Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park
Abstract
1. Introduction
2. Materials and Methods
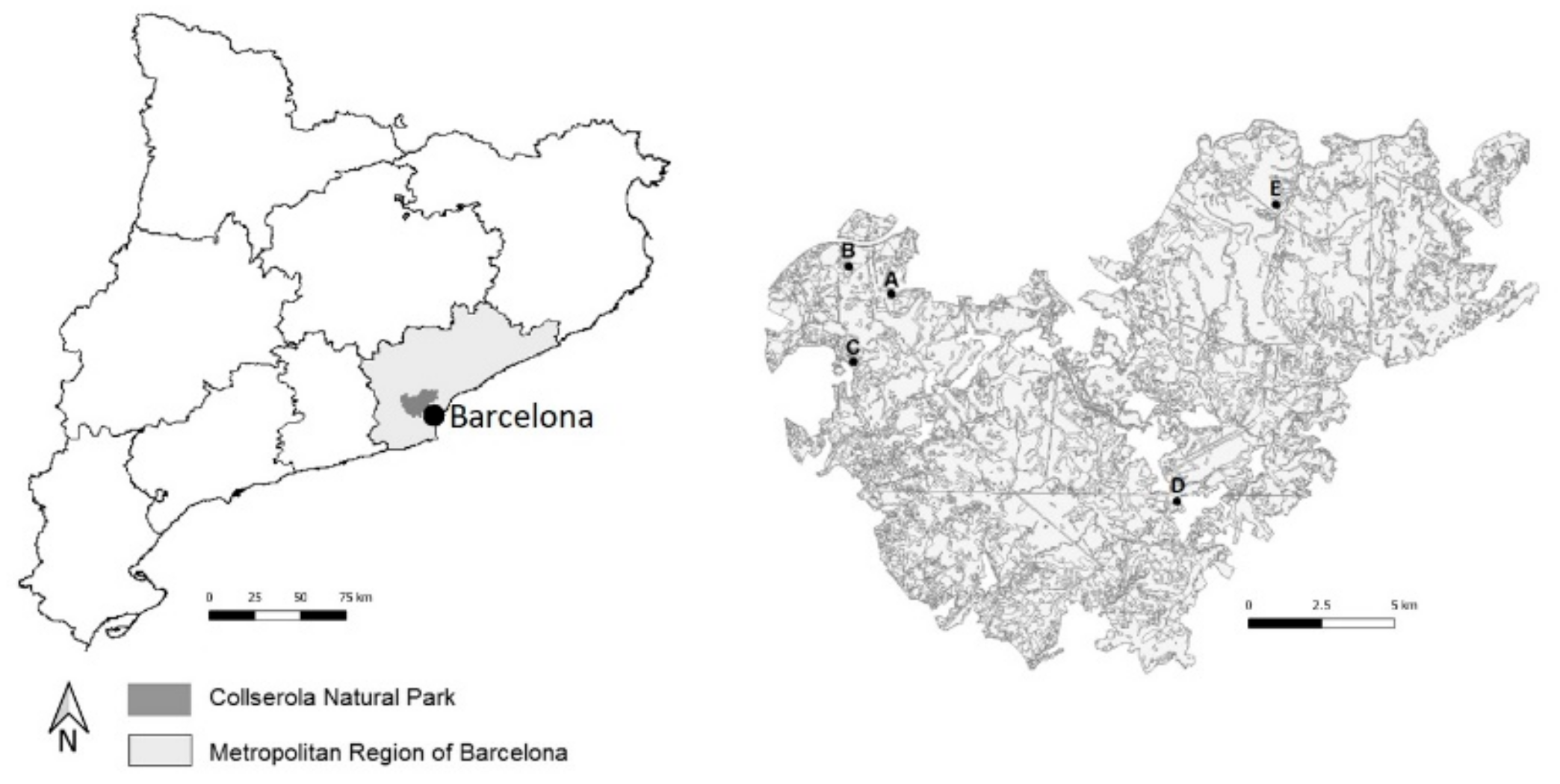
2.1. Plant Materials
2.2. Experimental Design
2.3. Phenotyping by Breeders
2.4. Phenotyping by Farmers
2.5. Phenotyping by Consumers
2.6. Statistical Analyses
3. Results
3.1. Farmers’ Ideotype
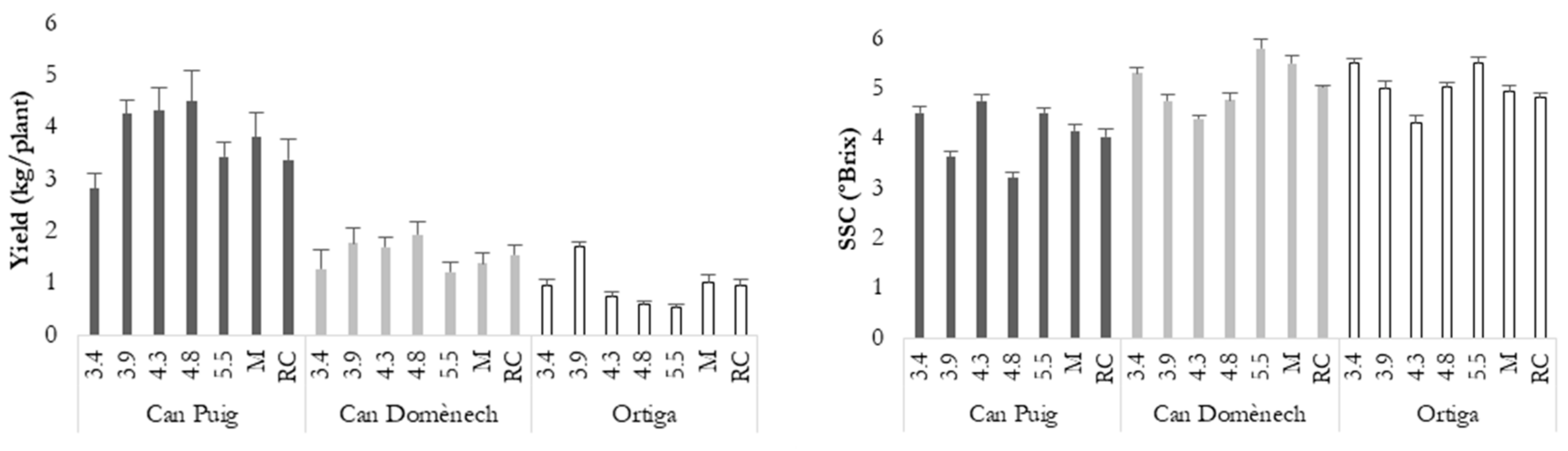
3.2. Genotype and Environmental Effects
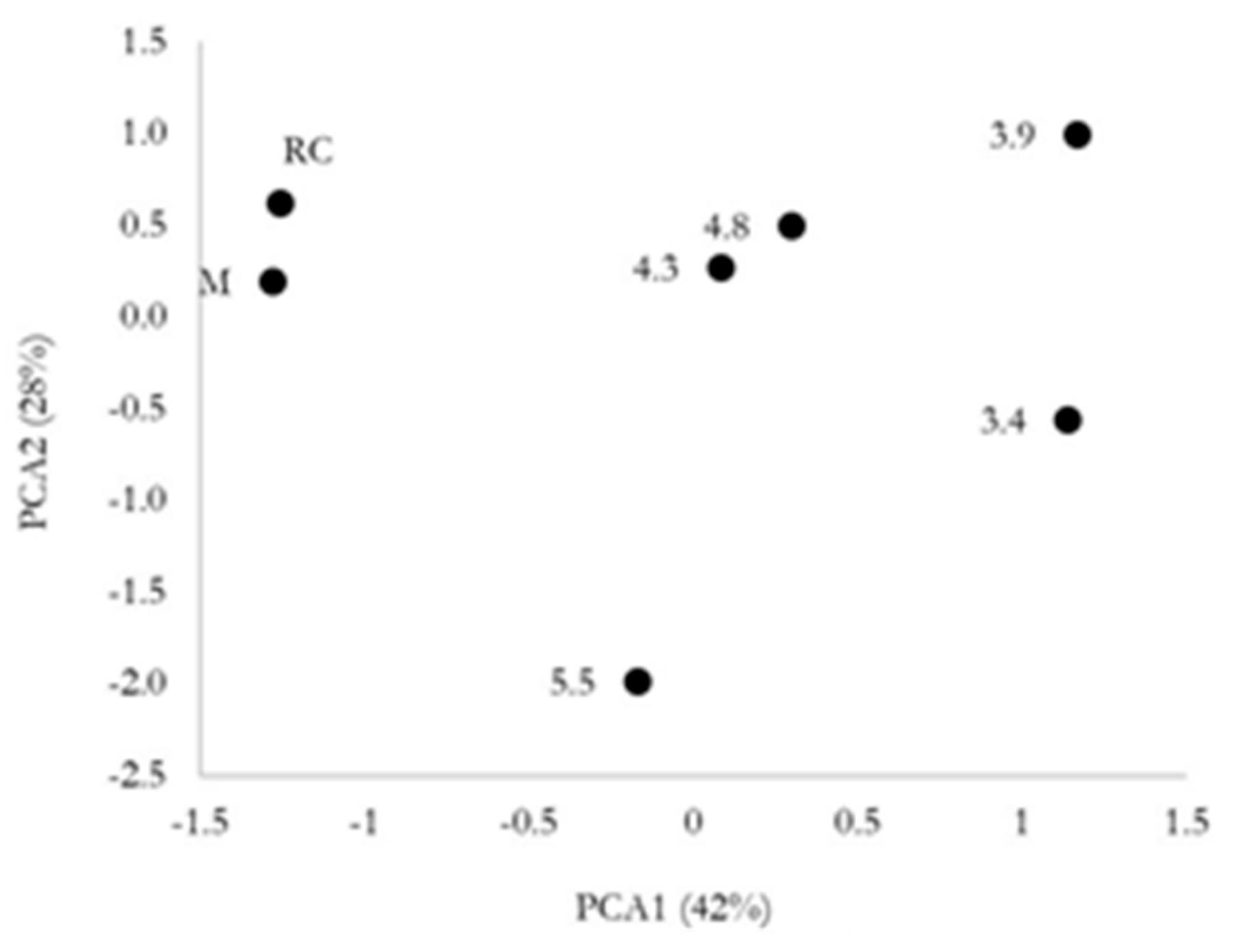
3.3. Farmers’ Evaluation and Yield Stability
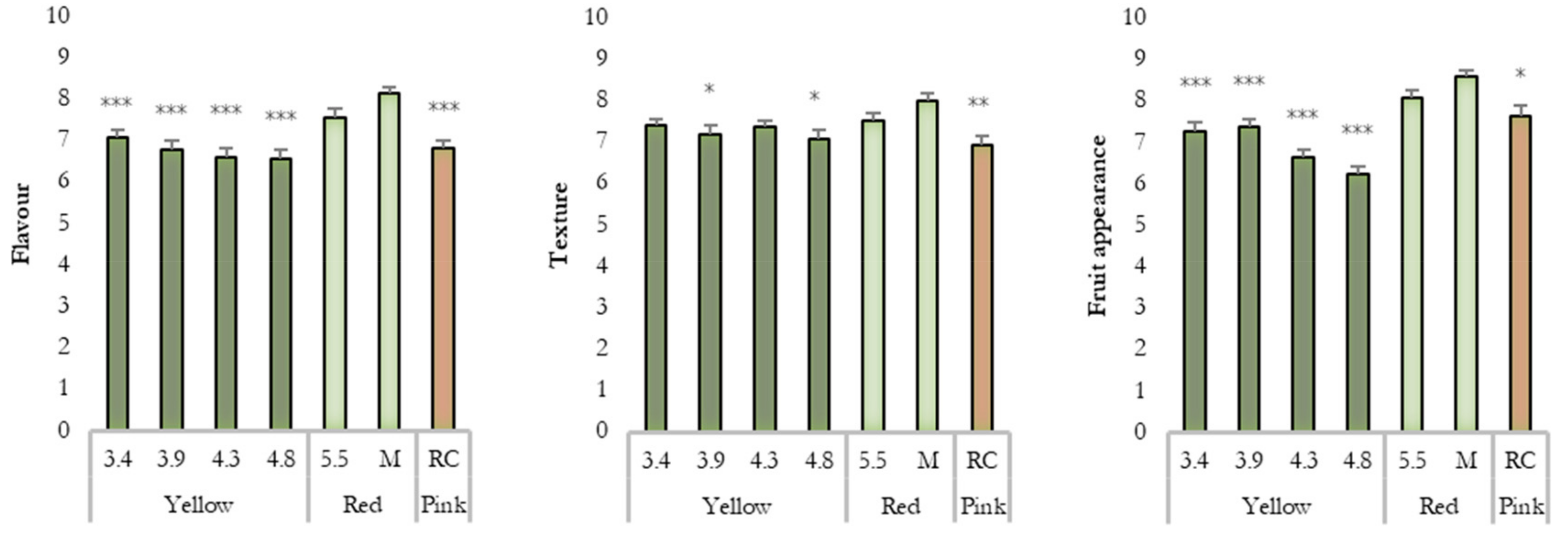
3.4. Consumer Preferences
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ceccarelli, S. Efficiency of Plant Breeding. Crop Sci. 2015, 55, 87–97. [Google Scholar] [CrossRef]
- Almekinders, C.J.M.; Elings, A. Collaboration of farmers and breeders: Participatory crop improvement in perspective. Euphytica 2001, 122, 425–438. [Google Scholar] [CrossRef]
- Dawson, J.C.; Goldberger, J.R. Assessing farmer interest in participatory plant breeding: Who wants to work with scientists? Renew. Agric. Food Syst. 2008, 23, 177–187. [Google Scholar] [CrossRef]
- Van Bueren, E.T.L.; Jones, S.S.; Tamm, L.; Murphy, K.M.; Myers, J.R.; Leifert, C.; Messmer, M.M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS-Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Decentralized-participatory plant breeding: An example of demand driven research. Euphytica 2007, 155, 349–360. [Google Scholar] [CrossRef]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- De Ponti, T.; Rijk, B.; van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Joshi, K.D.; Witcombe, J.R. Participatory varietal selection in rice in Nepal in favourable agricultural environments—A comparison of two methods assessed by varietal adoption. Euphytica 2002, 127, 445–458. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Participatory plant breeding: Who did it, who does it and where? Exp. Agric. 2019. [Google Scholar] [CrossRef]
