Effects of LED lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Total RNA Isolation and cDNA Synthesis
2.3. Quantitative Real-Time PCR Analysis
2.4. Analysis of Phenylpropanoid Content by HPLC
2.5. Statistical Analysis
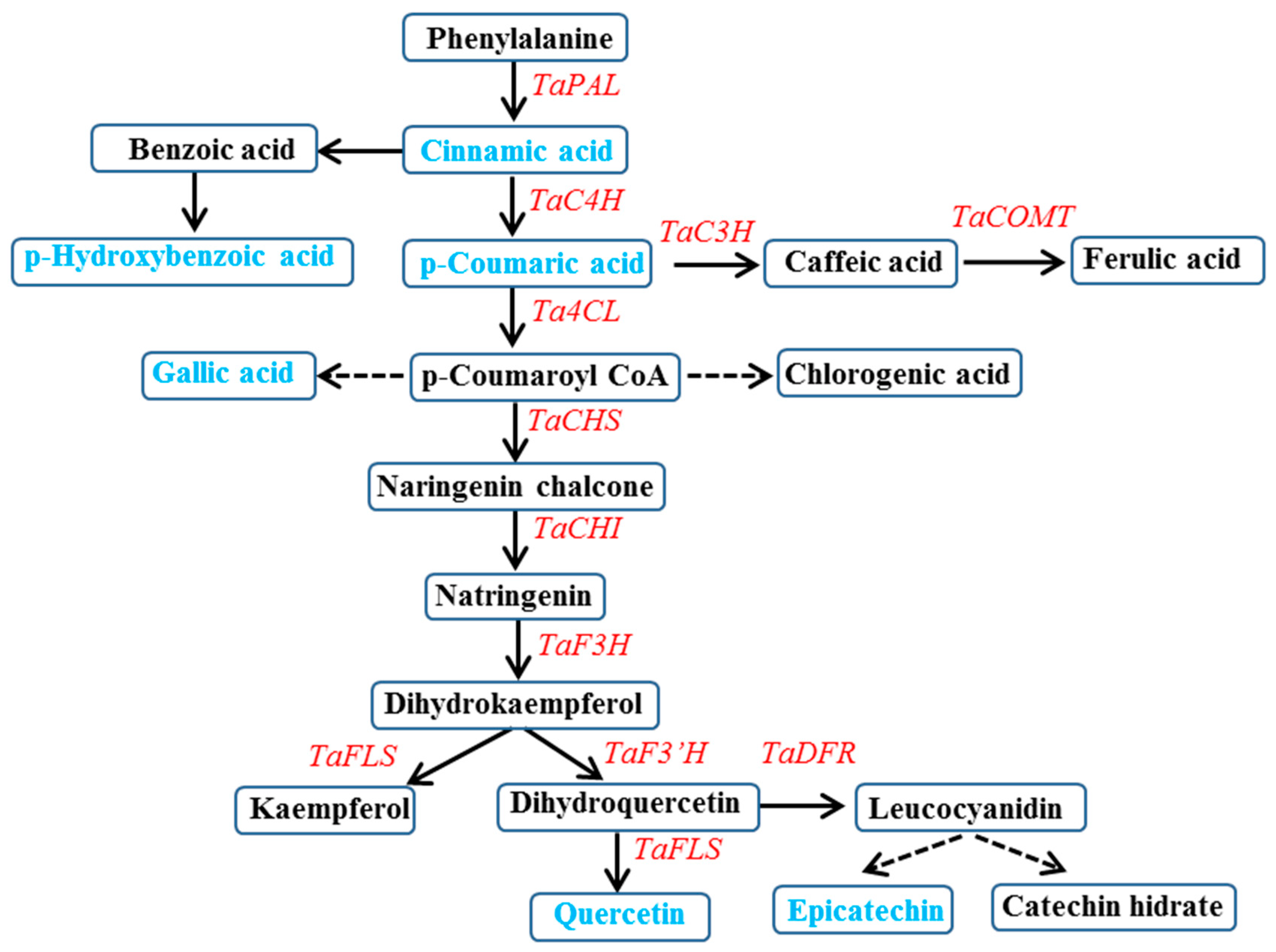
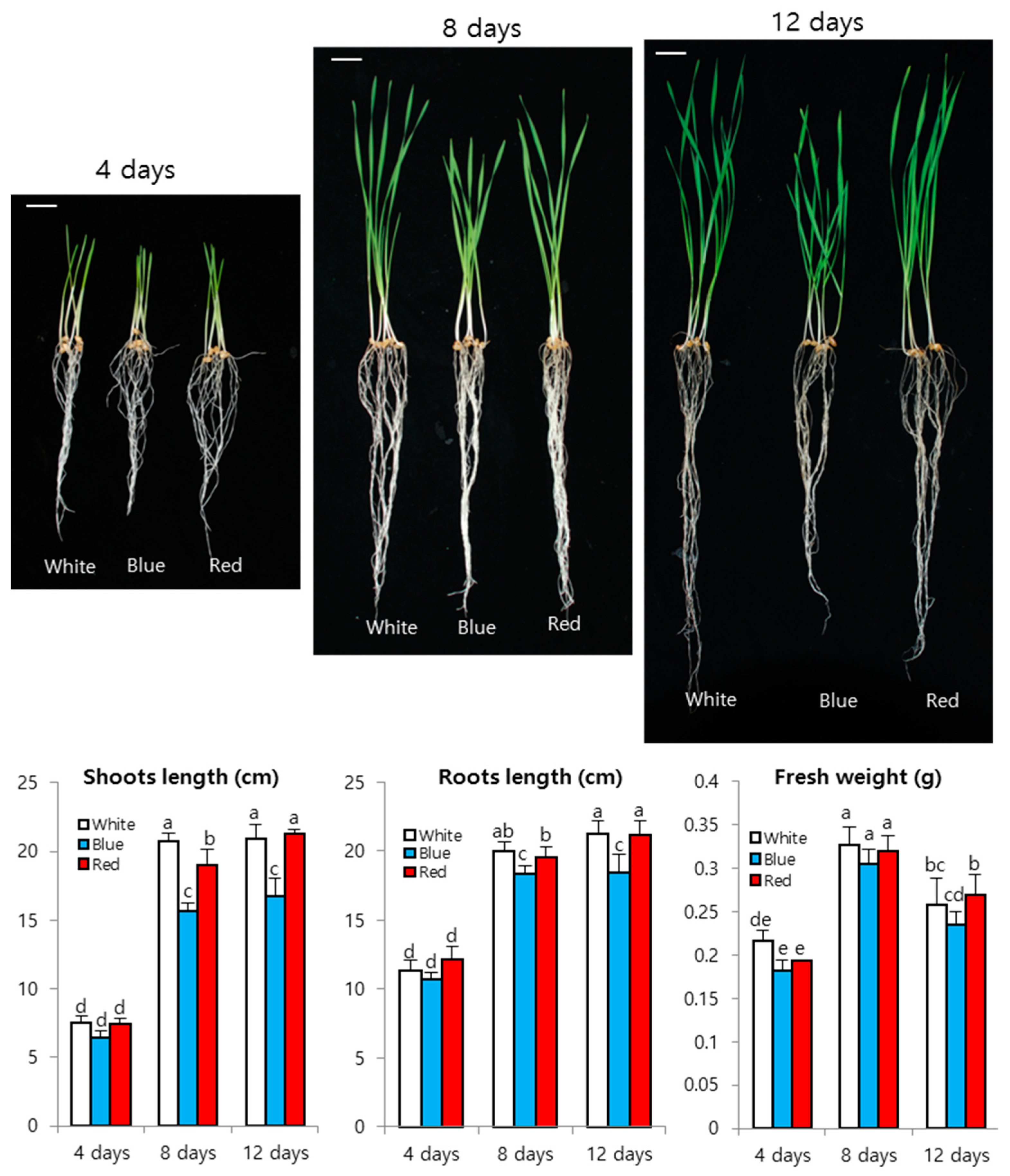
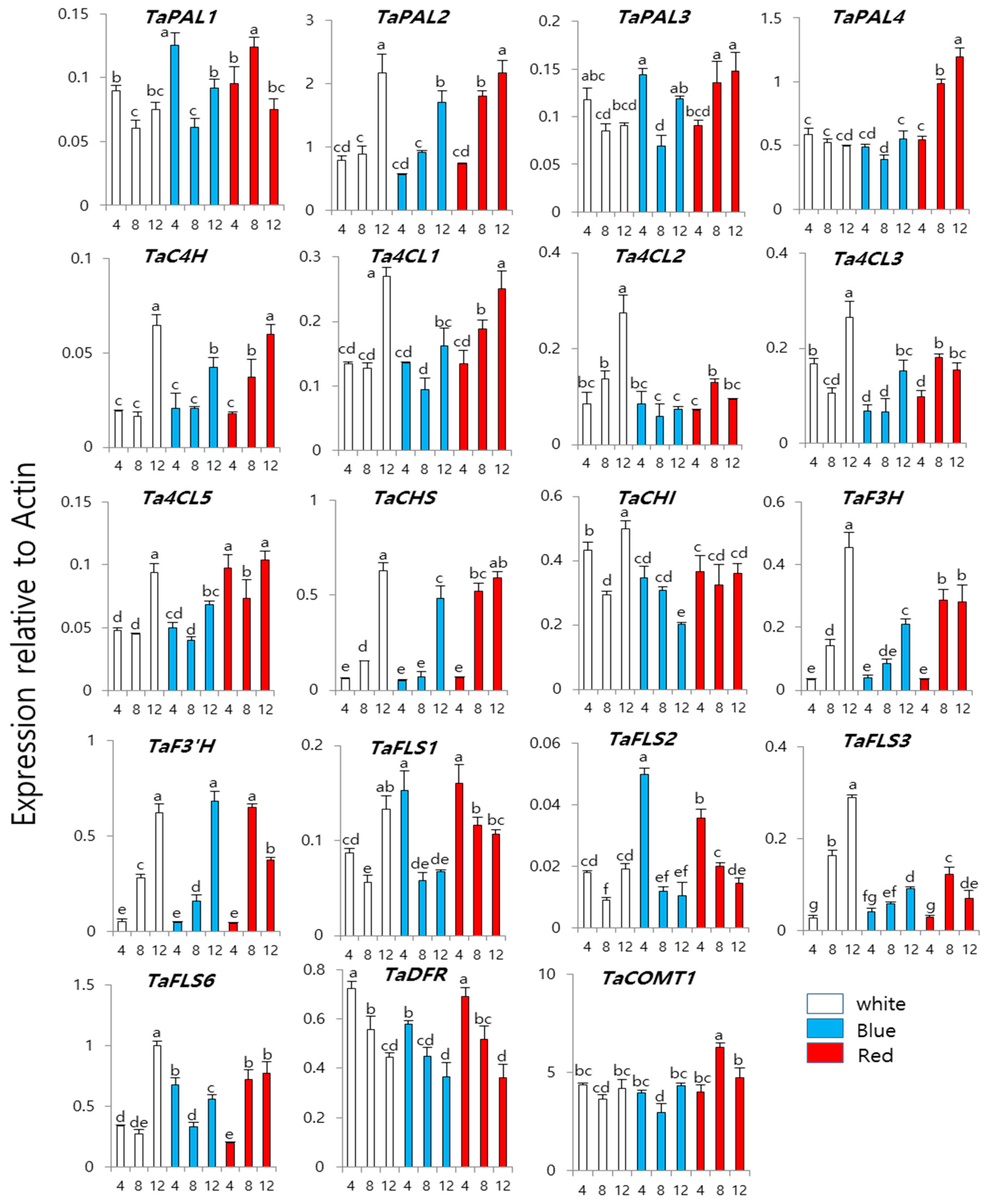
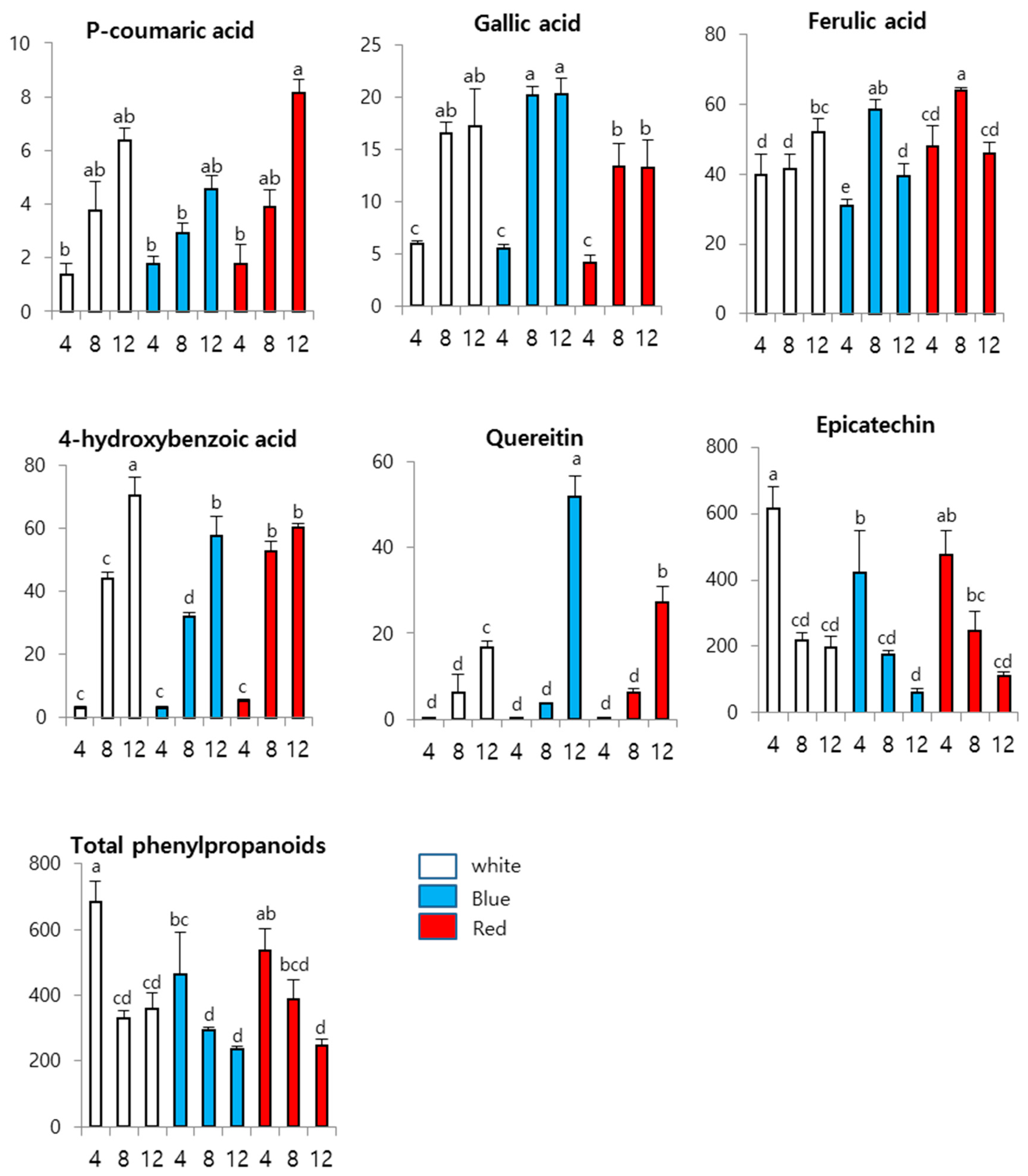
3. Results and Discussion
3.1. Wheat Sprout Growth under LED Light
3.2. Expression of Phenylpropanoid Biosynthesis Genes in Wheat Sprouts Grown under White, Blue, or Red Light
3.3. Effects of LEDs on Phenylpropanoid Content in Wheat Sprouts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.D.; Folta, K.M. Sequential light programs shape kale (Brassica napus) sprout appearance and alter metabolic and nutrient content. Hortic. Res. Engl. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to led lighting. Hortscience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. Led lighting in horticulture. Hortscience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortscience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. Hortscience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Hossen, M.Z. Light emitting diodes increase phenolics of buckwheat (Fagopyrum esculentum) sprouts. J. Plant Interact. 