Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Design, Set-Up, and Field Management
2.2. Seed Analysis, Reagents, and Chemicals
2.3. Extraction Procedures
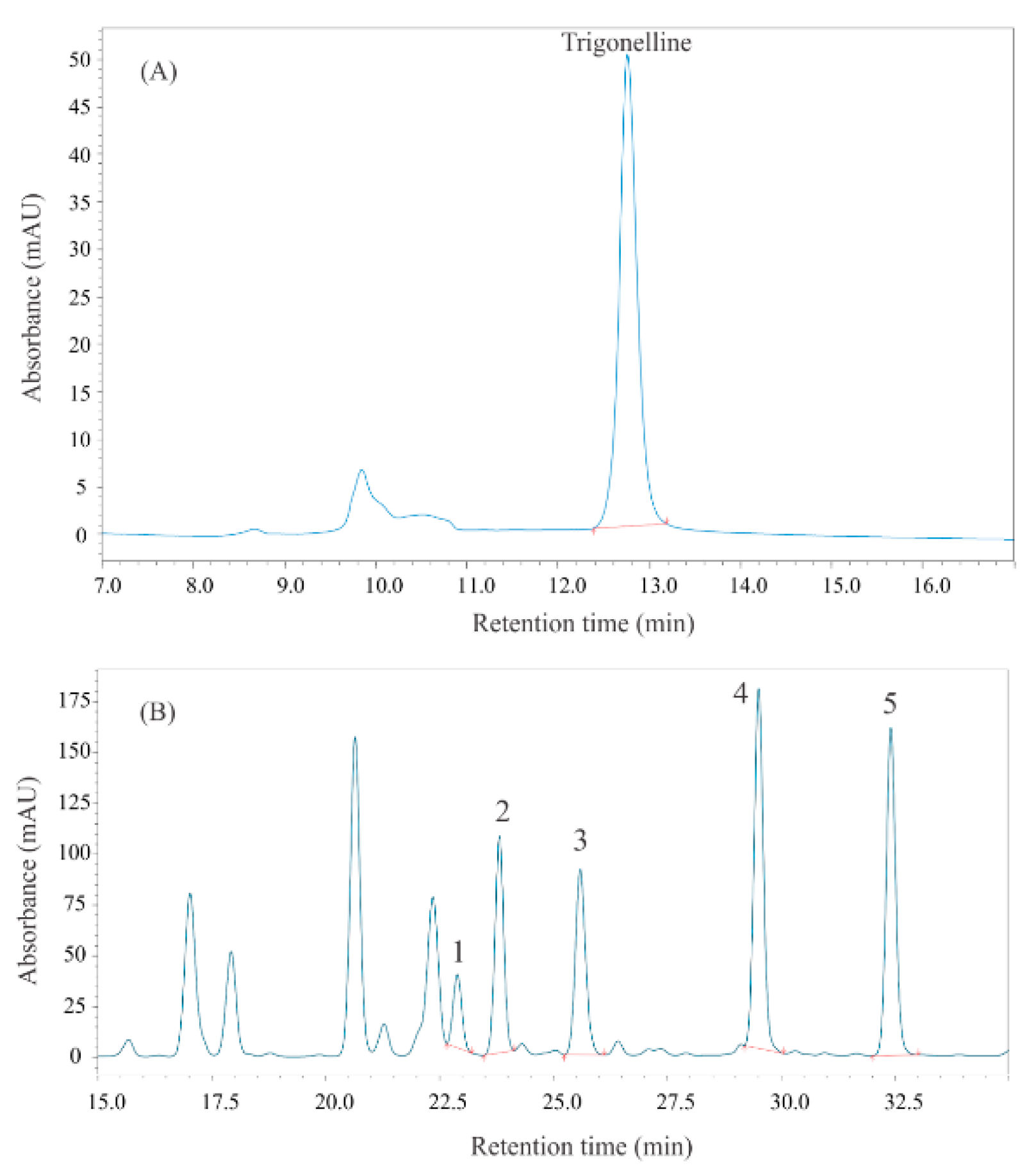
2.3.1. Preparation of Methanolic Extracts for Analysis of Trigonelline
2.3.2. Preparation Of Ethanolic Extracts for Antioxidant Activity and Flavonoids
2.4. Measurements
2.4.1. Quantitative Determination of Trigonelline Content by High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PDA)
2.4.2. Measurement of Antioxidant Activity
DPPH Assay
FRAP Assay
2.4.3. Measurement of Total Phenolic Content (TPC)
2.4.4. Measurement of Total Flavonoids Content (TFC)
2.4.5. Quantitative Measurement of Specific Flavonoids by High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PDA)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Trigonelline Content (TC)
3.2. Antioxidant Activity
3.2.1. DPPH Assay
3.2.2. FRAP Assay
3.3. Total Phenolic Content (TPC)
3.4. Total Flavonoid Content (TFC)
3.5. Flavonoids Compounds Content
3.5.1. Vitexin and Isovitexin
3.5.2. Orientin and Isoorientin
3.6. Correlations among Antioxidant Activity, Total Phenolic, Total Flavonoids, and Specific Flavonoid Compounds Content
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pacheco, A.L.; Pagliarini, M.F.; de Freitas, G.B.; Santos, R.H.; Serrão, J.E.; Zanuncio, J.C. Mineral composition of pulp and production of the yellow passion fruit with organic and conventional fertilizers. Food Chem. 2017, 217, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, K.; Somashekarappa, H.M. Estimation of radionuclides concentration and average annual committed effective dose due to ingestion for some selected medicinal plants of South India. J. Radiat. Res. Appl. Sci. 2016, 9, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological properties of fenugreek (Trigonella foenum graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-u’datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 2011, 6, 596–605. [Google Scholar] [CrossRef]
- Ahmad, A.; Alghamdi, S.S.; Mahmood, K.; Afzal, M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016, 23, 300–310. [Google Scholar] [CrossRef]
- Kang, L.P.; Zhao, Y.; Pang, X.; Yu, H.S.; Xiong, C.Q.; Zhang, J.; Gao, Y.; Yu, K.; Liu, C.; Ma, B.P. Characterization and identification of steroidal saponins from the seeds of Trigonella foenum-graecum by ultra high-performance liquid chromatography and hybrid timeof-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 257–267. [Google Scholar] [CrossRef]
- Chatterjee, S.; Variyar, P.S.; Sharma, A. Stability of lipid constituents in the radiation processed fenugreek seeds and turmeric: Role of phenolic antioxidants. J. Agric. Food Chem. 2009, 57, 9226–9233. [Google Scholar] [CrossRef]