- Hoagland, L.; Navazio, J.; Zystro, J.; Kaplan, I.; Vargas, J.G.; Gibson, K. Key traits and promising germplasm for an organic participatory tomato breeding program in the U.S. midwest. Hortscience 2015, 50, 1301–1308. [Google Scholar] [CrossRef]
- Campanelli, G.; Acciarri, N.; Campion, B.; Delvecchio, S.; Leteo, F.; Fusari, F.; Angelini, P.; Ceccarelli, S. Participatory tomato breeding for organic conditions in Italy. Euphytica 2015, 204, 179–197. [Google Scholar] [CrossRef]
- Campanelli, G.; Sestili, S.; Acciarri, N.; Montemurro, F.; Palma, D.; Leteo, F.; Beretta, M. Multi-parental advances generation inter-cross population, to develop organic tomato genotypes by participatory plant breeding. Agronomy 2019, 9, 119. [Google Scholar] [CrossRef]
- Mazzucato, A.; Ficcadenti, N.; Caioni, M.; Mosconi, P.; Piccinini, E.; Sanampudi, V.R.R.; Sestili, S.; Ferrari, V. Genetic diversity and distinctiveness in tomato (Solanum lycopersicum L.) landraces: The Italian case study of ‘A pera Abruzzese’. Sci. Hortic. 2010, 125, 55–62. [Google Scholar] [CrossRef]
- Casals, J.; Pascual, L.; Canizares, J.; Cebolla-Cornejo, J.; Casanas, F.; Nuez, F. The risks of success in quality vegetable markets: Possible genetic erosion in Marmande tomatoes (Solanum lycopersicum L.) and consumer dissatisfaction. Sci. Hortic. 2011, 130, 78–84. [Google Scholar] [CrossRef]
- Casals, J.; Rull, A.; Bernal, M.; González, R.; del Castillo, R.R.; Simó, J. Impact of grafting on sensory profile of tomato landraces in conventional and organic management systems. Hortic. Environ. Biotechnol. 2018, 59, 597–606. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Tanksley, S.D. High-resolution mapping and functional analysis of se2.1: A major stigma exsertion quantitative trait locus associated with the evolution from allogamy to autogamy in the genus Lycopersicon. Genetics 2004, 168, 1563–1573. [Google Scholar] [CrossRef]
