Roots of Lucerne Seedlings are More Resilient to a Water Deficit than Leaves or Stems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.3. Statistical Analysis
3. Results
3.1. Effect of Water Treatments on the Relative Water Content of Leaves, Stems and Roots
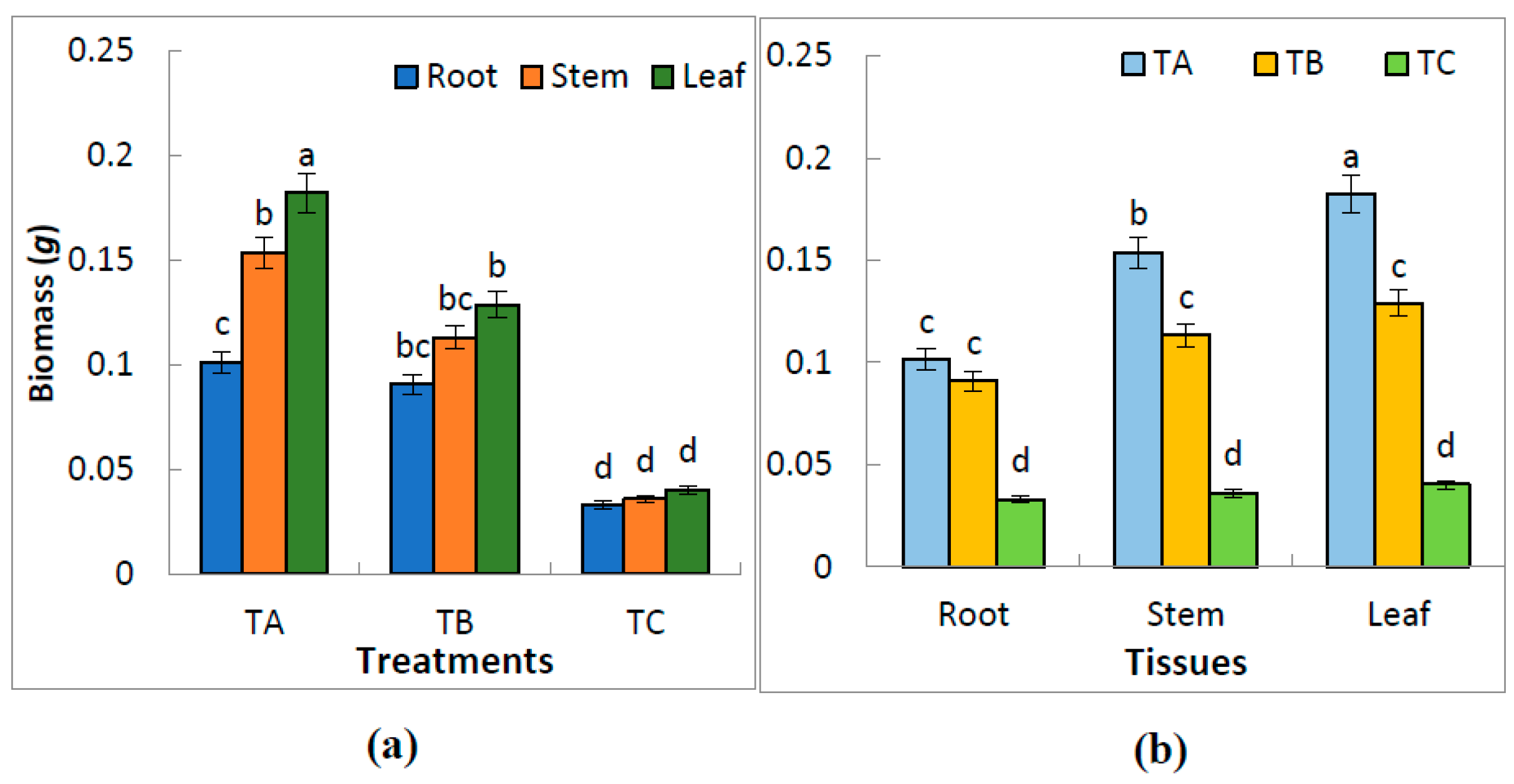
3.2. Effect of Water Treatments on the Biomass of Leaves, Stems and Roots
3.3. Effect of Water Treatments on the Proline Concentration of the Leaves, Stems and Roots
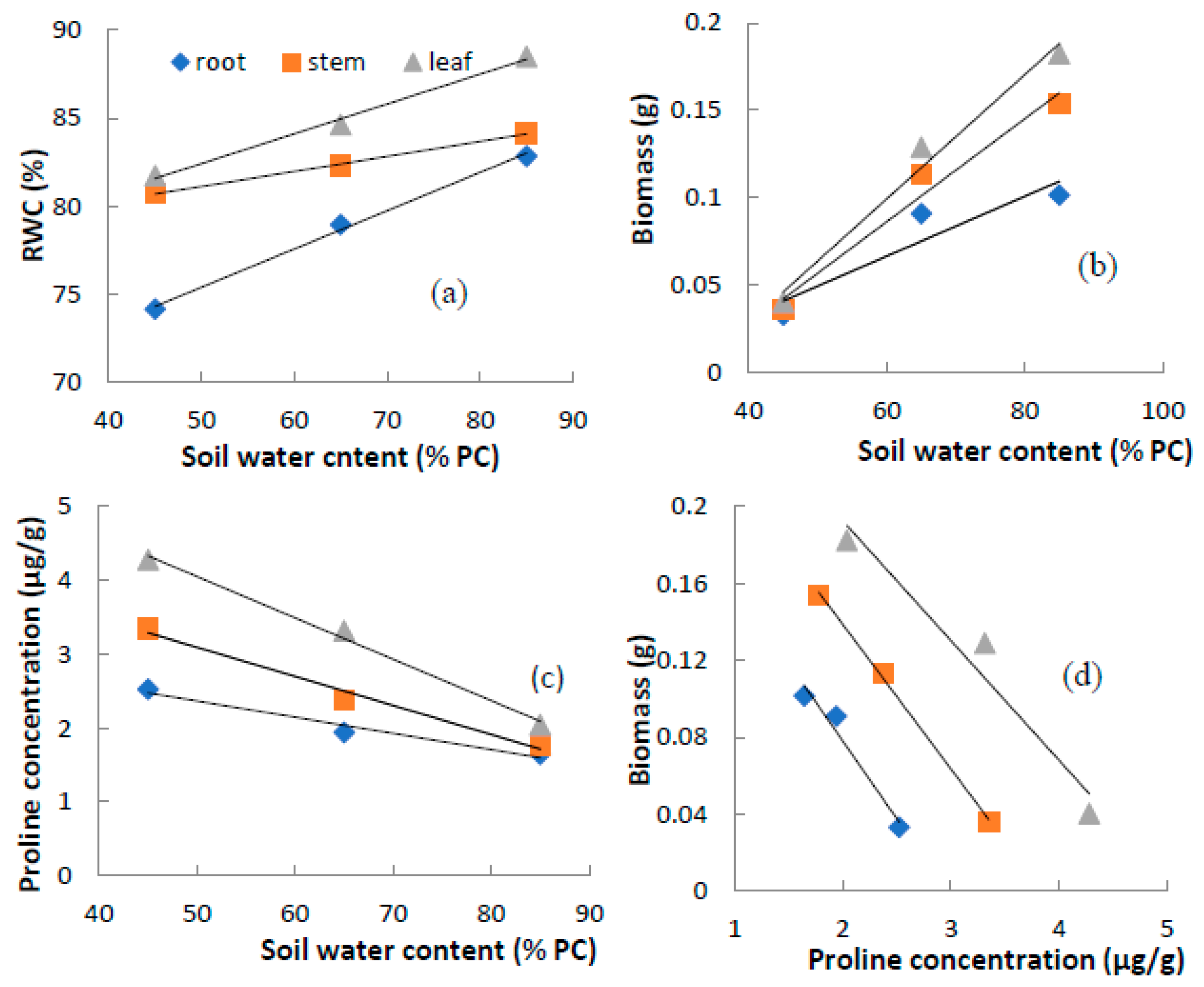
3.4. Correlation among Soil Water Content, Relative Water Content, Biomass and Proline Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doole, G.J.; Pannell, D.J. Role and value of including lucerne (Medicago sativa L.) phases in crop rotations for the management of herbicide-resistant Lolium rigidum in Western Australia. Crop Prot. 2008, 27, 497–504. [Google Scholar] [CrossRef]
- García, J.; Pérez-Alba, L.; Alvarez, C.; Rocha, R.; Ramos, M.; De Blas, C. Prediction of the nutritive value of lucerne hay in diets for growing rabbits. Anim. Feed Sci. Technol. 1995, 54, 33–44. [Google Scholar] [CrossRef]
- Bell, F.; Nutman, P.S. Experiments on nitrogen fixation by nodulated lucerne. Plant Soil 1971, 35, 231–264. [Google Scholar] [CrossRef]
- Jiang, H.M.; Jiang, J.P.; Jia, Y.; Li, F.M.; Xu, J.Z. Soil carbon pool and effects of soil fertility in seeded alfalfa fields on the semi-arid Loess Plateau in China. Soil Biol. Biochem. 2006, 38, 2350–2358. [Google Scholar] [CrossRef]
- Turner, N.C.; Molyneux, N.; Yang, S.; Xiong, Y.C.; Siddique, K.H. Climate change in south-west Australia and north-west China: Challenges and opportunities for crop production. Crop Pasture Sci. 2011, 62, 445–456. [Google Scholar] [CrossRef]