2007, 2, 71–78. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, Y.B.; Li, X.; Seo, J.M.; Kim, S.J.; Suzuki, T.; Chung, S.O.; Park, S.U. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in fagopyrum tataricum sprouts. J. Agric. Food Chem. 2014, 62, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Fraszczak, B.; Golcz, A.; Zawirska-Wojtasiak, R.; Janowska, B. Growth rate of sweet basil and lemon balm plants grown under fluorescent lamps and led modules. Acta Sci. Pol.-Hortoru. 2014, 13, 3–13. [Google Scholar]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of led light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Cuong, D.M.; Jeon, J.; Morgan, A.M.A.; Kim, C.; Kim, J.K.; Lee, S.Y.; Park, S.U. Accumulation of charantin and expression of triterpenoid biosynthesis genes in bitter melon (Momordica charantia). J. Agric. Food Chem. 2017, 65, 7240–7249. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of phenylpropanoids by white, blue, and red light irradiation and their organ-specific distribution in chinese cabbage (Brassica rapa ssp. Pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef]
- Li, H.M.; Tang, C.M.; Xu, Z.G. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic.-Amst. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Panda, P.; Appalashetti, M.; Judeh, Z.M.A. Phenylpropanoid sucrose esters: Plant-derived natural products as potential leads for new therapeutics. Curr. Med. Chem. 2011, 18, 3234–3251. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini-Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant. Food Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Eudes, A.; Liang, Y.; Mitra, P.; Loque, D. Lignin bioengineering. Curr. Opin. Biotechnol. 2014, 26, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant. Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Osbourn, A.; Ma, P.D. Myb transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant. 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Kwon, S.J.; Jeon, J.; Park, Y.J.; Park, J.S.; Park, S.U. Identification and characterization of phenylpropanoid biosynthetic genes and their accumulation in bitter melon (Momordica charantia). Molecules 2018, 23, 469. