Effects of Soil Tillage and Canopy Optimization on Grain Yield, Root Growth, and Water Use Efficiency of Rainfed Maize in Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Weather Data
2.2. Data Collection and Analyses
3. Results
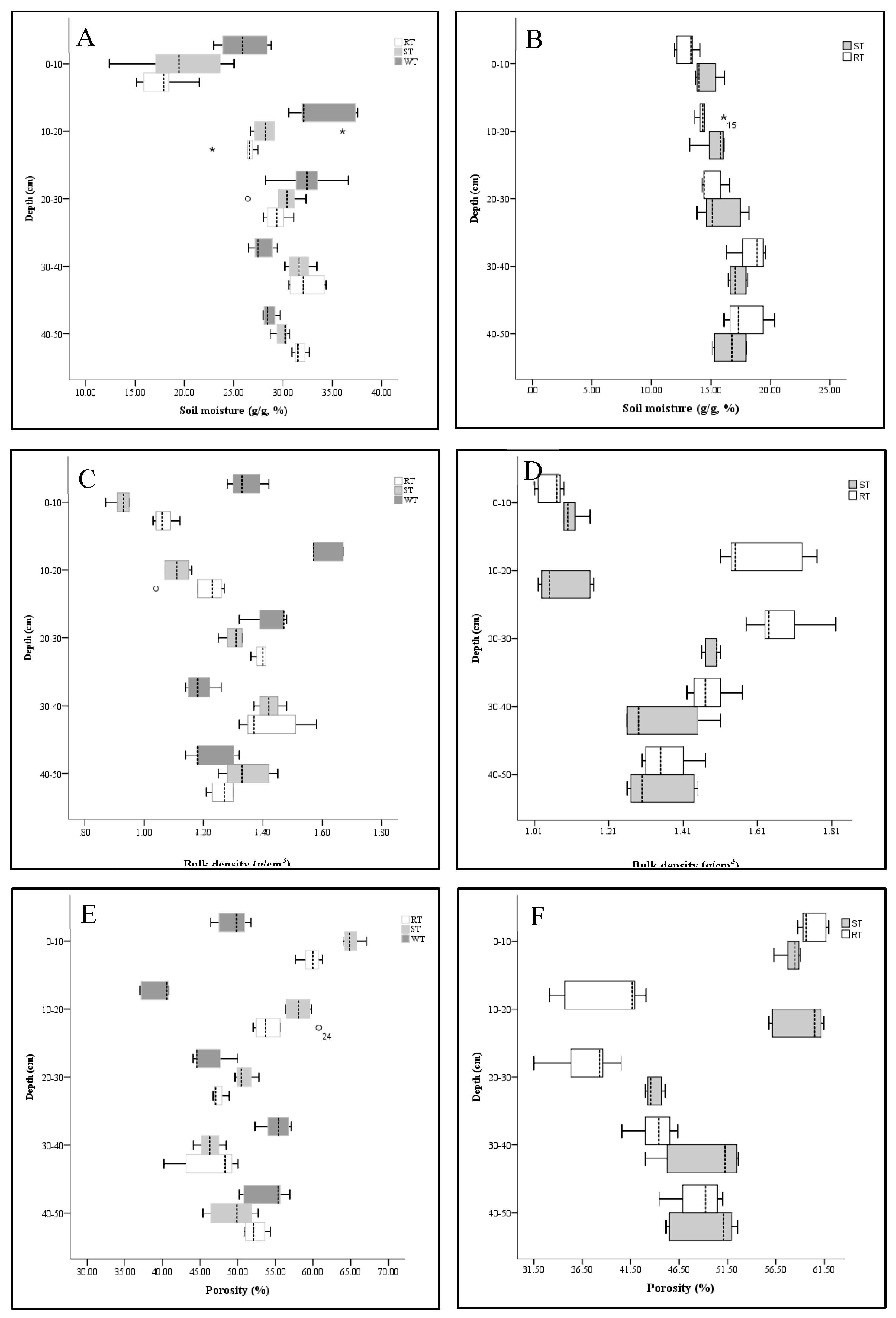
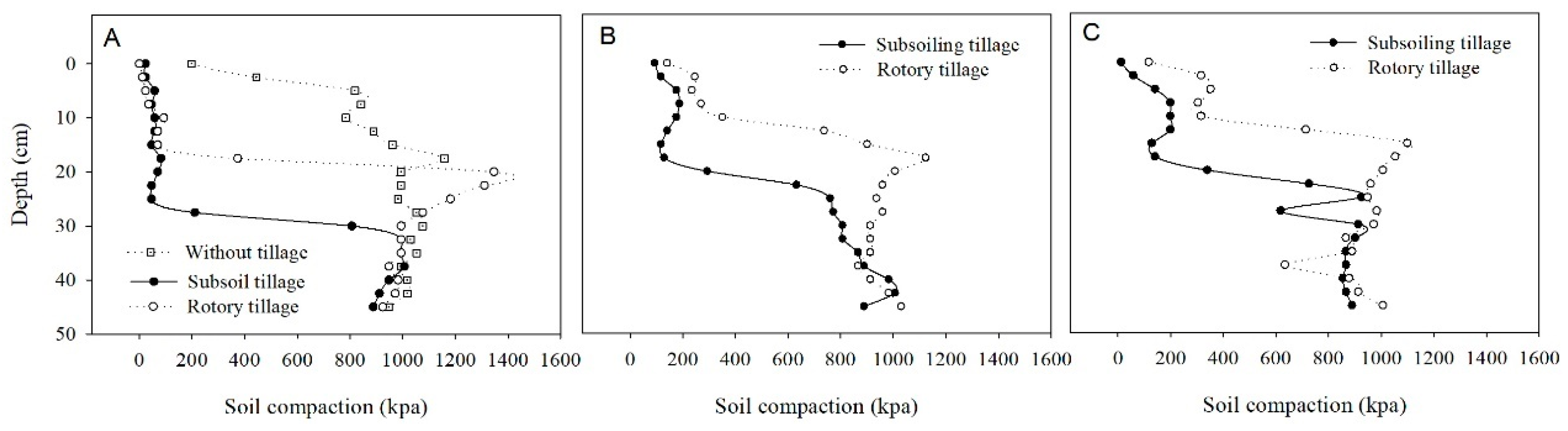
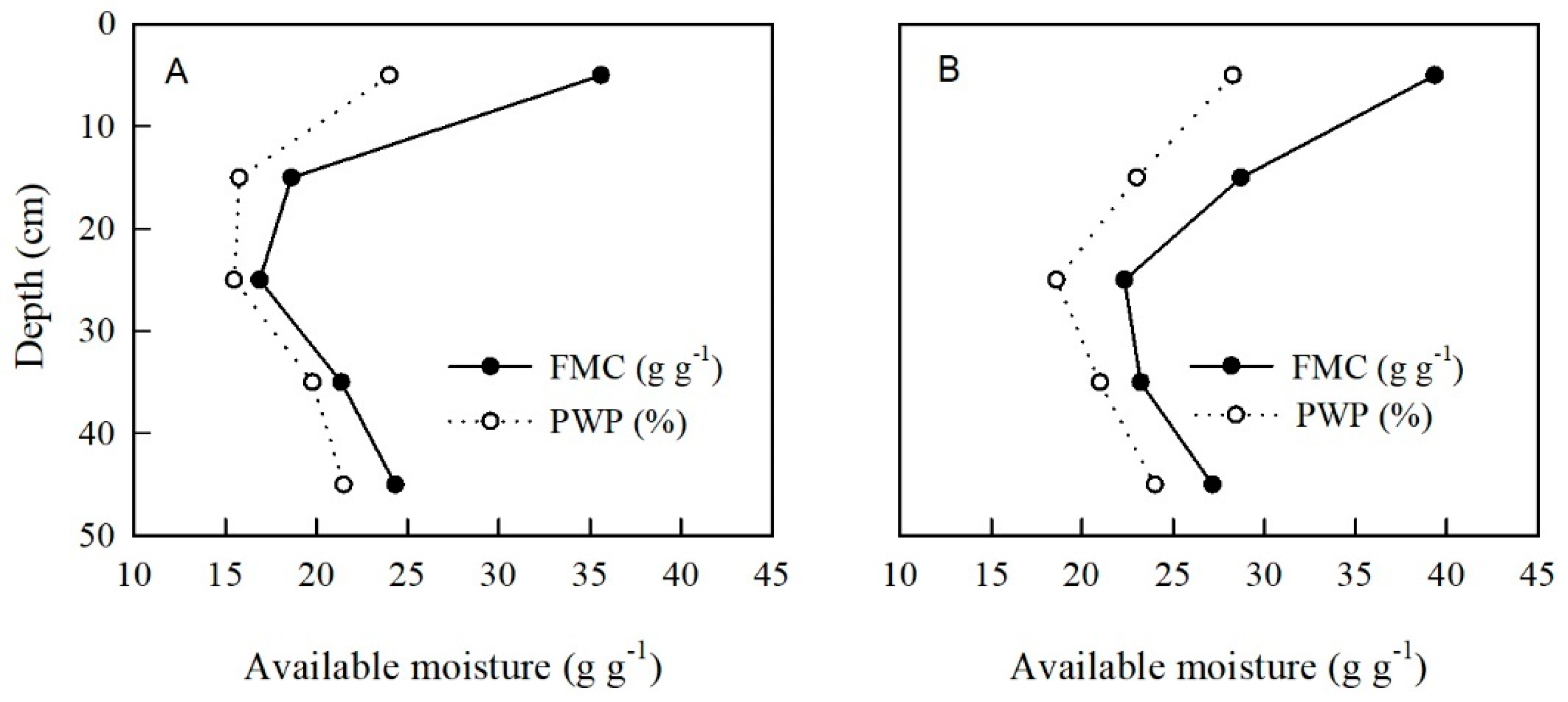
3.1. Soil Physical Properties
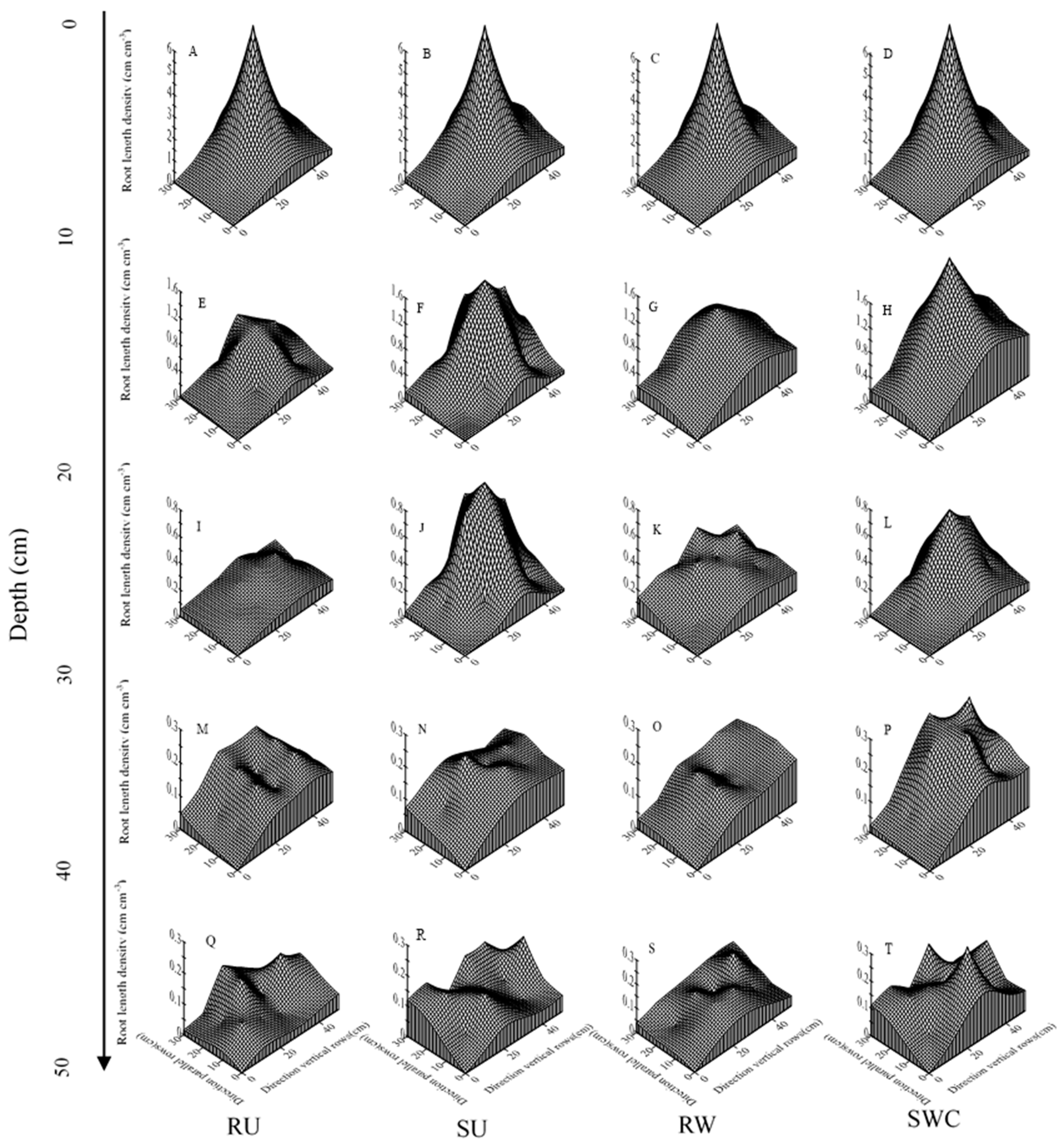
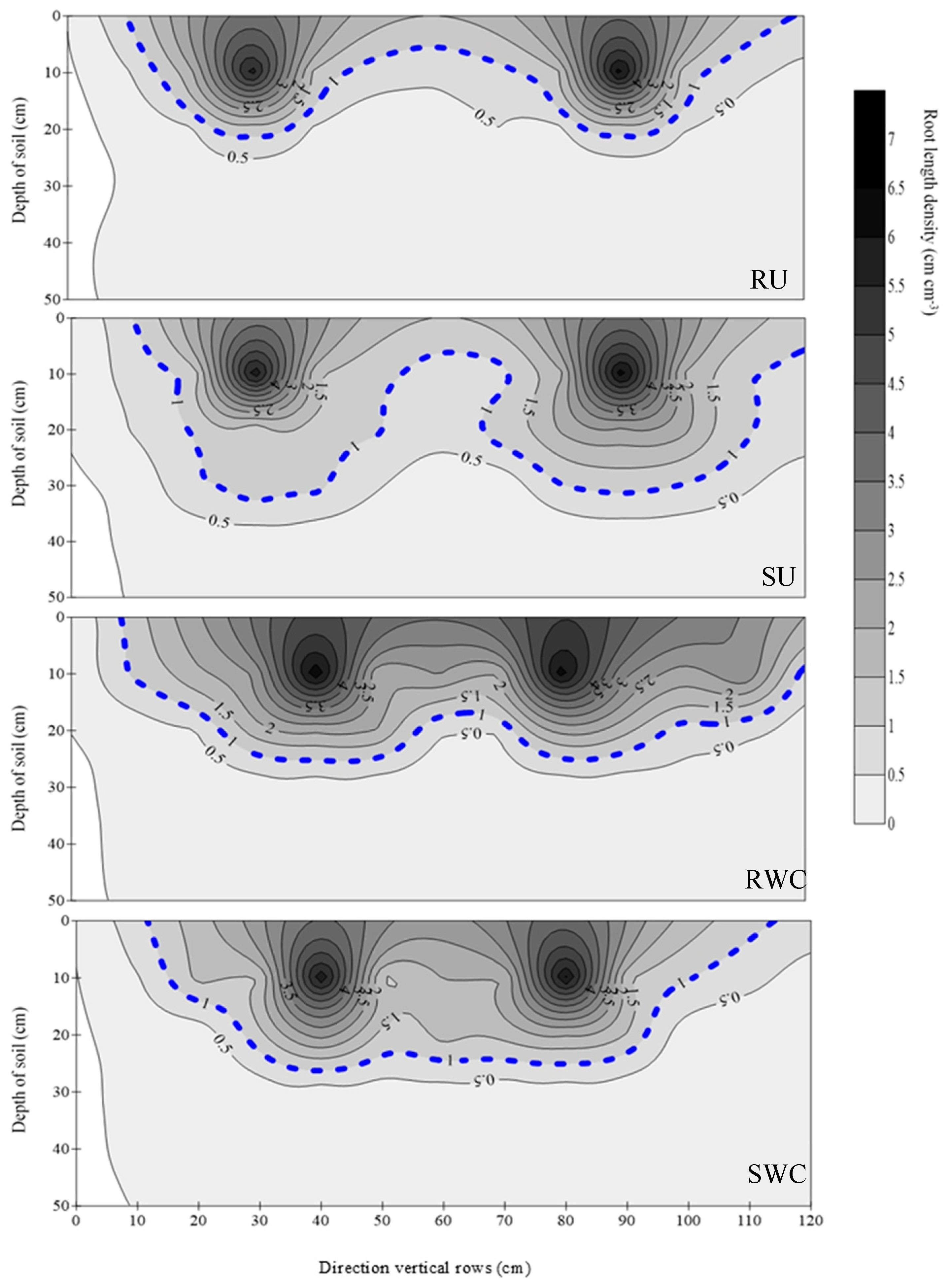
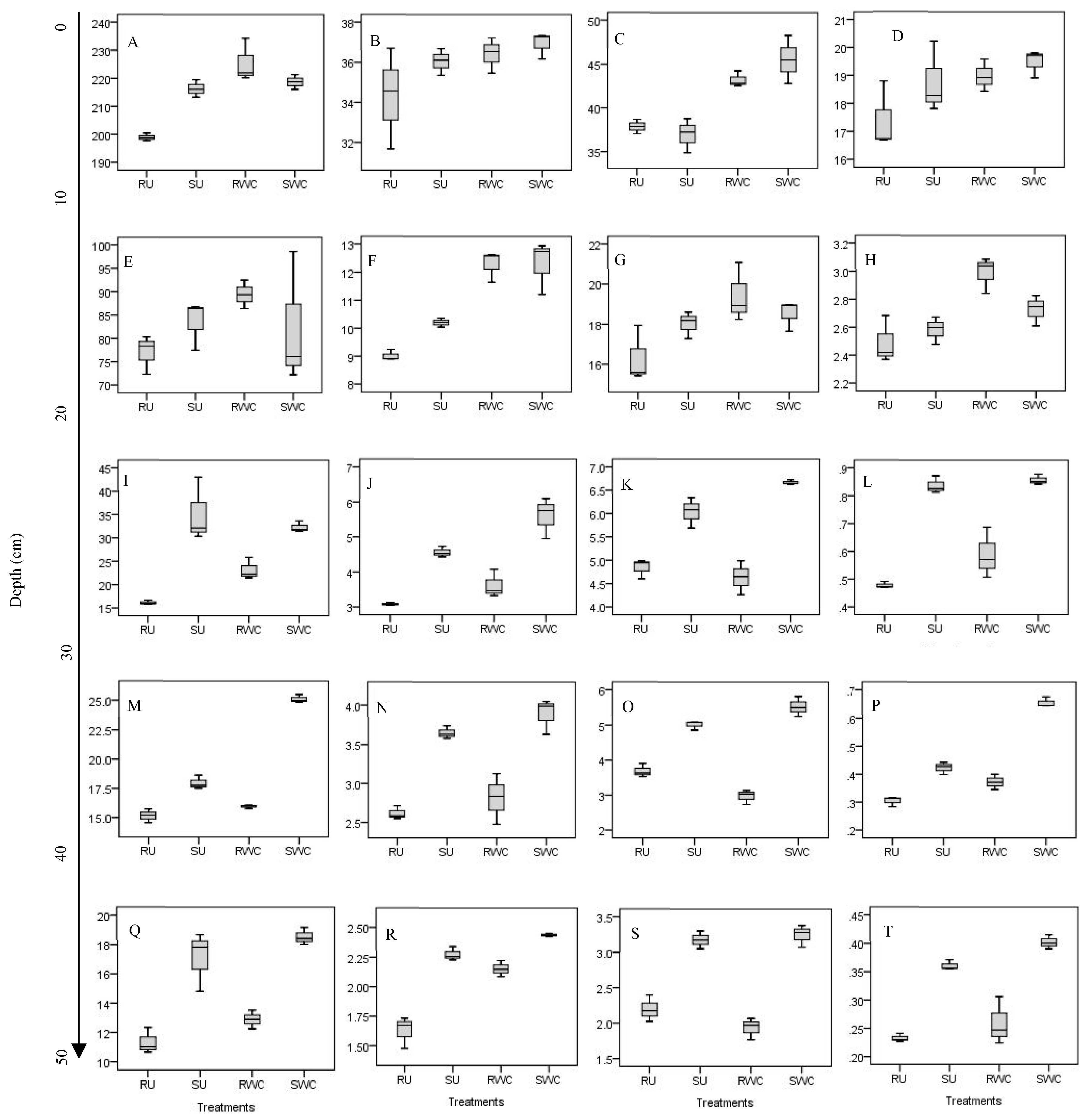
3.2. Root Morphology
3.3. Shoot to Root Ratios of Weight and Area
3.4. Yield and Water Use Efficiency (WUE)
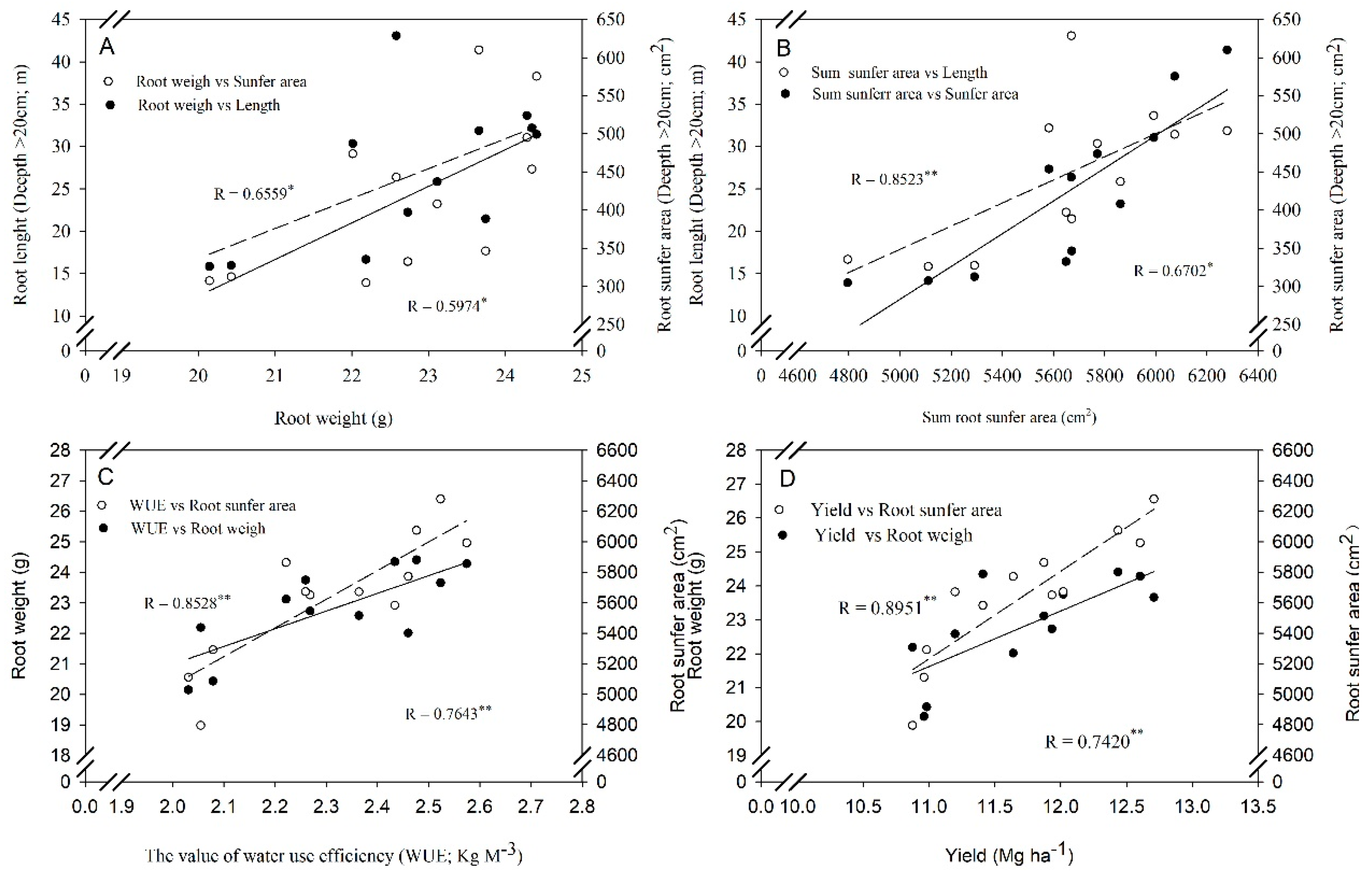
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J. China’s success in increasing per capita food production. J. Exp. Bot. 2011, 62, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Chen, X.P.; Vitousek, P. An experiment for the world. Nature 2013, 497, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Tokatlidis, I.S.; Koutroubas, S.D. A review of maize hybrids’ dependence on high plant populations and its implications for crop yield stability. Field Crops Res. 2004, 88, 103–114. