1. Introduction
Jerusalem artichoke (
Helianthus tuberosus L.) was initially domesticated in the temperate region of North America [
1]. It was important as a food crop like potato for native Americans and European settlers. The carbohydrate in its tubers, in the form of inulin, can be used as a raw material for health food products, animal feed, and bioethanol [
2,
3]. Jerusalem artichoke is currently grown in most parts of the world and it is successfully established as a food crop in tropical regions [
4]. However, production of Jerusalem artichoke in the tropics faces severe yield loss caused mainly by drought [
5], stem rot [
6], and leaf spot diseases. Stem rot caused by
Sclerotium rolfsii is an important disease of Jerusalem artichoke in tropical regions and yield losses as high as 60% have been estimated [
7]. Leaf spot is an emerging disease of Jerusalem artichoke in tropical regions. The disease causes severe leaf damage, lowers photosynthesis, and can reduce yield by up to 80% in
H. annuus [
8].
Jerusalem artichoke in temperate regions was shown to be moderately resistant to Alternaria leaf blight and stem spot caused by
Alternaria helianthi [
9], and it was used as a source of resistance to Alternaria leaf blight and stem spot in sunflower [
10]. Alternaria leaf spot on Jerusalem artichoke in Thailand appears as small yellow spots on leaves; the spots eventually turn brown and are surrounded by yellow haloes. Thereafter, the spots expand and coalesce. The leaves show leaf blight symptoms, and defoliation begins on mature leaves and spreads upward to younger leaves.
Methods for control of leaf spot incited by
Alternaria species have been investigated in sunflower and many of other crops. The disease can be controlled by several methods such as the use of resistant varieties, chemical control by fungicide applications [
11], and biological control [
12]. However, the lack of known resistant germplasm sources is an important constraint to the development of Jerusalem artichoke varieties with resistance to Alternaria leaf spot. The objectives of this study were to identify genotype variability of Jerusalem artichoke genotypes for resistance to Alternaria leaf spot under field conditions and to investigate the relationships among resistance characters, yield, and yield components for selection of resistant varieties.
4. Discussion
The studies of diversity in Jerusalem artichoke had been conducted for yield components [
24], inulin content [
25], morphological traits and agronomic traits [
26,
27], and stem rot resistance [
28]. For leaf spot disease of
Helianthus species, there was only one study in the temperate zone [
9]. To our knowledge, genotypic resistance to Alternaria leaf spot in tropical area has not been reported previously in Jerusalem artichoke. Alternaria leaf spot can destroy Jerusalem artichoke leaves, which are the main source of photosynthesis, reducing photosynthetic area and yield. Leaf spot disease caused by
A. alternata destroys the active leaf area and reduces yield of sunflower [
29]. Sunflowers with disease severity higher than 10% yielded less than 500 kg/ha [
30]. For mustard and rapeseed, leaf spot disease reduced yield up to 70% [
31].
In the present study, genotypic diversity of Jerusalem artichoke for resistance to Alternaria leaf spot was highly significant among accessions and was arranged into three groups including resistant, moderately resistant, and susceptible accessions. The varieties were classified into different 3 groups in two seasons. HEL335, HEL256, HEL317, HEL308, and JA86 showed high level of resistance to leaf spot disease in both seasons, whereas HEL 293 and HEL 246 showed susceptibility to leaf spot disease in both seasons. These groups of genotypes can be used as sources of resistance and standard susceptible checks, respectively, for leaf spot disease evaluations in breeding programs of Jerusalem artichoke. In sunflower, the genetic control of resistance to Alternaria leaf blight was polygenic and conferred by dominant genes [
32].
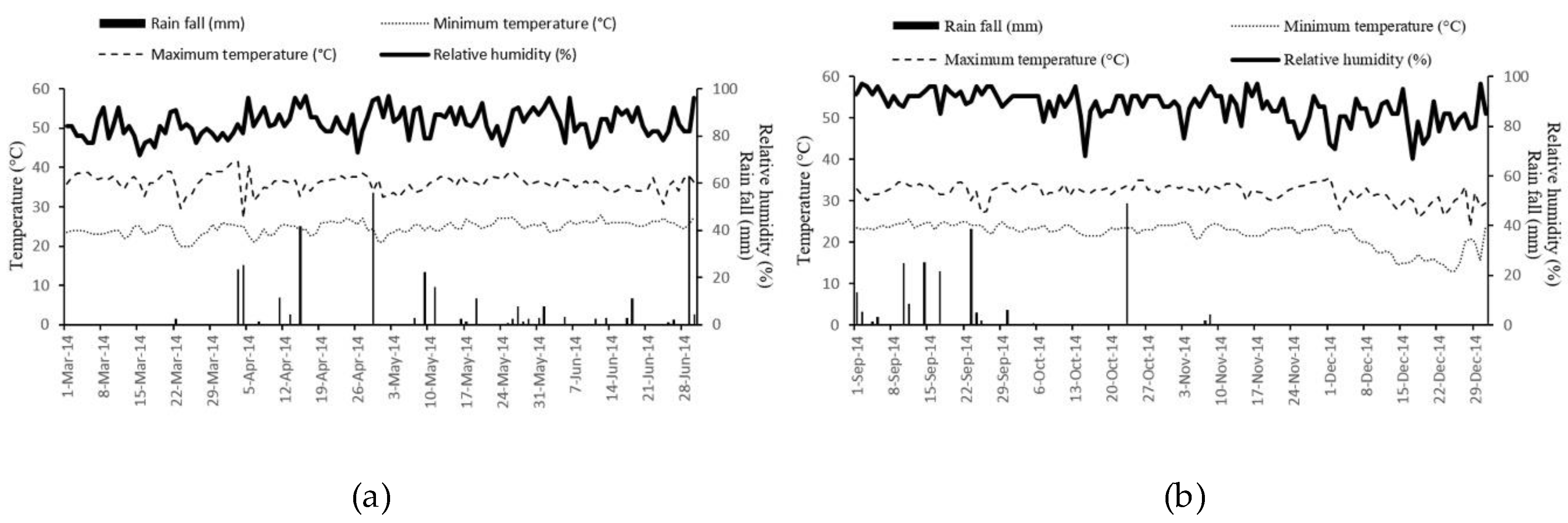
In this study, all disease parameters in the early rainy season were higher than in the late rainy season (
Table 3 and
Table 4). Season significantly affected disease incidence and disease severity index (
