Field Spectroscopy to Determine Nutritive Value Parameters of Individual Ryegrass Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population and Study Area
2.2. Spectral Collection
2.3. Laboratory Analysis
2.4. Model Building
3. Results
3.1. PLS Models Descriptive Statistic
| Statistic | ADF | ASH | CP | DM | IVVDMD | IVVOMD | NDF | WSC |
|---|---|---|---|---|---|---|---|---|
| Scatter correction | none | weighted MSC | derivative scale & offset | SNV | derivative scale & offset | remove, scale & offset | SNV | MSC |
| Derivative, gap, smooth 1, smooth 2 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 | 1,8,1,1 |
| Samples (N) | 103 | 102 | 105 | 103 | 104 | 104 | 104 | 105 |
| Mean | 24.42 | 9.82 | 11.59 | 24.63 | 76.77 | 72.89 | 46.17 | 24.25 |
| SD | 1.59 | 1.96 | 3.38 | 2.94 | 2.73 | 2.13 | 3.54 | 2.87 |
| Est. Min | 19.65 | 3.94 | 1.44 | 15.81 | 68.58 | 66.48 | 35.56 | 15.63 |
| Est. Max | 29.20 | 15.71 | 21.74 | 33.46 | 84.96 | 79.29 | 56.78 | 32.87 |
| SEC | 0.73 | 0.46 | 0.66 | 1.18 | 0.78 | 0.74 | 1.47 | 0.44 |
| R2 | 0.79 | 0.95 | 0.96 | 0.84 | 0.92 | 0.88 | 0.83 | 0.98 |
| SEPC | 1.37 | 0.98 | 1.38 | 2.11 | 1.69 | 1.56 | 2.87 | 2.46 |
| λN | 887 | 887 | 887 | 887 | 887 | 887 | 887 | 887 |
| Statistic | ADF | ash | CP | DM | IVVDMD | IVVOMD | NDF | WSC |
|---|---|---|---|---|---|---|---|---|
| N | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Slope | 0.75 | 0.72 | 0.84 | 0.54 | 0.95 | 0.87 | 0.68 | 0.50 |
| Y-intercept | 6.01 | 3.00 | 2.10 | 10.80 | 4.50 | 9.42 | 14.28 | 12.18 |
| Bias | −0.07 | 0.31 | 0.26 | −0.44 | 0.33 | 0.07 | −0.66 | 0.18 |
| SEC | 1.28 | 1.40 | 1.84 | 2.85 | 1.52 | 1.39 | 2.85 | 2.86 |
| SEP | 1.27 | 1.51 | 1.91 | 2.95 | 1.53 | 1.38 | 3.03 | 3.03 |
| SEPC | 1.28 | 1.49 | 1.92 | 2.95 | 1.51 | 1.39 | 2.99 | 3.06 |
| R2 | 0.22 | 0.51 | 0.74 | 0.11 | 0.69 | 0.52 | 0.35 | 0.64 |
| Predicted ave | 24.34 | 9.73 | 11.59 | 24.32 | 76.52 | 72.94 | 46.59 | 23.98 |