- Shang, M.; Cai, S.; Han, J.; Li, J.; Zhao, Y.; Zheng, J.; Namba, T.; Kadota, S.; Tezuka, Y.; Fan, W. Studies on flavonoids from fenugreek (Trigonaella foenum graecum L.). Zhongguo Zhong Yao Za Zhi 1998, 23, 614–639. [Google Scholar]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Vrchotova, N. The influence of organic and conventional crop management, variety and year on the yield and flavonoid level in common buckwheat groats. Food Chem. 2011, 127, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, E.A.E.; Salih, S.S.M.; Elhussein, A.A.; Babiker, E.E. Effects of intercropping, Bradyrhizobium inoculation and chicken manure fertilization on the chemical composition and physical characteristics of soybean seed. Food Chem. 2009, 112, 690–694. [Google Scholar] [CrossRef]
- Newton, G.L.; Bernard, J.K.; Hubbard, R.K.; Allison, J.R.; Lowrance, R.R.; Gascho, G.J.; Gates, R.N.; Vellidis, G. Managing manure nutrients through multi crop forage production. J. Dairy Sci. 2003, 86, 2243–2252. [Google Scholar] [CrossRef]
- Salehi, A.; Fallah, S.; Kaul, H.-P.; Zitterl-Eglseer, K. Antioxidant capacity and polyphenols in buckwheat seeds from fenugreek/buckwheat intercrops as influenced by fertilization. J. Cereal Sci. 2018, 84, 142–150. [Google Scholar] [CrossRef]
- Salehi, A.; Fallah, S.; Kaul, H.-P. Broiler litter and inorganic fertilizer effects on seed yield and productivity of buckwheat and fenugreek in row intercropping. Arch. Agron. Soil Sci. 2017, 63, 1121–1136. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczynska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Buhk, C.; Alt, M.; Steinbauer, M.J.; Beierkuhnlein, C.; Warren, S.D.; Jentsch, A. Homogenizing and diversifying effects of intensive agricultural land-use on plant species beta diversity in Central Europe-a call to adapt our conservation measures. Sci. Total Environ. 2017, 576, 225–233. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yan, X.; Li, J.; Jiao, N.; Hu, S. Biochar amendments increase the yield advantage of legume-based intercropping systems over monoculture. Agric. Ecosyst. Environ. 2017, 237, 16–23. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jornsgaard, B.; Kinane, J.; Jensen, E.S. Grain legume-cereal intercropping: The practical application of diversity, competition and facilitation in arable and organic cropping systems. Renew. Agric. Food Syst. 2008, 23, 3–12. [Google Scholar] [CrossRef]
- Costa, A.G.; Bertolucci, S.K.V.; Chagas, J.H.; Ferraz, F.O.; Pinto, J.E.B.P. Biomass production, yield and chemical composition of peppermint essential oil using different organic fertilizer sources. Ciência Agrotecnologia 2013, 37, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Kováčik, J.; Klejdus, B. Induction of phenolic metabolites and physiological changes in chamomile plants in relation to nitrogen nutrition. Food Chem. 2014, 142, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sinkovič, L.; Demšar, L.; Žnidarčič, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Aina, O.E.; Amoo, S.O.; Mugivhisa, L.L.; Olowoyo, J.O. Effect of organic and inorganic sources of nutrients on the bioactive compounds and antioxidant activity of tomato. Appl. Ecol. Environ. Res. 2019, 17, 3681–3694. