation is typical of all thermal weed control applications, such as steam, hot water and flaming, because the heat melts the membrane cuticle of the plant leaves and breaks down the plant cell structures, so the plant is unable to retain moisture and dehydrates within a few hours or days [
7]. A desiccation that requires a couple of days is consistent with the range of time observed for desiccation from hot foam.
Three days after the treatments only the 1.67 L m
−2 dose did not attain 0% of weed cover for
Festuca arundinacea (Schreb.) and in site II for
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) field experiments. In site I for
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) the dose of 3.33 L m
−2 did not attain 100% weed control (
Table 4 and
Table 5). Plant response to heat is known to be species-dependent [
28]. Weed leaf tissue death requires less heating exposure time when the maximum temperature increases. Both generative and vegetative propagules of most weed species are killed within a temperature range of 60 to 80 °C, and the duration of heating needed to reach mortality is determined by the thermal conductivity (e.g., higher with water than with gasses) and the maximum temperature reached (a high temperature can be effective within seconds) [
1]. As temperature increased linearly, the measurements show that the heat-killing time decreases exponentially [
29].
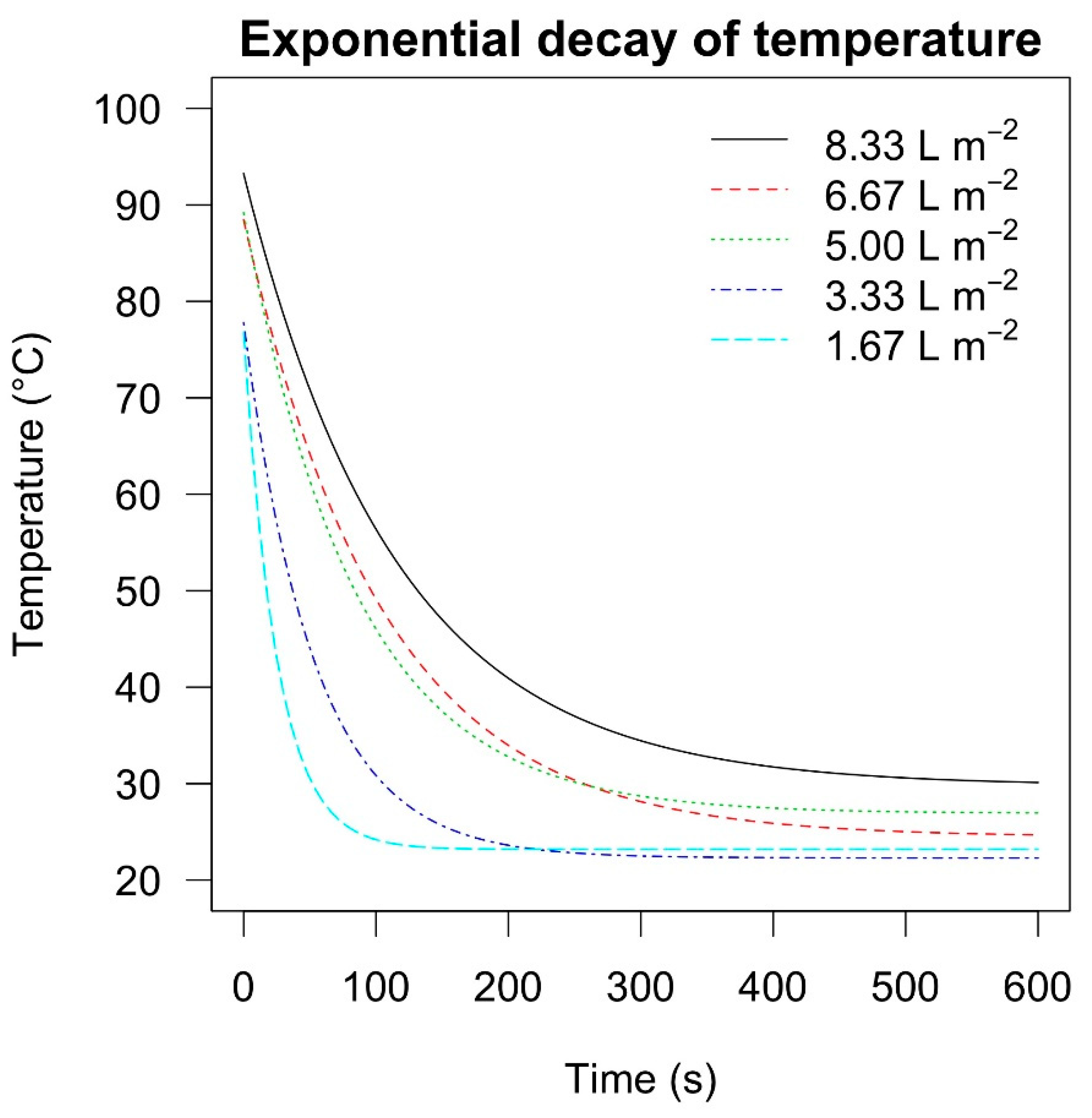
The highest maximum peak temperature (93 °C) estimated at the highest foam dose (i.e., 8.33 L m
−2) led to 100% weed devitalisation (0% of weed cover), and likewise, doses of 3.33 L m