| Actual ave | 24.27 | 10.03 | 11.86 | 23.88 | 76.85 | 73.01 | 45.94 | 24.16 |
| Predicted SD | 0.88 | 1.95 | 3.69 | 1.81 | 2.39 | 1.63 | 3.04 | 2.30 |
| Actual SD | 1.43 | 1.98 | 3.60 | 2.99 | 2.72 | 1.98 | 3.50 | 3.06 |
3.2. Robustness of the Predictive Model
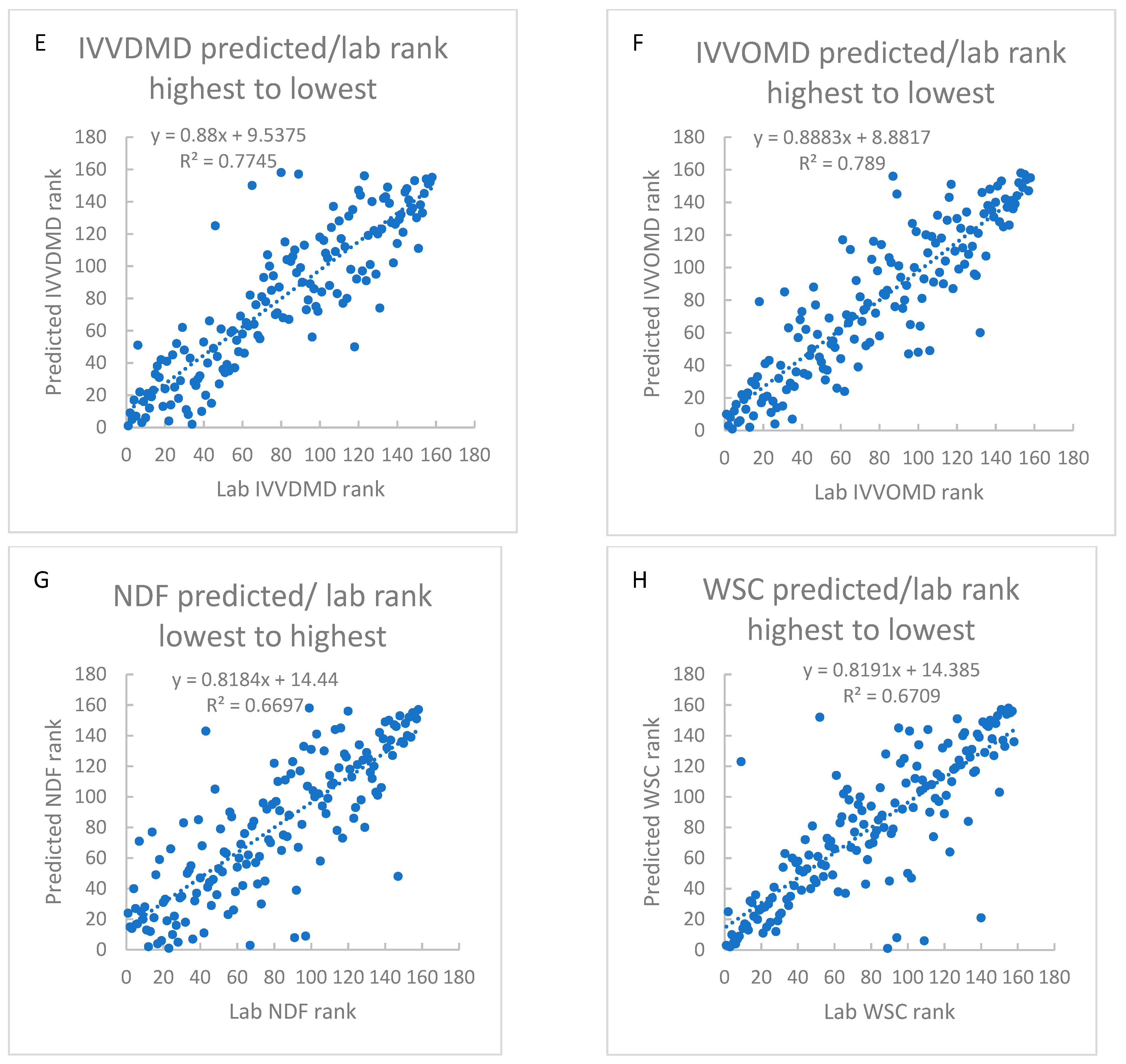
3.3. Predictive Ability of Field Model
3.4. Prediction of NV Parameters in Plants Using the Field Model
4. Discussion
4.1. Predictive Model Performance
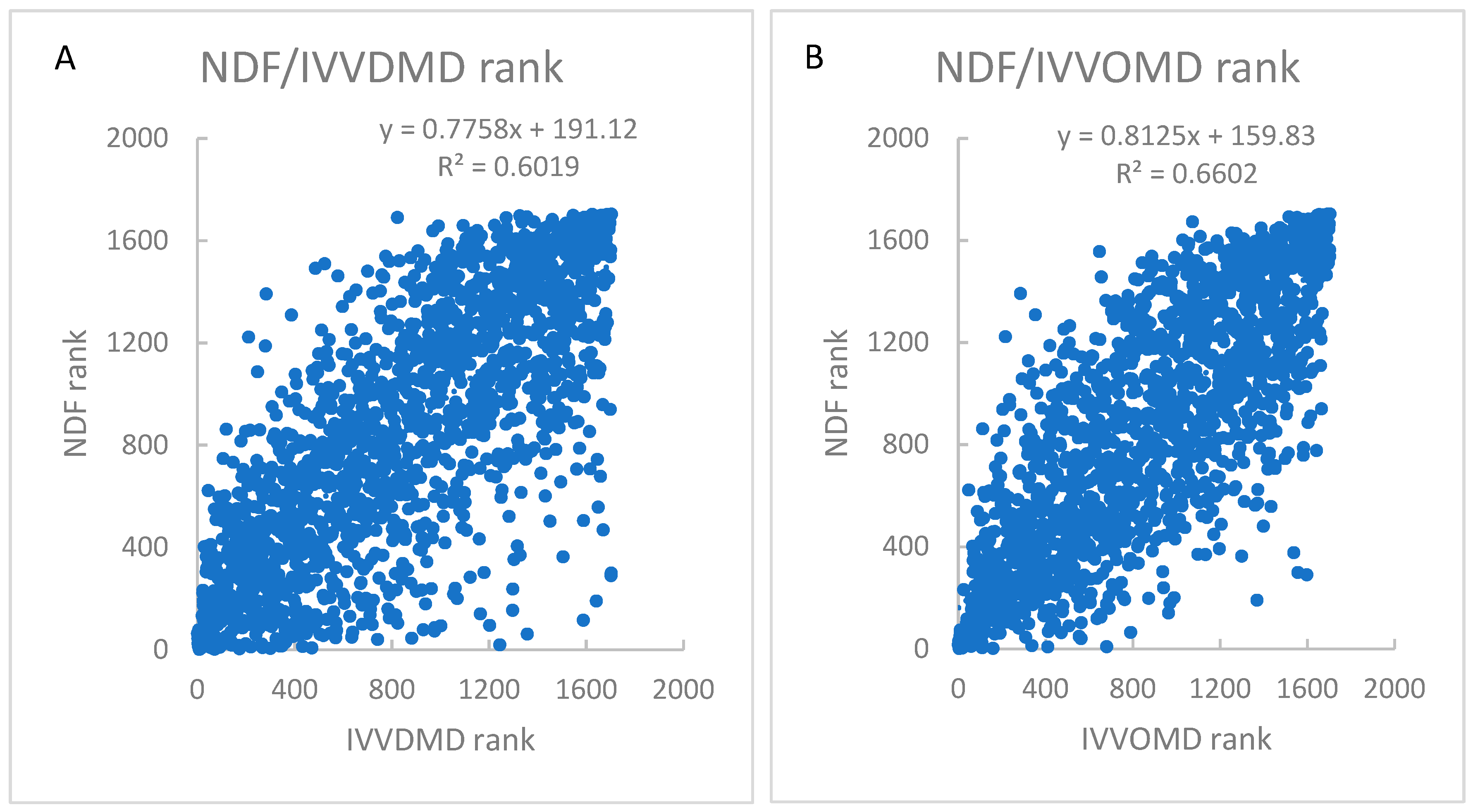
4.2. Interaction Between Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pullanagari, R.; Yule, I.; Tuohy, M.; Hedley, M.; Dynes, R.; King, W. In-field hyperspectral proximal sensing for estimating quality parameters of mixed pasture. Precis. Agric. 2012, 13, 351–369. [Google Scholar] [CrossRef]
- Tas, B.M.; Taweel, H.Z.; Smit, H.J.; Elgersma, A.; Dijkstra, J.; Tamminga, S. Effects of perennial ryegrass cultivars on intake, digestibility, and milk yield in dairy cows. J. Dairy Sci. 2005, 88, 3240–3248. [Google Scholar] [CrossRef]
- Smith, K.F.; Reed, K.F.M.; Foot, J.Z. An assessment of the relative importance of specific traits for the genetic improvement of nutritive value in dairy pasture. Grass Forage Sci. 1997, 52, 167–175. [Google Scholar] [CrossRef]
- Badenhorst, P.; Panter, S.; Palanisamy, R.; Georges, S.; Smith, K.; Mouradov, A.; Mason, J.; Spangenberg, G. Molecular breeding of transgenic perennial ryegrass (Lolium perenne L.) with altered fructan biosynthesis through the expression of fructosyltransferases. New Strateg. Plant Improv. 2018, 38, 1–13. [Google Scholar] [CrossRef]
- Starks, P.; Zhao, D.; Phillips, W.; Coleman, S. Development of canopy reflectance algorithms for real-time prediction of bermudagrass pasture biomass and nutritive values. Crop Sci. 2006, 46, 927–934. [Google Scholar] [CrossRef]
- Stewart, A.; Hayes, R. Ryegrass breeding—Balancing trait priorities. Ir. J. Agric. Food Res. 2011, 50, 31–46. [Google Scholar]