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Has, V.; Melidis, V.; Has, I.; Mylonas, I.; Evgenidis, G.; Copandean, A.; Ninou, E.; Fasoula, V.A. Maize hybrids less dependent on high plant densities improve resource-use efficiency in rainfed and irrigated conditions. Field Crops Res. 2011, 120, 345–351. [Google Scholar] [CrossRef]
- Piao, L.; Qi, H.; Li, C.; Zhao, M. Optimized tillage practices and row spacing to improve grain yield and matter transport efficiency in intensive spring maize. Field Crops Res. 2016, 198, 258–268. [Google Scholar] [CrossRef]
- Cai, H.; Ma, W.; Zhang, X.; Ping, J.Q.; Yan, X.; Liu, J.; Yuan, J.; Wang, L.; Ren, J. Effect of subsoil tillage depth on nutrient accumulation, root distribution, and grain yield in spring maize. Crop. J. 2014, 2, 297–307. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2006; p. 54. [Google Scholar]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- Wang, C.T.; Li, S.K. Assessment of limiting factors and techniques prioritization for maize production in China. Sci. Agric. Sin. 2010, 43, 1136–1146. [Google Scholar]
- Meng, Q.; Chen, X.; Lobell, D.B.; Cui, Z.; Zhang, Y.; Yang, H.; Zhang, F. Growing sensitivity of maize to water scarcity under climate change. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Pages, L. Links between root developmental traits and foraging performance. Plant Cell Environ. 2011, 34, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops Introduction. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Dweyer, L.M.; Zhou, X.; Dutilleul, P.; Hamel, C.; Reid, L.M.; Smith, D.L. Root morphology of contrasting maize genotypes. Agron. J. 2002, 94, 96–101. [Google Scholar] [CrossRef]
- Sharp, R.E.; Silk, W.K.; Hsaio, T.C. Growth of the maize primary root at low water potentials: I. Spatial distribution of expansive growth. Plant Physiol. 1988, 87, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Mermoud, A. Topsoil properties as affected by tillage practices in North China. Soil Tillage Res. 2001, 60, 11–19. [Google Scholar] [CrossRef]
- Amato, M.; Ritchie, J.T. Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure. Crop. Sci. 2002, 42, 773–780. [Google Scholar] [CrossRef]