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant. Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Goggin, D.E.; Steadman, K.J.; Powles, S.B. Green and blue light photoreceptors are involved in maintenance of dormancy in imbibed annual ryegrass (Lolium rigidum) seeds. New Phytol. 2008, 180, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (uplc) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar]
- Amici, M.; Bonfili, L.; Spina, M.; Cecarini, V.; Calzuola, I.; Marsili, V.; Angeletti, M.; Fioretti, E.; Tacconi, R.; Gianfranceschi, G.L.; et al. Wheat sprout extract induces changes on 20s proteasomes functionality. Biochimie 2008, 90, 790–801. [Google Scholar] [CrossRef] [PubMed]
- OuYang, F.; Mao, J.F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome analysis reveals that red and blue light regulate growth and phytohormone metabolism in norway spruce [Picea abies (L.) karst]. PLoS ONE 2015, 10, e0127896. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.C.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of blue and red led light irradiation on beta-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
| Gene | ID | Primer Sequence (5′ to 3′) | |
|---|---|---|---|
| Forward Primer | Reverse Primer | ||
| WpPAL1 | CJ628388.1 | GCTCTTTGAGGCCAATGTTCTT | GCTTCTATCTGTCCAGGGTGGT |
| WpPAL2 | CJ707202.1 | CTACATGGCACTCGCAAAGAAG | GTTGATCTCACGCTCGATTGAC |
| WpPAL3 | CJ805150.1 | TACATGAAGCAGGCAAAGAAGC | TTGTCGTTGACGGAGTTGATCT |
| WpPAL4 | BQ752712.1 | GTGAACTCTCTGGGCCTTGTGT | TCACGGAGGTCTTGATGTTCTC |
| WpC4H | HX132432.1 | CAACCACCGCAACCTCAT | GGTGAAGATGTCGAAGACGA |
| Wp4CL1 | GH727954.1 | GCCGCTGTTCCACATCTA | GGTCGCTCTTGGCGATCT |
| Wp4CL2 | CJ951387.1 | GAGGCCACAAAGAACACCAT | TTTGATTTCGGCGTGTGTAA |
| Wp4CL3 | CK163034.1 | AGTTCGCCTTCTCCTTCCTC | CGTACTCCAGCACCTTGTCC |
| Wp4CL5 | GH724596.1 | CAAGGGCGTCATGCTCA | AGCGAGTAGATGTGGAACAGC |
| WpCHS | HX091886.1 | CGTGGACGAAGTGATGAAGA | TTAGGTGTTCGCTGTTGGTG |
| WpCHI | CD890742.1 | ACAAGGTGACGGAGAACTGC | GAGTGGGTGAAGAGGATGGA |
| WpF3H | CJ659795.1 | CTGGAGAAGGTGATGGCTGT | CAGATAGTCCCGCCAGTTGT |
| WpF3′H | HX255892.1 | AAGGAGAGGCGCAATAGGAT | GTGATGGGGAAGCTACAAGC |
| WpFLS1 | BE423889.1 | TGAAGGATTTGGCTACTGTGG | GAGAAGACGCGGATGTCGT |
| WpFSL2 | BQ244276.1 | ATCCAAACTGACACGCATGA | AGTTCCCGGCCAAGTACAAG |
| WpFLS3 | HX107493.1 | CTACGGCTTCTTCCAGATCG | CAGATAGTCCCGCCAGTTGT |