Table 2). In the early rainy season, relative humidity was consistently higher than in the late rainy season. The range of relative humidity was 72%–97% in the early rainy season and 67%–97% in the late rainy season. In the late rainy season, during the critical time for disease development at 60 days after transplanting, relative humidity was lower than during the early rainy season. In the early rainy season, the AUDPC-DI and DSI was higher than in the late rainy season. In the early rainy season relative humidity was consistently higher throughout the testing season but in late rainy season, the relative humidity was lower after 60 days after transplanting. The relative humidity may be the main factor for conidia germination, leaf penetration, and development of the disease. Green and Bailey [
33] found that
A. cirsinoxia conidia germinated well under relative humidity higher than 90%. The temperature also may have affected disease progress. The temperature in the early rainy season (20–41.5 °C) was higher than in late rainy season (21–35.5 °C). The optimum temperature for germination of
Alternaria is 24 °C in laboratory conditions. The influence of temperature on Alternaria blight development of sunflower also varied between crop and season [
34]. Rainfall did not affect disease incidence and disease severity, possibly because the experiment was conducted under irrigation with a mini-sprinkler in both seasons. In sunflowers, development of Alternaria blight under field conditions was related to minimum and maximum temperature and relative humidity [
32]. Not only weather parameters, but also plant physiological growth stage affected Alternaria blight development in mustard [
35].
The results of combined analysis of variance shown highly significant of variety by season inter action for all disease parameters (
Table 2) indicated that the performance of the tested genotypes for disease resistance was inconsistent across seasons. A similar report of screening of a potato for resistance to early blight showed low correlation between the seasons [
36]. Therefore, screening of leaf spot disease resistance in Jerusalem artichoke for disease incidence and disease severity index should be conducted in at least two seasons.
Positive correlation was found between disease parameters in this experiment (
Table 7 and
Table 8). In early rainy season, AUDPC-DI and AUDPC-DSI was very strongly correlated (
Table 7) and in late rainy season, high correlations between AUDPC-DI with disease incidence and AUDPC-DSI with disease severity index were observed (
Table 8). In Alternaria blight of sunflower [
32], mustard [
31], and
Brassica [
37], disease incidence and disease severity index were used as resistant indexes. Correlation between disease parameters could help breeders use alternative traits as indirect selection indexes for improving resistant genotypes of Jerusalem artichoke.
In this study, Jerusalem artichoke grown in the early rainy season had lower yield and yield components than did the crop grown in the late rainy season. Season was the main source of variation in number of tubers per plant, tuber size, and tuber yield. The variations in these traits as affected by seasonal variations would be due to the fact that quantitative traits are controlled by multiple genes with combined effect, and expression of these traits can vary greatly depending on environment [
38]. Several quantitative traits such as tuber yield, tuber size, inulin content, and maturity are economically important [
25]. HEL278 and HEL280 had the highest yield and yield components in both seasons. HEL 278 showed susceptibility to leaf spot disease whereas HEL 280 showed moderate resistance to disease. These genotypes can be used as sources for breeding programs to improve yield and yield components in Jerusalem artichoke. In general, no significant correlation was found between disease parameters and yield and yield components in both seasons. The results indicated that selection for high yield and desirable yield components with Alternaria resistance is possible with the tested materials. It is possible that severity of Alternaria leaf spot would need to be higher than in our study in order to increase yield loss.
5. Conclusions
In conclusion, variation of Jerusalem artichoke genotypes for Alternaria leaf spot was grouped into three groups including resistant, moderately resistant, and susceptible. HEL335, HEL256, HEL317, HEL308, and JA86 were resistant genotypes and HEL 293 and HEL 246 were classified to susceptible genotypes. These groups can be used as sources of resistance and susceptible check, respectively, for breeding of leaf spot disease resistance. HEL278 and HEL280 had the highest yield and yield components in both seasons. These genotypes can be used as sources for breeding programs to improve yield and yield components in Jerusalem artichoke. Selection of Jerusalem artichoke for high yield and desirable yield components with Alternaria resistance is possible because of no correlation between agronomic traits with leaf spot disease resistance.