- Liu, S.; He, W.; Yan, C.; Liu, Q. Effects of different tillage managements on soil physical properties in dryland. Agric. Res. Arid Areas 2010, 28, 65–70. [Google Scholar]
- Guan, D.; Al-Kaisi, M.M.; Zhang, Y.; Duan, L.; Tan, W.; Zhang, M.; Li, Z. Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crops Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B.; Sun, X.; Yue, Y.; Ma, W.; Zhao, M. Soil Tillage Management Affects Maize Grain Yield by Regulating Spatial Distribution Coordination of Roots, Soil Moisture and Nitrogen Status. PLoS ONE 2015, 10, e0129231. [Google Scholar] [CrossRef]
- Martin, T.; Oswald, O.; Graham, I.A. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: Nitrogen availability. Plant Physiol. 2002, 128, 472–481. [Google Scholar] [CrossRef]
- O’Brien, E.E.; Gersani, M.; Brown, J.S. Root proliferation and seed yield in response to spatial heterogeneity of below-ground competition. New Phytol. 2005, 168, 401–412. [Google Scholar] [CrossRef] [PubMed]
- China Meteorological Administration. Available online: http://www.cma.gov.cn (accessed on 22 December 2017).
- Böhm, W. Methods of Studying Root Systems; Springer Science & Business Media: Berlin, Germany, 1979; Volume 33, p. 188. [Google Scholar]