−2, 5.00 L m
−2, and 6.67 L m
−2 in both sites of the the
Festuca arundinacea (Schreb.) and site II of
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) field experiments, whereas the maximum temperatures estimated at the lowest dose (i.e., 1.67 L m
−2) provided a significantly higher level of weed cover percentage. This suggests that the average peak temperature of 77 °C (obtained with the dose of 1.67 L m
−2), and the temperatures of 70 °C, 60 °C and 50 °C, reached after 3 s, 9 s and 17 s, respectively, were not enough to completely desiccate these weed species (
Table 1). Instead, an average peak temperature of 78 °C, and temperatures of 70 °C, 60 °C, and 50 °C, reached after 8 s, 21 s, and 37 s, respectively, obtained 100% of weed control (
Table 1). However, these temperatures did not result in 100% of weed devitalisation in site I with
Taraxacum officinale (Weber) and
Plantago lanceolata (L.), where the average peak temperature was 89 °C (obtained with the dose of 5.00 L m
−2), and temperatures of 70 °C, 60 °C, and 50 °C reached after 31 s, 54 s and 84 s, respectively, were needed to obtain 100% weed control (
Table 1).
The analysis of the effect of doses on weed cover regrowth and weed dry biomass at the end of the experiment enabled an evaluation of whether the higher hot foam doses damaged not only the leaves, but also the protected meristems, which are typical of grasses and perennial weeds such as
Festuca arundinacea (Schreb.),
Taraxacum officinale (Weber), and
Plantago lanceolata (L.) [
8]. Most weeds with protected meristems will regrow after a single hot water treatment [