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Impact of Organic and Inorganic Fertilizers Application on the Phytochemical and Antioxidant Activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Fallah, S.; Neugschwandtner, R.; Mehdi, B.; Kaul, H.P. Growth analysis and land equivalent ratio of fenugreek- buckwheat intercrops at different fertilizer types. Die Bodenkultur J. Land Manag. Food Environ. 2018, 69, 105–119. [Google Scholar] [CrossRef]
- Salehi, A.; Mehdi, B.; Fallah, S.; Kaul, H.P.; Neugschwandtner, R.W. Productivity and nutrient use efficiency with integrated fertilization of buckwheat–fenugreek intercrops. Nutr. Cycl. Agroecosyst. 2018, 110, 407–425. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu, sreagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Leontowicz, M.; Gorinstein, S.; Leontowicz, H.; Krzeminski, R.; Lojek, A.; Katrich, E. Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed cholesterol-containing diets. J. Agric. Food Chem. 2003, 51, 5780–5785. [Google Scholar] [CrossRef] [PubMed]
- Dadrasan, M.; Chaichi, M.R.; Pourbabaee, A.A.; Yazdani, D.; Keshavarz-Afshar, R. Deficit irrigation and biological fertilizer influence on yield and trigonelline production of fenugreek. Ind. Crops Prod. 2015, 77, 156–162. [Google Scholar] [CrossRef]
- Hassanzadeh, E.; Chaichi, M.R.; Mazaheri, D.; Rezazadeh, S.; Badi, H.A.N. Physical and chemical variabilities among domestic Iranian fenugreek (Trigonella foenum-graceum) seeds. Asian J. Plant Sci. 2011, 10, 323–330. [Google Scholar] [CrossRef]
- Baghbani-Arani, A.; Modarres-Sanavy, S.A.M.; Mashhadi-Akbar-Boojar, M.; Mokhtassi-Bidgoli, A. Towards improving the agronomic performance, chlorophyll fluorescence parameters and pigments in fenugreek using zeolite and vermicompost under deficit water stress. Ind. Crops Prod. 2017, 109, 346–357. [Google Scholar] [CrossRef]
- Abdelkader, M.A.I.; Hamad, E.H.A. Evaluation of productivity and competition indices of safflower and fenugreek as affected by intercropping pattern and foliar fertilization rate. Middle East J. Agric. Res. 2015, 4, 956–966. [Google Scholar]
- Facchini, P.J. Alkaloid biosynthesis in plants: Biochemistry cell biology, molecular regulation, and metabolic engineering applications. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 29–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Abbasi Surki, A. The effect of organic manure and chemical fertilizer on essential oil, chemical compositions and antioxidant activity of dill (Anethum graveolens) in sole and intercropped with soybean (Glycine max). J. Clean. Prod. 2018, 199, 18–26. [Google Scholar] [CrossRef]
- Moghbeli, T.; Bolandnazar, S.; Panahande, J.; Raei, Y. Evaluation of yield and its components on onion and fenugreek intercropping ratios in different planting densities. J. Clean. Prod. 2019, 213, 634–641. [Google Scholar] [CrossRef]