- Ruiz-Sanchez, M.C.; Plana, V.; Ortuno, M.F.; Tapia, L.M.; Abrisqueta, J.M. Spatial root distribution of apricot trees in different soil tillage practices. Plant Soil 2005, 272, 211–221. [Google Scholar] [CrossRef]
- Hou, X.; Li, R.; Jia, Z.; Han, Q.; Wang, W.; Yang, B. Effects of rotational tillage practices on soil properties, winter wheat yields and water-use efficiency in semi-arid areas of north-west China. Field Crops Res. 2012, 129, 7–13. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.; Hao, S. Effects of subsoil bulk density on late growth stage photosynthetic characteristics and grain yield of maize (Zea mays L.). Chin. J. Appl. Ecol. 2008, 19, 787–793. [Google Scholar]
- Trouwborst, G.; Oosterkamp, J.; Hogewoning, S.W.; Harbinson, J.; van Ieperen, W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant 2010, 138, 289–300. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Sui, P.; Gao, W.; Yan, P.; Tao, Z. Effect of planting geometries on canopy structure of spring maize under high-density condition in North China Plain. Chin. J. Ecol. 2015, 34, 18–24. [Google Scholar]
- Jiao, L.; Dong, Z.; Gao, J.; Chen, C.; Lu, L.; Dong, X.; Li, G.; Xu, Y. Effects of plant growth regulators on canopy structure in spring maize under different plant densities. J. Maize Sci. 2014, 22, 51–58. [Google Scholar]
- Hammer, G.L.; Dong, Z.S.; McLean, G.; Doherty, A.; Messina, C.; Schusler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can Changes in Canopy and/or Root System Architecture Explain Historical Maize Yield Trends in the US Corn Belt? Crop. Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Wu, Q.; Lai, N.; Yuan, L.; Zhang, F. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci. China Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef]
- King, J.; Gay, A.; Sylvester-Bradley, R.; Bingham, I.; Foulkes, J.; Gregory, P.; Robinson, D. Modelling cereal root systems for water and nitrogen capture: Towards an economic optimum. Ann. Bot. 2003, 91, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
| Organic Matter (g kg−1) | Total Nitrogen (N; g kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | |
|---|---|---|---|---|---|
| Soil fertility | 19.66 | 1.12 | 132.8 | 33.26 | 161.5 |
| Fertilizers supply | Year | N (kg ha−1) | P2O5 (kg ha−1) | K2O (kg ha−1) | |