- Council of the European Union Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91; Oficial Journal of the European Union: Brussels, Belgium, 2007; p. L189/1-23.
- Casals, J.; Rivera, A.; Sabaté, J.; Romero del Castillo, R.; Simó, J. Cherry and fresh market tomatoes: Differences in chemical, morphological, and sensory traits and their implications for consumer acceptance. Agronomy 2019, 9, 9. [Google Scholar] [CrossRef]
- Sinesio, F.; Cammareri, M.; Moneta, E.; Navez, B.; Peparaio, M.; Causse, M.; Grandillo, S. Sensory quality of fresh French and Dutch market tomatoes: A preference mapping study with Italian consumers. J. Food Sci. 2010, 75, S55–S67. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Pereira-Dias, L.; Casanova, C.; García-Martínez, M.D.; Rosa, E.; Soler, E.; Plazas, M.; Soler, S. Insights into the adaptation to greenhouse cultivation of the traditional Mediterranean long shelf-life tomato carrying the alc mutation: A multi-trait comparison of landraces, selections, and hybrids in open field and greenhouse. Front. Plant. Sci. 2018, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop. Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Ortiz, R.; Izquierdo, J. Yield stability differences among tomato genotypes grown in Latin America and the Caribbean. HortScience 1994, 29, 1175–1177. [Google Scholar] [CrossRef]
- Causse, M.; Friguet, C.; Coiret, C.; LéPicier, M.; Navez, B.; Lee, M.; Holthuysen, N.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer preferences for fresh tomato at the European scale: A common segmentation on taste and firmness. J. Food Sci. 2010, 75, S531–S541. [Google Scholar] [CrossRef] [PubMed]