13], and a delay in the regrowth of the studied weed species may suggest higher injury at the meristem level.
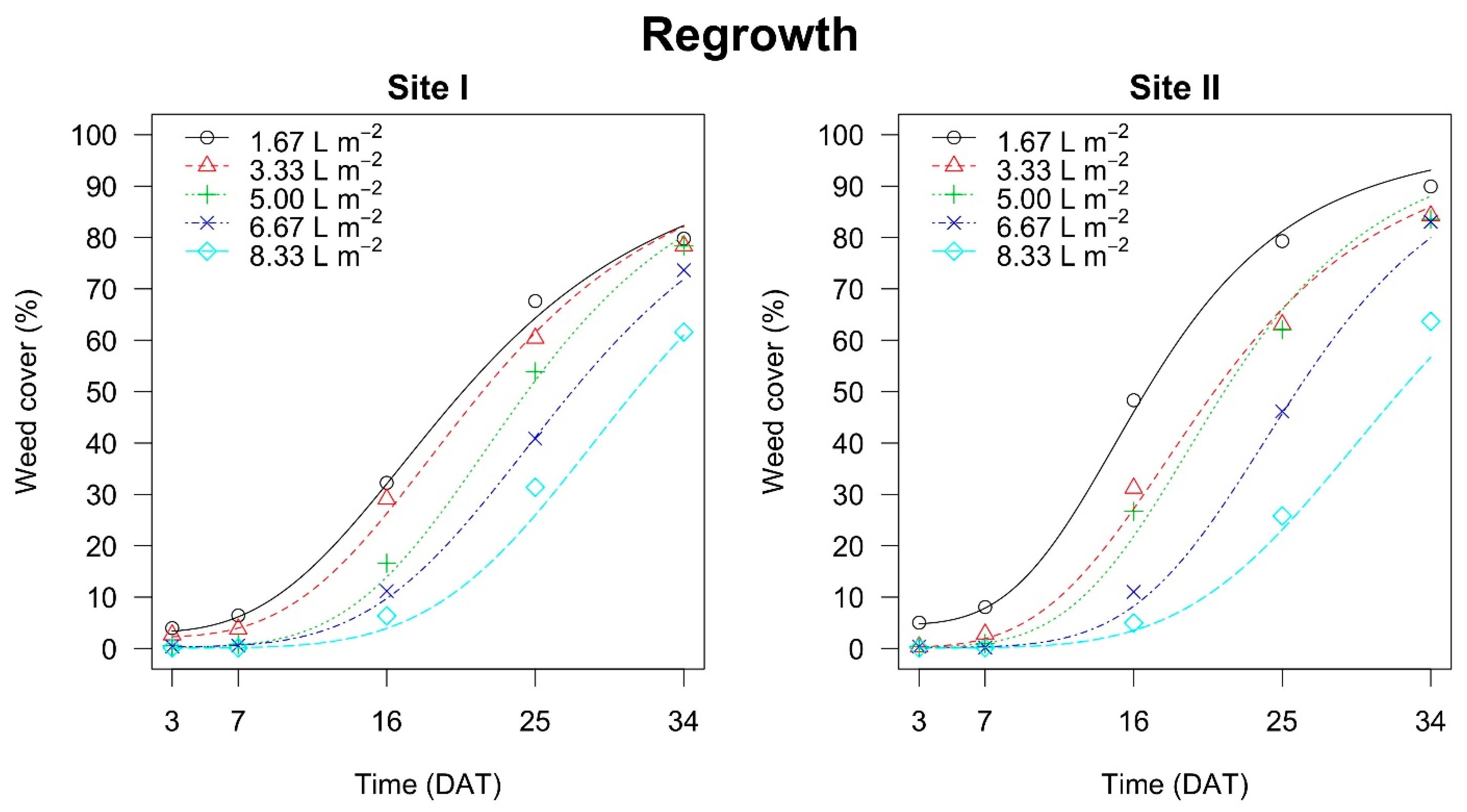
In both the
Festuca arundinacea (Schreb.) field experiment sites the effect of the higher hot foam doses only exhibited a significant delay when attaining 10% and 25% weed cover, whereas after about 30 days all doses again attained 50% weed cover. In site II, with the
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) field, the highest dose (i.e., 8.33 L m
−2) significantly delayed the regrowth of weeds (10%, 25% and 50%, respectively), compared to the other doses, probably because the temperatures recorded when this dose was used was more effective in damaging the meristems of these weed species than those at lower doses. In site I, the effect of the highest dose on weed regrowth when reaching 10% and 25% of weed cover was the same as in site II, whereas the time for reaching 50% of weed cover was similar for doses of 6.67 L m
−2 and 8.33 L m
−2 (
Table 6 and
Table 7).
When lower hot foam doses where used, regrowth was faster for the
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) than for the
Festuca arundinacea (Schreb.) infested fields (
Table 6 and
Table 7), probably because the former two have prostrate and large leaves with creeping stems, and if their meristems are less injured by lower temperatures, they may contribute to a higher coverage than a grassy weed [
13]. This effect was not observable with the highest doses, where the times of regrowth were similar between species.