- Chapman, D.; Edwards, G.; Stewart, A.; McEvoy, M.; O’Donovan, M.; Waghorn, G. Valuing forages for genetic selection: What traits should we focus on? Anim. Prod. Sci. 2015, 55, 869–882. [Google Scholar] [CrossRef]
- Surmen, M.; Yavuz, T.; Albayrak, S.; Cankaya, N. Forage yield and quality of perennial ryegrass (Lolium perenne L.) lines in the black sea coastal area of turkey. Turk. J. Field Crop. 2013, 18, 40–45. [Google Scholar]
- Casler, M.; Vogel, K. Accomplishments and impact from breeding for increased forage nutritional value. Crop Sci. 1999, 39, 12–20. [Google Scholar] [CrossRef]
- Vogel, K.; Gabrielsen, B.; Ward, J.; Anderson, B.; Mayland, H.; Masters, R. Forage quality, mineral constituents, and performance of beef yearlings grazing two crested wheatgrasses. Agron. J. 1993, 85, 584. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Casler, M.D. Breeding forage crops for increased nutritional value. Adv. Agron. 2001, 71, 51–107. [Google Scholar]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M. Field-based phenomics for plant genetics research. Field Crop. Res 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Thulin, S.M. Hyperspectral Remote Sensing of Temperate Pasture Quality; RMIT University Melbourne: Melbourne, Australia, 2008. [Google Scholar]
- Pembleton, L.W.; Inch, C.; Baillie, R.C.; Drayton, M.C.; Thakur, P.; Ogaji, Y.O.; Spangenberg, G.C.; Forster, J.W.; Daetwyler, H.D.; Cogan, N.O. Exploitation of data from breeding programs supports rapid implementation of genomic selection for key agronomic traits in perennial ryegrass. Theor. Appl. Genet. 2018, 131, 1891–1902. [Google Scholar] [CrossRef]
- Wilkins, P. Breeding perennial ryegrass for agriculture. Euphytica 1991, 52, 201–214. [Google Scholar] [CrossRef]
- Humphreys, M.O. Genetic improvement of forage crops past, present and future. J. Agric. Sci. 2005, 143, 441–448. [Google Scholar] [CrossRef]