- Pacicco, L.; Bodesmo, M.; Torricelli, R.; Negri, V. A methodological approach to identify agro-biodiversity hotspots for priority in situ conservation of plant genetic resources. PLoS ONE 2018, 13, e0197709. [Google Scholar] [CrossRef] [PubMed]
- Pico, B.; Herraiz, J.; Ruiz, J.J.; Nuez, F. Widening the genetic basis of virus resistance in tomato. Sci. Hortic. 2002, 94, 73–89. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sarli, G.; Logozzo, G.; Spagnoletti Zeuli, P. Landraces in inland areas of the Basilicata region, Italy: Monitoring and perspectives for on farm conservation. Genet. Resour. Crop Evol. 2012, 59, 701–716. [Google Scholar] [CrossRef]
- Casals, J.; Casañas, F.; Simó, J. Is it still necessary to continue to collect crop genetic resources in the Mediterranean area? A case study in Catalonia. Econ. Bot. 2017, 71, 330–341. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Kik, C.; van Hintum, T.; van Treuren, R.; Visser, B. Genetic erosion in crops: Concept, research results and challenges. Plant Genet. Resour. 2010, 8, 1–15. [Google Scholar] [CrossRef]
- Maeder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Desclaux, D.; Nolot, J.M.; Chiffoleau, Y.; Gozé, E.; Leclerc, C. Changes in the concept of genotype × environment interactions to fit agriculture diversification and decentralized participatory plant breeding: Pluridisciplinary point of view. Euphytica 2008, 163, 533. [Google Scholar] [CrossRef]
- Georgelis, N.; Scott, J.W.; Baldwin, E.A. Relationship of tomato fruit sugar concentration with physical and chemical traits and linkage of RAPD markers. J. Am. Soc. Hortic. Sci. 2004, 129, 839–845. [Google Scholar] [CrossRef]