| 2013 | 315.75 | 147.38 | 236.25 | ||
| 2014 | 275 | 140 | 230 | ||
| Treatment | Root Weight (RW) (g/m2) | Root Surface Area (RSA) (m2 10−2) | Shoot Weight (SW) (g/m2) | Leaf Area (LA) (m2 10−2) | RW/SW (%) | LA/RSA (%) |
|---|---|---|---|---|---|---|
| RU | 20.92 b | 50.66 c | 203.6 a | 42.37 c | 0.103 b | 0.836 b |
| SU | 22.98 a | 56.75 b | 222.8 a | 52.04 b | 0.103 b | 0.917 ab |
| RWC | 23.19 a | 57.28 b | 210.6 a | 56.89 ab | 0.110 ab | 0.993 a |
| SWC | 24.11 a | 61.15 a | 210.4 a | 60.97 a | 0.115 a | 0.997 a |
| Year | Treatment | Yield (Mg·ha−1) | ET (mm) | WUE (kg M−3) |
|---|---|---|---|---|
| 2013 | RU | 10.03 d | 721.8 a | 1.389 d |
| SU | 10.51 c | 717.0 a | 1.466 c | |
| RWC | 11.36 b | 721.8 a | 1.575 b | |
| SWC | 13.16 a | 712.6 a | 1.847 a | |
| 2014 | RU | 10.94 d | 532.5 a | 2.055 d |
| SU | 11.41 c | 471.8 c | 2.419 b | |
| RWC | 11.94 b | 530.9 a | 2.250 c | |
| SWC | 12.58 a | 498.5 b | 2.525 a | |
| ANOVA | ||||
| Root optimized (RO) | ** | ns | ns | |
| Canopy optimized (CO) | ** | ns | ns | |