- Ruckelshausen, A.; Biber, P.; Dorna, M.; Gremmes, H.; Klose, R.; Linz, A.; Rahe, F.; Resch, R.; Thiel, M.; Trautz, D. Bonirob: An autonomous field robot platform for individual plant phenotyping. Precis. Agric. 2009, 9, 1. [Google Scholar]
- Smith, K.F.; Simpson, R.J.; Armstrong, R.D. Using near infrared reflectance spectroscopy to estimate the nutritive value of senescing annual ryegrass (Lolium rigidum): A comparison of calibration methods. Aust. J. Exp. Agric. 1998, 38, 45–54. [Google Scholar] [CrossRef]
- Stuth, J.; Jama, A.; Tolleson, D. Direct and indirect means of predicting forage quality through near infrared reflectance spectroscopy. Field Crop. Res. 2003, 84, 45–56. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2013, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Coppens, F.; Wuyts, N.; Inzé, D.; Dhondt, S. Unlocking the potential of plant phenotyping data through integration and data-driven approaches. Curr. Opin. Syst. Biol. 2017, 4, 58–63. [Google Scholar] [CrossRef]
- Alomar, D.; Montero, R.; Fuchslocher, R. Effect of freezing and grinding method on near-infrared reflectance (nir) spectra variation and chemical composition of fresh silage. Anim. Feed Sci. Technol. 1999, 78, 57–63. [Google Scholar] [CrossRef]
- Seelan, S.K.; Laguette, S.; Casady, G.M.; Seielstad, G.A. Remote sensing applications for precision agriculture: A learning community approach. Remote Sens. Environ. 2003, 88, 157–169. [Google Scholar] [CrossRef]
- Kumar, J.; Pratap, A.; Kumar, S. Phenomics in Crop Plants: Trends, Options and Limitation; Springer India: New Delhi, India, 2015; Volume 1, p. 310. [Google Scholar]
- Vigneau, N.; Ecarnot, M.; Rabatel, G.; Roumet, P. Potential of field hyperspectral imaging as a non destructive method to assess leaf nitrogen content in wheat. Field Crop. Res. 2011, 122, 25–31. [Google Scholar] [CrossRef]