- Ho, L.C. The mechanism of assimilate partitioning and carbohydrate compartmentation in fruit in relation Ito the quality and yield of tomato. J. Exp. Bot. 1996, 47, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Panthee, D.; Labate, J.; McGrath, M.; Breksa III, A.; Robertson, L. Genotype and environmental interaction for fruit quality traits in vintage tomato varieties. Euphytica 2013, 193, 169–182. [Google Scholar] [CrossRef]
- Panthee, D.R.; Cao, C.; Debenport, S.J.; Rodríguez, G.R.; Labate, J.A.; Robertson, L.D.; Breksa, A.P.; van der Knaap, E.; McSpadden Gardener, B.B. Magnitude of genotype × environment interactions affecting tomato fruit quality. HortScience 2012, 47, 721–726. [Google Scholar] [CrossRef]
- Prudent, M.; Causse, M.; Genard, M.; Tripodi, P.; Grandillo, S.; Bertin, N. Genetic and physiological analysis of tomato fruit weight and composition: Influence of carbon availability on QTL detection. J. Exp. Bot. 2009, 60, 923–937. [Google Scholar] [CrossRef]
- Sperling, L.; Loevinsohn, M.E.; Ntabomvura, B. Rethinking the farmer’s role in plant breeding: Local bean experts and on-station selection in Rwanda. Exp. Agric. 1993, 29, 509–519. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Russi, L.; Romani, M.; Pecetti, L.; Nazzicari, N. Farmer-participatory vs. conventional market-oriented breeding of inbred crops using phenotypic and genome-enabled approaches: A pea case study. Field Crops Res. 2019, 232, 30–39. [Google Scholar] [CrossRef]
- Casals, J.; Rivera, A.; Figàs, M.R.; Casanova, C.; Camí, B.; Soler, S.; Simó, J. A comparison of landraces vs. modern varieties of lettuce in organic farming during the winter in the Mediterranean area: An approach considering the viewpoints of breeders, consumers, and farmers. Front. Plant Sci. 2018, 9, 1491. [Google Scholar]