- Pandey, V.; Patel, A.; Patra, D.D. Integrated nutrient regimes ameliorate crop productivity, nutritive value, antioxidant activity and volatiles in basil (Ocimum basilicum L.). Ind. Crops Prod. 2016, 87, 124–131. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.P.; Wahab, P.E.M.; Sinniah, U.R. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The impact of organic farming on quality of tomatoes is associated to increased oxidative stress during fruit development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef] [PubMed]
- Hamouz, K.; Lachman, J.; Hejtmánková, K.; Pazderů, K.; Čížek, M.; Dvořák, P. Effect of natural and growing conditions on the content of phenolics in potatoes with different flesh colour. Plant Soil Environ. 2010, 56, 368–374. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Fathy Zaki, M. Antioxidant activities of phenolics, flavonoids and vitamin C in two cultivars of fennel (Foeniculum vulgare Mill.) in responses to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Sousa, C.; Pereira, D.M.; Pereira, J.A.; Bento, A.; Rodrigues, M.A.; Dopico-García, S.; Valentão, P.; Lopes, G.; Ferreres, F.; Seabra, R.M.; et al. Multivariate analysis of tronchuda cabbage (Brassica oleracea L. var. costata DC) phenolics: Influence of fertilizers. J. Agric. Food Chem. 2008, 56, 2231–2239. [Google Scholar] [CrossRef]
- Naguib, A.E.-M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, flavonoids and glucosinolates of Broccoli (Brassica olaracea var. italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef]
- Lundegårdh, B.; Mårtensson, A. Organically produced plant foods—Evidence of health benefits. Acta Agric. Scand. B 2003, 53, 3–15. [Google Scholar] [CrossRef]
- Qin, H.; Lu, K.; Strong, P.J.; Xu, Q.; Wu, Q.; Xu, Z.; Wang, H. Long-term fertilizer application effects on the soil, root arbuscular mycorrhizal fungi and community composition in rotation agriculture. Appl. Soil Ecol. 2015, 89, 35–43. [Google Scholar] [CrossRef]
- Sutrisno, S.; Yusnawan, E. Effect of manure and inorganic fertilizers on vegetative, generative characteristics, nutrient, and secondary metabolite con-tents of mungbean. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 56–65. [Google Scholar] [CrossRef]


| Sources of Variation | df | Trigonelline | DPPH | FRAP | Total Phenolic | Total Flavonoids | Vitexin | Isovitexin | Orientin | Isoorientin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | Replication | 2 | 0.008 | 0.2536 | 0.1139 | 0.2317 | 0.0065 | 0.0038 | 0.7968 | 0.3584 | 0.0872 |
| Cropping system (CS) | 3 | <0.0001 | <0.0001 | <0.0001 | 0.0033 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | 0.0001 | |
| Fertilizer types (FT) | 2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| CS × FT | 6 | 0.0396 | 0.0003 | 0.0306 | 0.0401 | 0.0116 | 0.0532 | 0.0420 | 0.0523 | 0.0329 | |
| Error | 22 | - | - | - | - | - | - | - | - | - | |
| CV (%) | - | 6.12 | 5.59 | 7.52 | 5.70 | 6.35 | 7.82 | 7.11 | 6.41 | 8.44 | |
| 2015 | Replication | 2 | 0.1562 | 0.7369 | 0.2285 | 0.9867 | 0.3762 | 0.1619 | 0.9605 | 0.8543 | 0.4649 |