- Perbandt, D.; Fricke, T.; Wachendorf, M. Effects of changing simulated sky cover on hyperspectral reflectance measurements for dry matter yield and forage quality prediction. Comput. Electron. Agric. 2010, 73, 230–239. [Google Scholar] [CrossRef]
- Restaino, E.A.; Fernandez, E.G.; La Manna, A.; Cozzolino, D. Prediction of the nutritive value of pasture sliage by near infrared spectroscopy (nirs). Chil. J. Agric. Res. 2009, 69, 560–566. [Google Scholar] [CrossRef]
- Rabatel, G.; Al Makdessi, N.; Ecarnot, M.; Roumet, P. A spectral correction method for multi-scattering effects in close range hyperspectral imagery of vegetation scenes: Application to nitrogen content assessment in wheat. Adv. Anim. Biosci. 2017, 8, 353–358. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R. A tutorial on near infrared spectroscopy and its calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- Esquerre, C.; Gowen, A.A.; Burger, J.; Downey, G.; O’Donnell, C.P. Suppressing sample morphology effects in near infrared spectral imaging using chemometric data pre-treatments. Chemom. Intell. Lab. Syst. 2012, 117, 129–137. [Google Scholar] [CrossRef]
- Zhao, D.; Starks, P.J.; Brown, M.A.; Phillips, W.A.; Coleman, S.W. Assessment of forage biomass and quality parameters of bermudagrass using proximal sensing of pasture canopy reflectance. Grassl. Sci. 2007, 53, 39–49. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Pullanagari, R.; Yule, I.; Hedley, M.; Tuohy, M.; Dynes, R.; King, W. Multi-spectral radiometry to estimate pasture quality components. Int. J. Adv. Precis. Agric. 2012, 13, 442–456. [Google Scholar] [CrossRef]
- Hueni, A.; Damm, A.; Kneubuehler, M.; Schläpfer, D.; Schaepman, M.E. Field and airborne spectroscopy cross-validation—some considerations. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 1117–1135. [Google Scholar] [CrossRef]
- Shetty, N.; Gislum, R.; Jensen, A.M.D.; Boelt, B. Development of nir calibration models to assess year-to-year variation in total non-structural carbohydrates in grasses using plsr. Chemom. Intell. Lab. Syst. 2012, 111, 34–38. [Google Scholar] [CrossRef]