| RO × CO | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, L.; Li, M.; Xiao, J.; Gu, W.; Zhan, M.; Cao, C.; Zhao, M.; Li, C. Effects of Soil Tillage and Canopy Optimization on Grain Yield, Root Growth, and Water Use Efficiency of Rainfed Maize in Northeast China. Agronomy 2019, 9, 336. https://doi.org/10.3390/agronomy9060336
Piao L, Li M, Xiao J, Gu W, Zhan M, Cao C, Zhao M, Li C. Effects of Soil Tillage and Canopy Optimization on Grain Yield, Root Growth, and Water Use Efficiency of Rainfed Maize in Northeast China. Agronomy. 2019; 9(6):336. https://doi.org/10.3390/agronomy9060336
Chicago/Turabian StylePiao, Lin, Ming Li, Jialei Xiao, Wanrong Gu, Ming Zhan, Cougui Cao, Ming Zhao, and Congfeng Li. 2019. "Effects of Soil Tillage and Canopy Optimization on Grain Yield, Root Growth, and Water Use Efficiency of Rainfed Maize in Northeast China" Agronomy 9, no. 6: 336. https://doi.org/10.3390/agronomy9060336
APA StylePiao, L., Li, M., Xiao, J., Gu, W., Zhan, M., Cao, C., Zhao, M., & Li, C. (2019). Effects of Soil Tillage and Canopy Optimization on Grain Yield, Root Growth, and Water Use Efficiency of Rainfed Maize in Northeast China. Agronomy, 9(6), 336. https://doi.org/10.3390/agronomy9060336