| Cropping system (CS) | 3 | <0.0001 | 0.0016 | 0.0006 | 0.0558 | <0.0001 | <0.0001 | 0.0176 | 0.0002 | <0.0001 | |
| Fertilizer types (FT) | 2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| CS × FT | 6 | 0.0448 | 0.0302 | 0.0157 | 0.0360 | 0.0447 | 0.0454 | 0.0387 | 0.0504 | 0.0004 | |
| Error | 22 | - | - | - | - | - | - | - | - | - | |
| CV (%) | - | 7.08 | 10.37 | 8.86 | 5.03 | 10.30 | 9.59 | 8.45 | 8.32 | 6.47 |
| Sole F | F:B = 2:1 | F:B = 1:1 | F:B = 1:2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | ||
| Vitexin | CF | 7.38e | 8.30f | 10.88c | 11.42cde | 9.64cd | 9.80ef | 7.84e | 8.44f |
| IF | 7.15e | 11.80cd | 13.13ab | 14.06ab | 12.34b | 11.17cde | 9.41d | 10.36de | |
| BL | 9.82cd | 10.50de | 14.24a | 15.0a | 14.45a | 14.68a | 10.49cd | 12.72bc | |
| Isovitexin | CF | 0.29e | 0.33f | 0.34de | 0.35def | 0.36cd | 0.34ef | 0.36cd | 0.34ef |
| IF | 0.37cd | 0.40cd | 0.41bc | 0.39cd | 0.38bcd | 0.40bc | 0.35de | 0.39cdef | |
| BL | 0.35de | 0.37cdef | 0.47a | 0.50a | 0.44ab | 0.46ab | 0.39bcd | 0.41bc | |
| Orientin | CF | 0.33f | 0.34f | 0.44bc | 0.42cde | 0.36ef | 0.37ef | 0.39def | 0.37ef |
| IF | 0.35ef | 0.41cde | 0.47b | 0.44bc | 0.45bc | 0.42cde | 0.44bcd | 0.43cd | |
| BL | 0.40cde | 0.39def | 0.56a | 0.56a | 0.47b | 0.50b | 0.44bcd | 0.44bc | |
| Isoorientin | CF | 0.70f | 0.69e | 0.82bde | 0.86cd | 0.87bcd | 0.86cd | 0.83cde | 0.84cd |
| IF | 0.76ef | 0.78de | 0.94b | 0.96b | 0.92bcd | 0.93bc | 0.91bcd | 0.92bc | |
| BL | 0.89bcd | 0.88bc | 1.20a | 1.30a | 0.94b | 0.97b | 0.93bc | 0.94bc | |
| Antioxidant Activity | ||||
|---|---|---|---|---|
| DPPH | FRAP | |||
| 2014 | 2015 | 2014 | 2015 | |
| Total phenolic content (TPC) | 0.44 ** | 0.74 *** | 0.72 *** | 0.60 *** |
| Total flavonoids content (TFC) | 0.78 *** | 0.82 *** | 0.81 *** | 0.59 ** |
| Flavonoid compounds | ||||
| Vitexin | 0.72 *** | 0.77 *** | 0.70 *** | 0.57 *** |
| Isovitexin | 0.70 *** | 0.70 *** | 0.65 *** | 0.47 ** |
| Orientin | 0.80 *** | 0.79 *** | 0.80 *** | 0.48 ** |
| Isoorientin | 0.75 *** | 0.65 *** | 0.69 *** | 0.46 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, A.; Fallah, S.; Zitterl-Eglseer, K.; Kaul, H.-P.; Abbasi Surki, A.; Mehdi, B. Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat. Agronomy 2019, 9, 367. https://doi.org/10.3390/agronomy9070367
Salehi A, Fallah S, Zitterl-Eglseer K, Kaul H-P, Abbasi Surki A, Mehdi B. Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat. Agronomy. 2019; 9(7):367. https://doi.org/10.3390/agronomy9070367
Chicago/Turabian StyleSalehi, Aliyeh, Sina Fallah, Karin Zitterl-Eglseer, Hans-Peter Kaul, Ali Abbasi Surki, and Bano Mehdi. 2019. "Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat" Agronomy 9, no. 7: 367. https://doi.org/10.3390/agronomy9070367
APA StyleSalehi, A., Fallah, S., Zitterl-Eglseer, K., Kaul, H.-P., Abbasi Surki, A., & Mehdi, B. (2019). Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat. Agronomy, 9(7), 367. https://doi.org/10.3390/agronomy9070367