- Lundberg, K.; Hoffman, P.; Bauman, L.; Berzaghi, P. Prediction of forage energy content by near infrared reflectance spectroscopy and summative equations. Prof. Anim. Sci. 2004, 20, 262–269. [Google Scholar] [CrossRef]
- Gebremedhin, A.; Badenhorst, P.E.; Wang, J.; Spangenberg, G.C.; Smith, K.F. Prospects for measurement of dry matter yield in forage breeding programs using sensor technologies. Agronomy 2019, 9, 65. [Google Scholar] [CrossRef]
- Deaville, E.R.; Givens, D.I. Regions of normalised near infrared reflectance difference spectra related to the rumen degradation of fresh grass, grass silage and maize silage. Anim. Feed Sci. Technol. 1998, 72, 41–51. [Google Scholar] [CrossRef]
- Vogel, K.; Pedersen, J.F. Breeding Systems for Cross-Pollinated Perennial Grasses; FAO: Rome, Italy, 1993. [Google Scholar]
- Chapman, D.F.; Bryant, J.R.; Kerr, G.A.; Judson, G.; Cookson, T.; Edwards, G.R.; McMillan, W.H. Economic values for perennial ryegrass traits in new zealand dairy farm systems. In Proceedings of the 22nd International Grasslands Congress, Sydney, Australia, 15–19 September 2013; pp. 822–823. [Google Scholar]

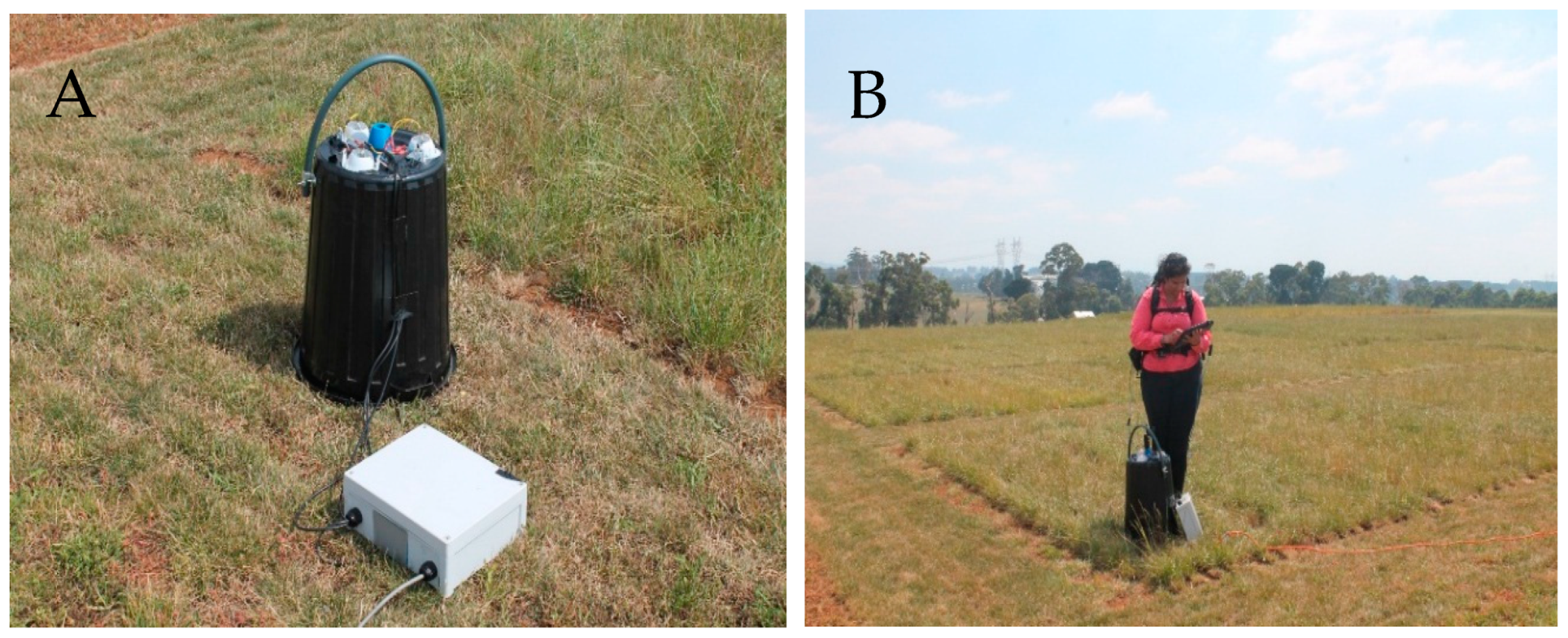


| Date | Breeding Line | Spectra Collected | NV Lab Results |
|---|---|---|---|
| 23/08/2018 | A | 316 | 0 |
| 24/08/2018 | D | 27 | 27 |
| 24/08/2018 | E | 18 | 18 |
| 24/08/2018 | F | 31 | 31 |
| 27/09/2018 | A | 288 | 0 |
| 12/10/2018 | B | 454 | 84 |
| 11/10/2018 | C | 474 | 0 |
| 30/11/2018 | B | 94 | 30 |
| total | 1704 | 190 |
| Statistic | ADF | ash | CP | DM | IVVDMD | IVVOMD | NDF | WSC |
|---|---|---|---|---|---|---|---|---|
| Samples (N) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Slope | 0.61 | 0.47 | 0.68 | 0.69 | 0.70 | 0.64 | 1.08 | 0.78 |
| Intercept | 9.33 | 3.69 | 3.02 | 13.85 | 21.58 | 23.81 | −2.30 | 5.23 |