- Folta, K.M.; Klee, H.J. Sensory sacrifices when we mass-produce mass produce. Hortic. Res. 2016, 3, 16032. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2017, 22, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef] [PubMed]


| Locality | Can Domènech | Can Puig | Ortiga |
|---|---|---|---|
| pH | 8.32 | 8.41 | 8.34 |
| Electrical conductivity (ds/m) | 0.274 | 0.351 | 0.236 |
| Organic matter (%) | 3.49 | 4.29 | 2.1 |
| Nitrogen (NO3-) (mg/kg) | 13.9 | 40.0 | 13.9 |
| Phosphorus (P) (mg/kg) | 85 | 95 | 62 |
| Potassium (K) (mg/kg) | 609 | 942 | 203 |
| Calcium (Ca) (mg/kg) | 6883 | 7267 | 7172 |
| Magnesium (Mg) (mg/kg) | 383 | 430 | 277 |
| Textural class | Loam | Loam | Loam |
| Sand (0.05< D <2 mm) (%) | 38.3 | 38.8 | 42.2 |
| Silt coarse (0.02< D< 0.05 mm) (%) | 22.9 | 20.4 | 20.6 |
| Silt fine and medium (0.002< D< 0.02 mm) (%) | 15.9 | 18.3 | 16.6 |
| Clay (D< 0.002 mm) (%) | 22.9 | 22.6 | 20.6 |
| Trait | Mean score | |
|---|---|---|
| Flavor | 9.6 | a |
| Fruit color and shape | 9 | ab |
| Resistance to physiological disorders | 8.7 | bc |
| Yield | 8.6 | bc |
| Storability | 8.4 | bc |
| Disease resistance | 8.4 | bc |
| Nutritional quality | 7.9 | cd |
| Water use efficiency | 7.1 | de |
| Nutrient use efficiency | 6.6 | de |
| Plant architecture (growth habit) | 6.4 | de |
| Weed competitiveness | 5.6 | e |
| Agronomic Traits | |||||
| Locality | Genotype | Locality*Genotype | Block (Locality) | Residual | |
| Df 1 | 2 | 6 | 12 | 3 | 176 |
| Yield | 65.7 *** | 4.2 *** | 0.4 *** | 4.7 *** | 25.0 |
| Number of fruits per plant | 37.3 *** | 15.7 *** | 8.0 *** | 9.4 *** | 29.5 |
| Fruit weight | 21.4 *** | 47.5 *** | 8.2 *** | 0.3 ns | 22.5 |
| Cracking | 8.5 *** | 7.6 ** | 8.0 * | 8.7 *** | 67.1 |
| Blossom-end rot | 2.0 ns | 5.3 ns | 11.6 * | 3.3 ns | 77.8 |
| Fruit Quality Traits | |||||