- Yousfi, N.; Slama, I.; Ghnaya, T.; Savouré, A.; Abdelly, C. Effects of water deficit stress on growth, water relations and osmolyte accumulation in Medicago truncatula and M. laciniata populations. C. R. Biol. 2010, 333, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F.M.; Wang, X.L. Soil quality responses to alfalfa watered with a field micro-catchment technique in the Loess Plateau of China. Field Crops Res. 2006, 95, 64–74. [Google Scholar] [CrossRef]
- Guan, X.K.; Zhang, X.H.; Turner, N.C.; Xu, B.C.; Li, F.M. Two perennial legumes (Astragalus adsurgens Pall. and Lespedeza davurica S.) adapted to semiarid environments are not as productive as lucerne (Medicago sativa L.), but use less water. Grass Forage Sci. 2013, 68, 469–478. [Google Scholar] [CrossRef]
- Turner, N.C. Adaptation to water deficits: A changing perspective. Funct. Plant Biol. 1986, 13, 175–190. [Google Scholar] [CrossRef]
- Thiyagarajan, G.; Rajakumar, D.; Kumaraperumal, R.; Manikandan, M. Physiological responses of groundnut (Arachis hypogaea L.) to moisture stress: A review. Agric. Rev. 2009, 30, 192–198. [Google Scholar]
- Guo, Z.G.; Liu, H.X.; Wang, S.M.; Tian, F.P.; Cheng, G.D. Biomass, persistence and drought resistance of nine lucerne varieties in the dry environment of west China. Aust. J. Exp. Agric. 2005, 45, 59–64. [Google Scholar] [CrossRef]
- Petcu, E.; Schitea, M.; Cîrstea, V.E. The effect of water stress on cuticular transpiration and its association with alfalfa yield. Rom. Agric. Res. 2009, 26, 53–56. [Google Scholar]
- Liu, H.S.; Li, F.M.; Xu, H. Deficiency of water can enhance root respiration rate of drought-sensitive but not drought-tolerant spring wheat. Agric. Water Manag. 2004, 64, 41–48. [Google Scholar] [CrossRef]
- Turner, N.C.; Begg, J.E. Plant-water relations and adaptation to stress. Plant Soil 1981, 58, 97–131. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Stewart, C.R.; Hanson, A.D. Proline accumulation as a metabolic response to water stress. In Adaptation of Plants to Water and High Temperature Stress; Turner, N.C., Kramer, P.J., Eds.; John-Wiley & Sons: New York, NY, USA, 1980; pp. 173–189. [Google Scholar]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.E.; Moot, D.J.; Fletcher, A.L.; Jamieson, P.D. A framework for quantifying water extraction and water stress responses of perennial lucerne. Crop Pasture Sci. 2009, 60, 785–794. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Pecetti, L.; Tava, A. Physiological and morphological traits associated with adaptation of lucerne (Medicago sativa) to severely drought-stressed and to irrigated environments. Ann. Appl. Biol. 2013, 162, 27–40. [Google Scholar] [CrossRef]
- Petcu, E.; Schitea, M.; Dragan, L. The effect of water stress on stomatal resistance and chlorophyll fluorescence and their association with alfalfa yield. Rom. Agric. Res. 2014, 31, 113–119. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- French, R.J.; Turner, N.C. Water deficits change dry matter partitioning and seed yield in narrow-leafed lupins (Lupinus angustifolius L.). Aust. J. Agric. Res. 1991, 42, 471–484. [Google Scholar] [CrossRef]
- Haffani, S.; Mezni, M.; Slama, I.; Ksontini, M.; Chaïbi, W. Plant growth, water relations and proline content of three vetch species under water-limited conditions. Grass Forage Sci. 2014, 69, 323–333. [Google Scholar] [CrossRef]
- Porter, M.A.; Bidlack, J.E. Morphology, biomass, and vessel diameter of pigeon pea subjected to water stress. Commun. Soil Sci. Plant Anal. 2011, 42, 2334–2343. [Google Scholar] [CrossRef]
- Talluto, G.; Gugliuzza, G.; Massenti, R.; Lo Bianco, R. Growth and biomass partitioning of young loquat plants under water deficit. Acta Hort. 2015, 1092, 199–204. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Pacheco, C.M.A.; Chaves, M.M. Soil-plant water relations, root distribution and biomass partitioning in Lupinus albus L. under drought conditions. J. Exp. Bot. 1995, 46, 947–956. [Google Scholar] [CrossRef]