| Bias | 0.19 | −0.80 | −0.15 | 6.03 | −1.30 | −2.32 | 1.22 | −0.91 |
| SEC | 1.59 | 0.79 | 1.11 | 1.60 | 2.25 | 2.14 | 3.41 | 2.48 |
| SEP | 1.58 | 1.16 | 1.13 | 6.24 | 2.55 | 3.13 | 3.51 | 2.59 |
| SEPC | 1.59 | 0.86 | 1.14 | 1.63 | 2.23 | 2.14 | 3.35 | 2.47 |
| R2 | 0.10 | 0.14 | 0.29 | 0.26 | 0.07 | 0.09 | 0.15 | 0.24 |
| Predicted Ave | 23.51 | 8.45 | 10.02 | 25.44 | 75.33 | 72.62 | 45.11 | 27.38 |
| Actual Ave | 23.70 | 7.65 | 9.88 | 31.47 | 74.03 | 70.30 | 46.33 | 26.48 |
| Predicted SD | 0.85 | 0.67 | 1.02 | 1.34 | 0.90 | 1.04 | 1.33 | 1.76 |
| Actual SD | 1.64 | 0.84 | 1.30 | 1.83 | 2.30 | 2.21 | 3.64 | 2.79 |
| ADF | Ash | CP | DM | IVVDMD | NDF | IVVOMD | WSC | |
|---|---|---|---|---|---|---|---|---|
| average | 24.24 | 9.86 | 11.24 | 24.71 | 76.37 | 45.37 | 72.94 | 23.81 |
| minimum | 20.35 | 5.50 | 4.03 | 19.11 | 68.44 | 29.83 | 63.42 | 12.92 |
| maximum | 29.45 | 15.71 | 23.42 | 33.37 | 90.65 | 55.61 | 82.83 | 31.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, C.; Cogan, N.; Badenhorst, P.; Spangenberg, G.; Smith, K. Field Spectroscopy to Determine Nutritive Value Parameters of Individual Ryegrass Plants. Agronomy 2019, 9, 293. https://doi.org/10.3390/agronomy9060293
Smith C, Cogan N, Badenhorst P, Spangenberg G, Smith K. Field Spectroscopy to Determine Nutritive Value Parameters of Individual Ryegrass Plants. Agronomy. 2019; 9(6):293. https://doi.org/10.3390/agronomy9060293
Chicago/Turabian StyleSmith, Chaya, Noel Cogan, Pieter Badenhorst, German Spangenberg, and Kevin Smith. 2019. "Field Spectroscopy to Determine Nutritive Value Parameters of Individual Ryegrass Plants" Agronomy 9, no. 6: 293. https://doi.org/10.3390/agronomy9060293
APA StyleSmith, C., Cogan, N., Badenhorst, P., Spangenberg, G., & Smith, K. (2019). Field Spectroscopy to Determine Nutritive Value Parameters of Individual Ryegrass Plants. Agronomy, 9(6), 293. https://doi.org/10.3390/agronomy9060293