| WpFLS6 | CD454732.1 | GTACCAGCATCCGTCCTTGA | GTGGTGCTCCTCCAGAAGAT |
| WpDFR | CJ714375.1 | TACGACCAGGACAACTGGAG | GGGATGATGCTGATGAAGTC |
| WpCOMT1 | CJ858964.1 | CCATCAAGGGCATCAACTTC | CAAGGCGTCGTAGCAGTTCT |
| WpActin | GQ339780 | CGTGTTGGATTCTGGTGATG | CGAGCTTCTCCTTGATGTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout. Agronomy 2019, 9, 307. https://doi.org/10.3390/agronomy9060307
Cuong DM, Ha TW, Park CH, Kim NS, Yeo HJ, Chun SW, Kim C, Park SU. Effects of LED lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout. Agronomy. 2019; 9(6):307. https://doi.org/10.3390/agronomy9060307
Chicago/Turabian StyleCuong, Do Manh, Tai Wook Ha, Chang Ha Park, Nam Su Kim, Hyeon Ji Yeo, Se Won Chun, Changsoo Kim, and Sang Un Park. 2019. "Effects of LED lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout" Agronomy 9, no. 6: 307. https://doi.org/10.3390/agronomy9060307
APA StyleCuong, D. M., Ha, T. W., Park, C. H., Kim, N. S., Yeo, H. J., Chun, S. W., Kim, C., & Park, S. U. (2019). Effects of LED lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout. Agronomy, 9(6), 307. https://doi.org/10.3390/agronomy9060307