- Turner, N.C. Imposing and maintaining soil water deficits in drought studies in pots. Plant Soil 2018, 1–11. [Google Scholar] [CrossRef]
- Sobrado, M.A.; Turner, N.C. A comparison of the water relations characteristics of Helianthus annuus and Helianthus petiolaris when subjected to water deficits. Oecologia 1983, 58, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.; Larsen, E.K.; Davies, W.J.; Dodd, I.C. Applying ‘drought’ to potted plants by maintaining suboptimal soil moisture improves plant water relations. J. Exp. Bot. 2017, 68, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought tolerance in Pinus halepensis seed sources as identified by distinctive physiological and molecular markers. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, D.; Paleg, L.G. Proline accumulation: Physiological aspects. In Physiology and Biochemistry of Drought Resistance in Plants; Paleg, L.G., Aspinall, D., Eds.; Academic Press: New York, NY, USA, 1981; pp. 205–207. [Google Scholar]
- Sharma, S.; Villamor, J.G.C.; Verslues, P.E. Essential role of tissue specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.H.; Dandekar, A.M. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Verbruggen, N.; Villarroel, R.; Van Montagu, M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol. 1993, 103, 771–781. [Google Scholar] [CrossRef] [PubMed]


| RWC (%) | |||||
|---|---|---|---|---|---|
| Root | Stem | Leaf | Mean(T) | LSD(T) | |
| TA | 82.5 ± 4.3 aA | 84.2 ± 2.8 aA | 88.5 ±3.4 aA | 85.0 ± 3.1 a | ns |
| TB | 78.9 ± 4.6 aA | 82.3 ± 0.5 aA | 84.6 ± 1.4 abA | 81.9 ± 2.9 ab | ns |
| TC | 74.1 ± 2.9 aA | 80.7 ± 2.2 aA | 81.7 ± 0.9 bA | 78.9 ± 4.1 b | ns |
| Mean(W) | 78.5 ± 8.2 B | 82.4 ± 1.7 AB | 85.0 ± 3.4 A | 81.9 ± 4.0 b | |
| LSD(W) | ns | ns | 3.99 | T × W: ns | |
| Treatment | Total Biomass (g plant−1) | Root to Total Biomass (%) | Stem to Total Biomass (%) | Leaf to Total Biomass (%) | Root:Shoot Ratio |