| Locality | Genotype | Locality*genotype | Residual | ||
| df | 2 | 6 | 12 | 80 | |
| SSC 2 | 36.9 *** | 20.5 *** | 17.9 *** | 24.7 | |
| Firmness | 13.3 *** | 30.0 *** | 6.2 ns | 50.6 | |
| pH | 24.7 *** | 45.4 *** | 21.6 *** | 8.3 | |
| Dry matter | 38.4 *** | 18.0 *** | 17.6 *** | 26.0 | |
| Titratable acidity | 4.3 ** | 52.6 *** | 12.5 *** | 30.6 | |
| L *,3 | 8.5 *** | 38.5 *** | 18.7 *** | 34.3 | |
| a * | 2.5 *** | 44.6 *** | 24.4 *** | 28.4 | |
| b * | 3.6 *** | 68.3 *** | 9.4 *** | 18.7 | |
| PCA1 (42%) | PCA2 (28%) | |
|---|---|---|
| Potential yield_Ortiga | 0.589 | 0.358 |
| Fruit appearance_Ortiga | 0.226 | 0.943 |
| Fruit marketability_Ortiga | 0.752 | −0.143 |
| Potential yield_CanPuig | 0.648 | −0.398 |
| Fruit appearance_CanPuig | 0.205 | 0.826 |
| Fruit marketability_CanPuig | −0.416 | −0.017 |
| Potential yield_Can Domènech | 0.916 | −0.296 |
| Fruit appearance_Can Domènech | 0.728 | 0.570 |
| Fruit marketability_Can Domènech | 0.876 | −0.432 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casals, J.; Rull, A.; Segarra, J.; Schober, P.; Simó, J. Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park. Agronomy 2019, 9, 486. https://doi.org/10.3390/agronomy9090486
Casals J, Rull A, Segarra J, Schober P, Simó J. Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park. Agronomy. 2019; 9(9):486. https://doi.org/10.3390/agronomy9090486
Chicago/Turabian StyleCasals, Joan, Aurora Rull, Joel Segarra, Philipp Schober, and Joan Simó. 2019. "Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park" Agronomy 9, no. 9: 486. https://doi.org/10.3390/agronomy9090486
APA StyleCasals, J., Rull, A., Segarra, J., Schober, P., & Simó, J. (2019). Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park. Agronomy, 9(9), 486. https://doi.org/10.3390/agronomy9090486