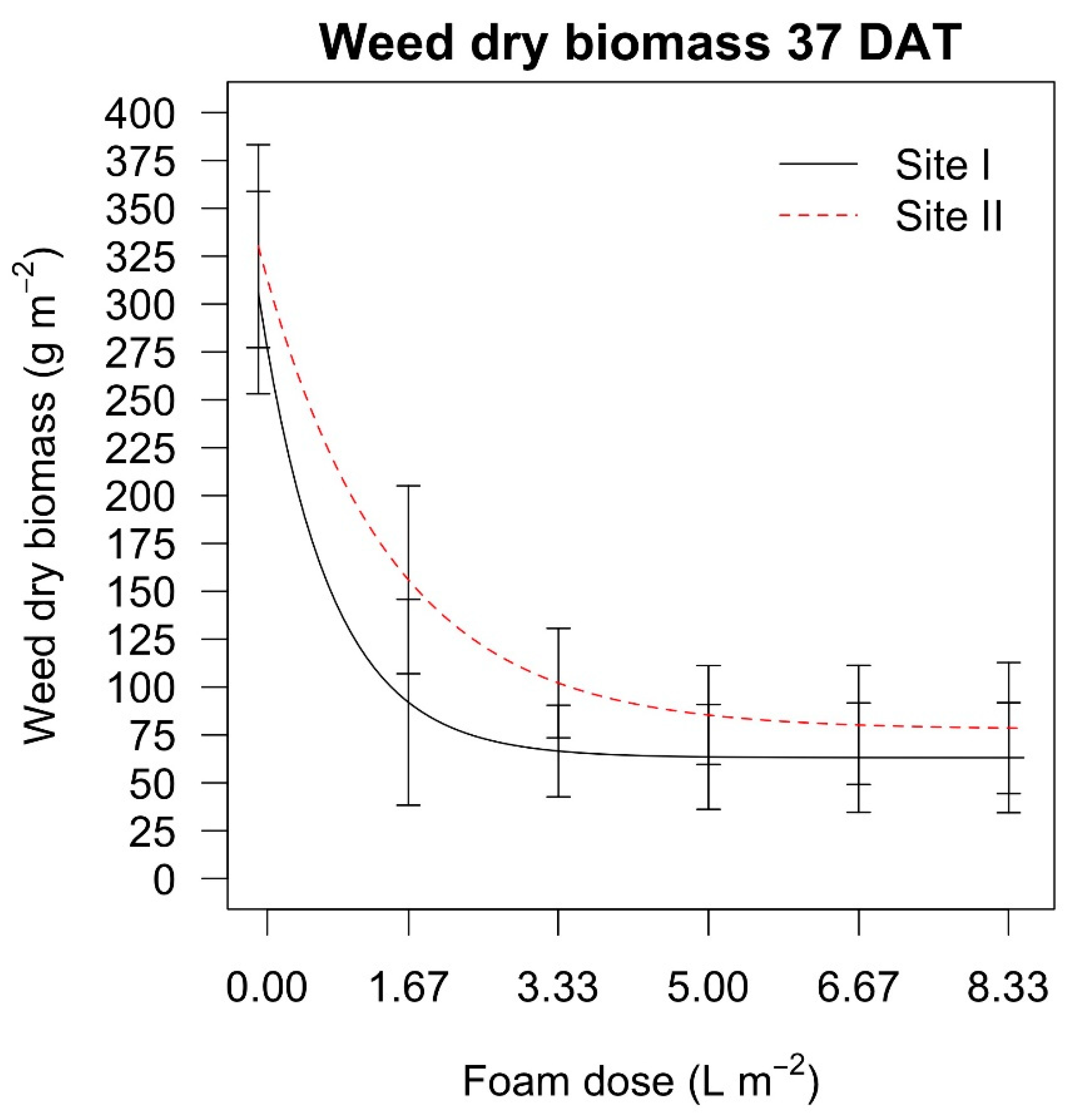
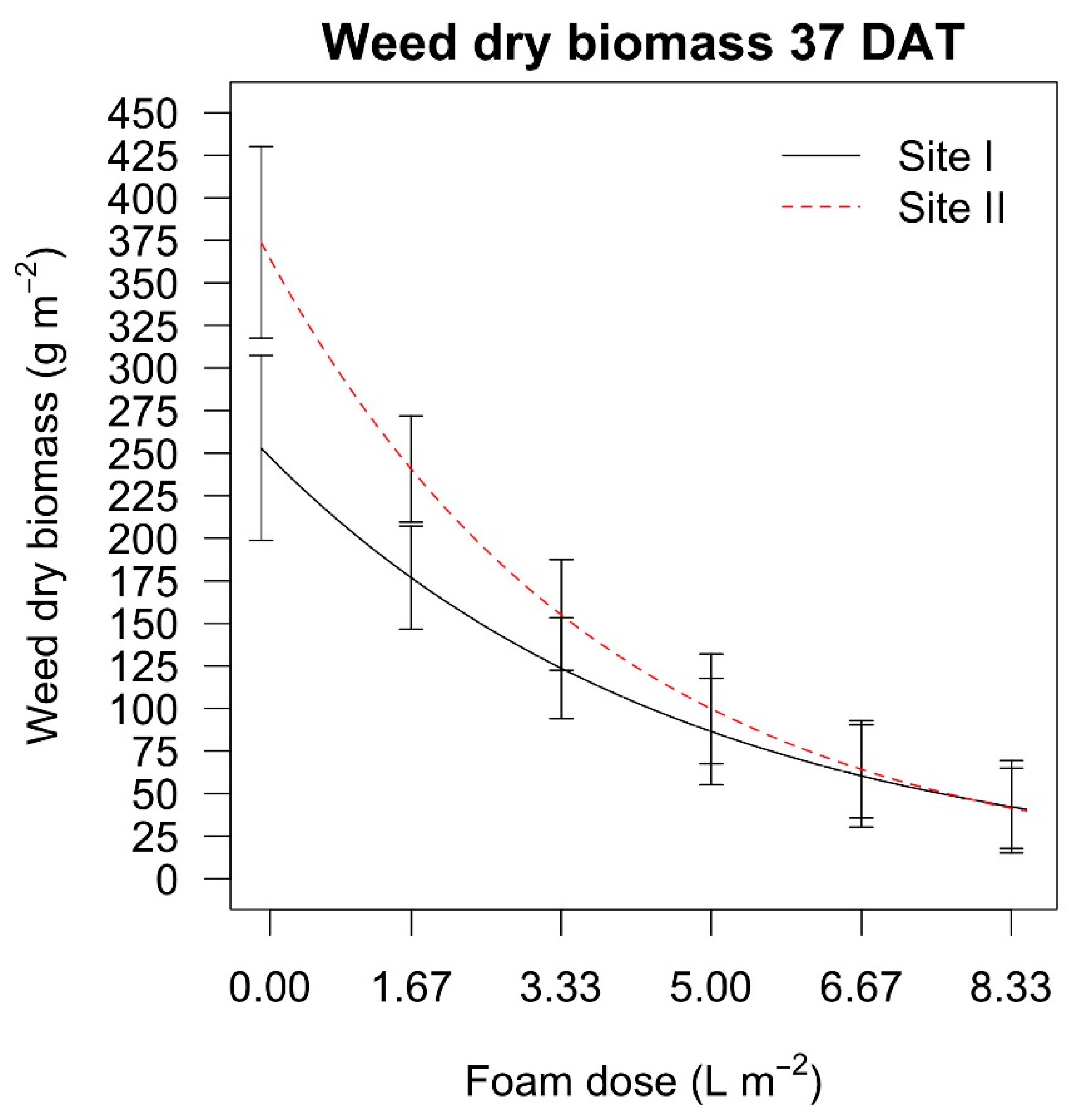
The dry weed biomass 37 days after the treatments in the
Festuca arundinacea (Schreb.) infested field experiment was higher only in site II when the dose of 1.67 L m
−2 was applied, whereas the other doses led to similar biomasses. In the
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) infested field experiment the effect of the hot foam dose was more marked than for the grassy species, and the dry biomass decreased when increasing the dose (
Table 8).
The highest estimated time needed for weeds to regrow and provide 50% soil cover was on average 30 days. This time was estimated for all hot foam doses in the
Festuca arundinacea (Schreb.) infested fields, and for the doses of 6.67 L m
−2 and 8.33 L m
−2 in site I and 8.33 L m
−2 in site II in the
Taraxacum officinale (Weber) and
Plantago lanceolata (L.) infested field. One month for 50% of weed regrowth in an open field study is a long time, considering that the weeds came from a real infested field (not seeded or transplanted in pots before the application of the hot foam), and thus the weeds had developed roots in ideal conditions. A window of 30 days before a weed cover regrowth of 50% is sufficient to guarantee a competitive advantage against weeds for a transplanted plant. Thus, hot foam may have the potential for future use not only in urban areas and railways (where its application is common in north European countries such as Sweden) [
2,
12], but also as a desiccant for controlling weeds in no-till soil bands before the transplant of high-income vegetable crops.