|---|---|---|---|---|---|
| TA | 0.44 ± 0.09 a | 23.2 ± 2.3 a | 35.1 ± 6.4 a | 41.7 ± 7.3 a | 0.30 ± 0.007 a |
| TB | 0.33 ± 0.18 b | 27.3 ± 3.2 a | 33.0 ± 7.2 b | 38.7 ± 6.8 a | 0.38 ± 0.009 b |
| TC | 0.11 ± 0.04 c | 30.2 ± 4.2 b | 33.0 ± 5.4 c | 36.8 ± 6.3 b | 0.43 ± 0.005 c |
| Index | Plant Tissue | Liner Regression | Correlation Coefficient |
|---|---|---|---|
| RWC | Leaf | Y = 0.169X + 74.0 | R =0.84 ** |
| Stem | Y = 0.086X + 76.8 | R = 0.61 * | |
| Root | Y = 0.209X + 64.9 | R = 0.58 * | |
| Biomass | Leaf | Y = 0.004X − 0.114 | R = 0.98 ** |
| Stem | Y = 0.003X − 0.090 | R = 0.98 ** | |
| Root | Y = 0.002X − 0.036 | R = 0.91 ** | |
| Proline concentration | Leaf | Y = −0.056X + 6.80 | R = 0.99 ** |
| Stem | Y = −0.039X + 5.06 | R = 0.99 ** | |
| Root | Y = −0.029X + 3.36 | R = 0.98 ** | |
| Biomass-proline concentration | Leaf | Y = −15.20X + 4.99 | R = −0.97 ** |
| Stem | Y = −13.11X + 3.82 | R = −0.97 ** | |
| Root | Y = −11.56X + 2.90 | R = −0.95 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-Z.; Liu, H.; Yan, G.; Li, G.; Turner, N.C. Roots of Lucerne Seedlings are More Resilient to a Water Deficit than Leaves or Stems. Agronomy 2019, 9, 123. https://doi.org/10.3390/agronomy9030123
Luo Y-Z, Liu H, Yan G, Li G, Turner NC. Roots of Lucerne Seedlings are More Resilient to a Water Deficit than Leaves or Stems. Agronomy. 2019; 9(3):123. https://doi.org/10.3390/agronomy9030123
Chicago/Turabian StyleLuo, Yong-Zhong, Hui Liu, Guijun Yan, Guang Li, and Neil C. Turner. 2019. "Roots of Lucerne Seedlings are More Resilient to a Water Deficit than Leaves or Stems" Agronomy 9, no. 3: 123. https://doi.org/10.3390/agronomy9030123
APA StyleLuo, Y.-Z., Liu, H., Yan, G., Li, G., & Turner, N. C. (2019). Roots of Lucerne Seedlings are More Resilient to a Water Deficit than Leaves or Stems. Agronomy, 9(3), 123. https://doi.org/10.3390/agronomy9030123





