Ammonium Sorbed to Zeolite Is Partly Available to Wheat in the First Growth Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Zeolite
2.2. Zeolite and Labelled N
2.3. Growth Trials and Treatments
2.4. Analyses
2.5. Calculations and Statistics
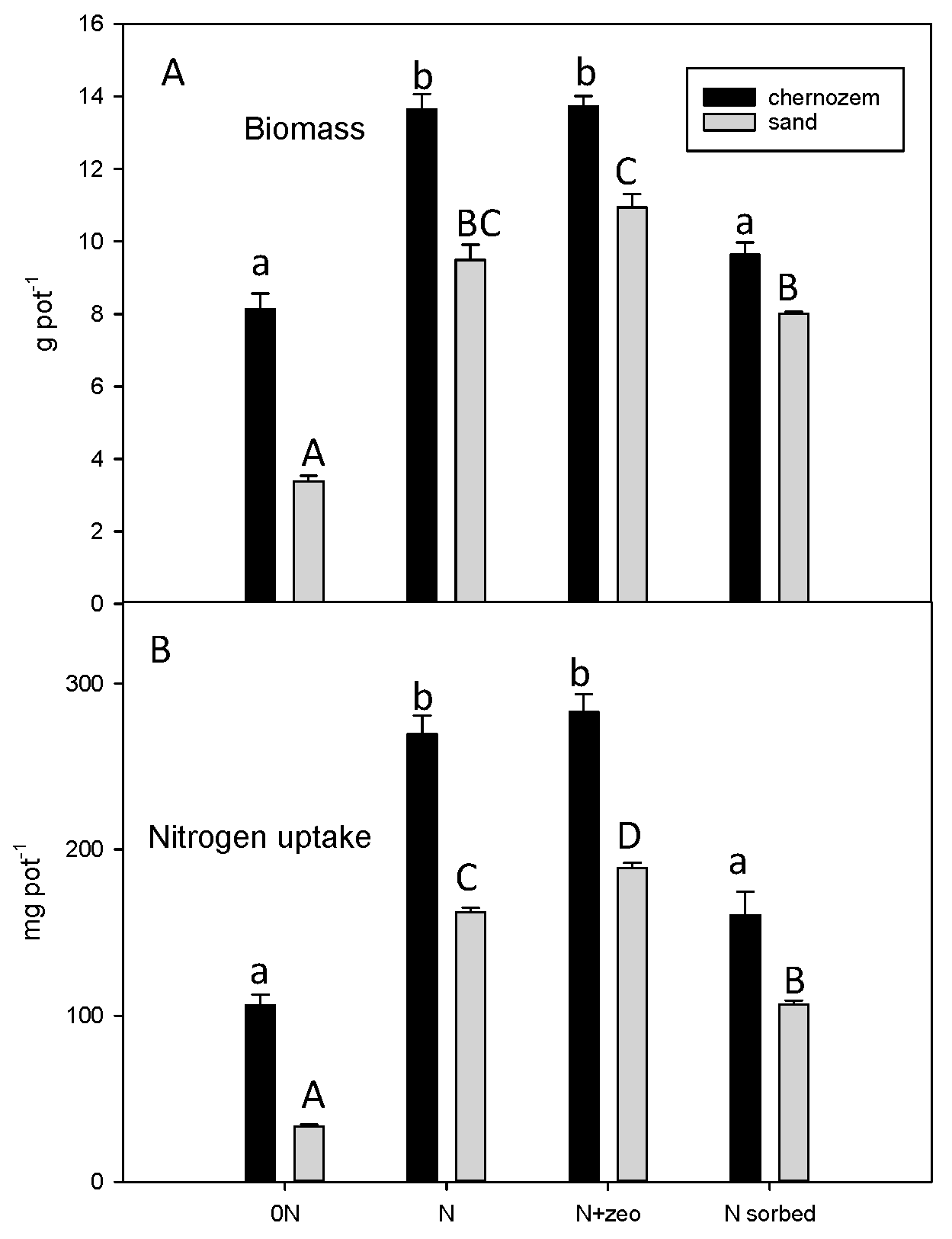
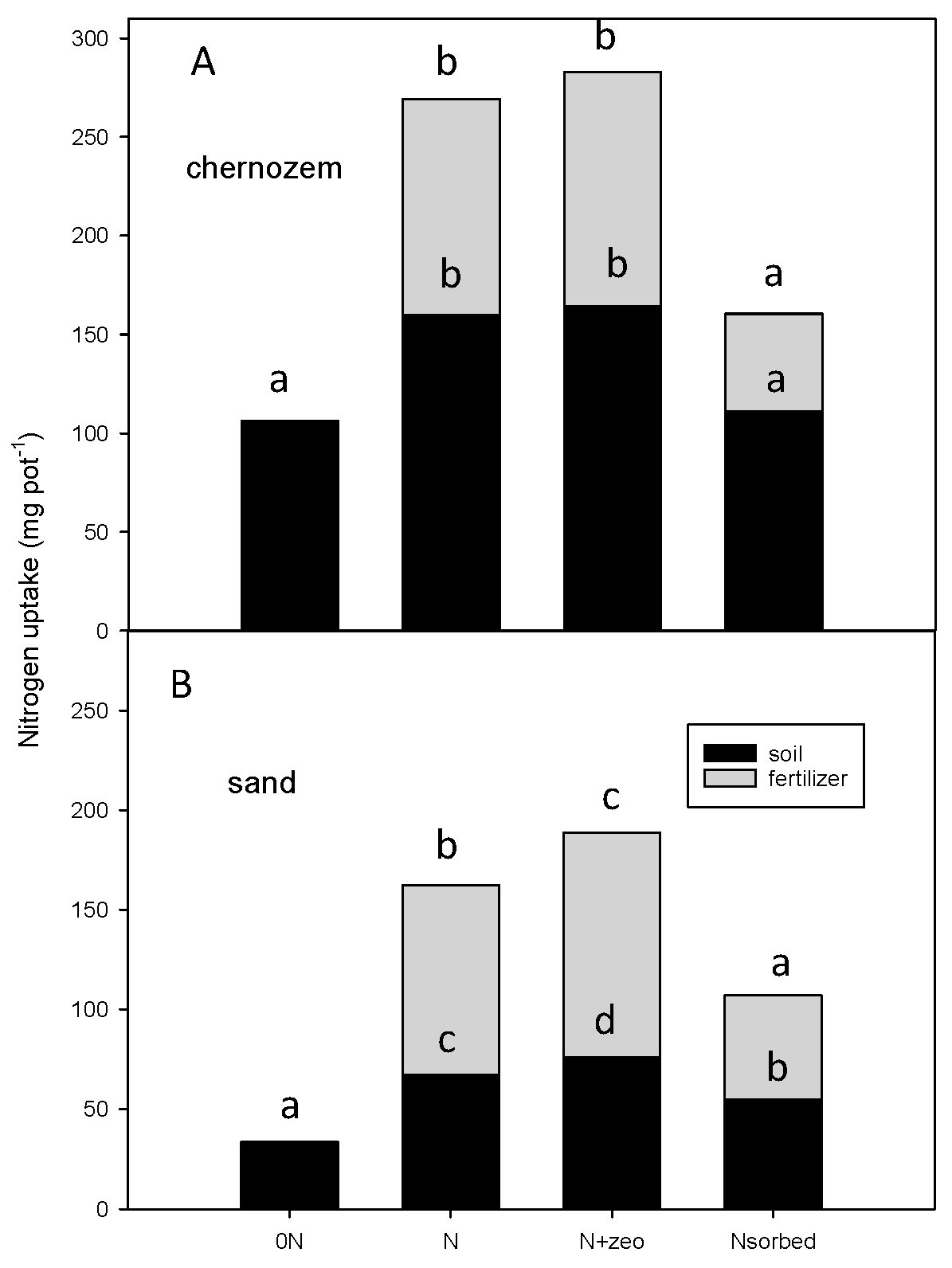
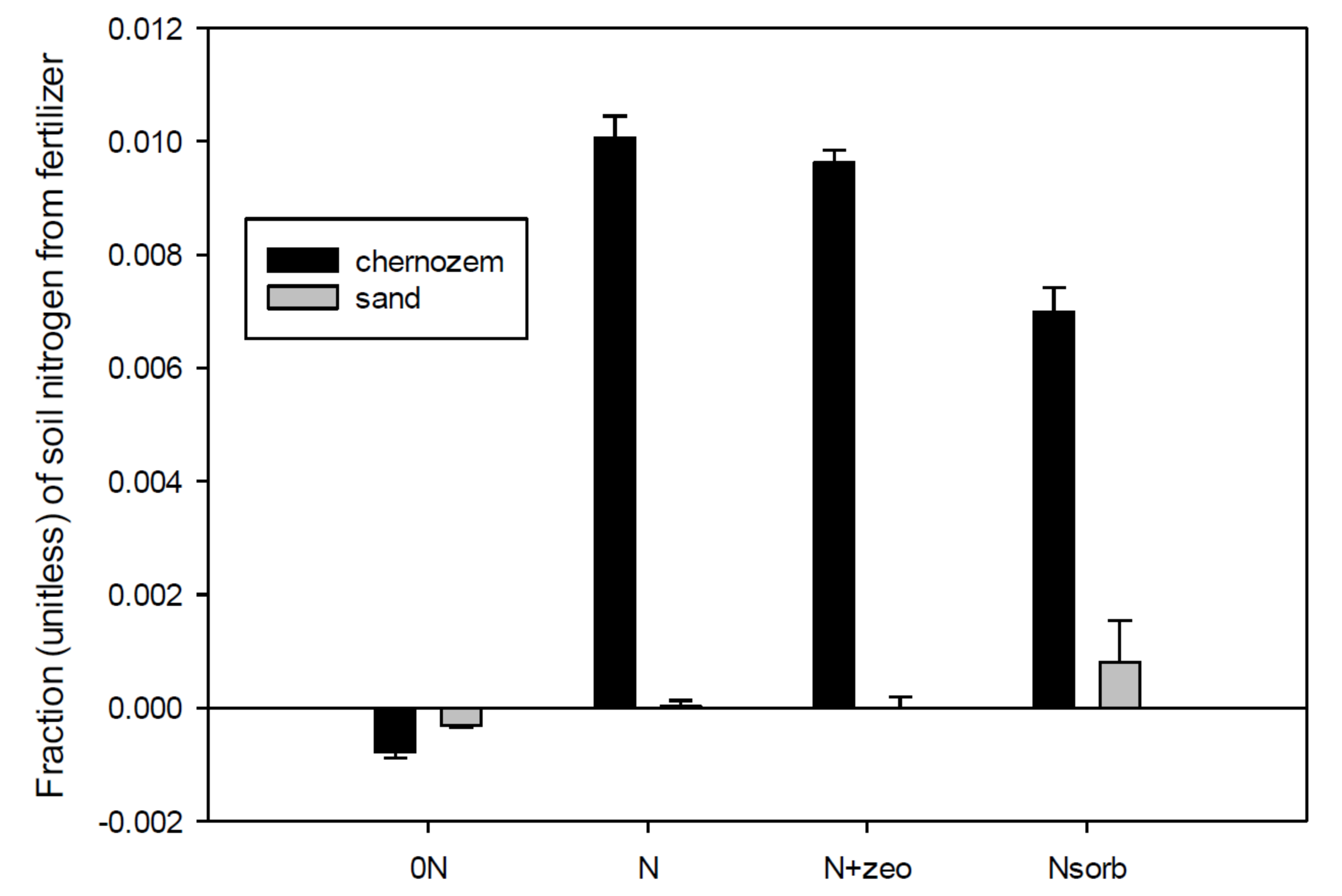
3. Results
3.1. Biomass and N Uptake
3.2. N Uptake from Soil and Fertilizer
3.3. Soil N
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoornweg, D.; Bhada-Tata, P.; Kennedy, C. Environment: Waste production must peak this century. Nature 2013, 502, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Metha, C.M.; Khunjar Nguyen, V.; Tait, S.; Bastone, D.J. Technologies to recover nutrients from waste streams: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar]
- Crini, G.; Badot, P.-M. Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents for Pollutant Removal from Wastewaters; Presses universitaires de Franche-Comté: Besançon, France, 2010. [Google Scholar]
- Estevez, M.M.; Sapci, Z.; Linjordet, R.; Morken, J. Incorporation of fish by-product into the semi-continuous anaerobic co-digestion of pre-treated lignocellulose and cow manure, with recovery of digestate’s nutrients. Renew. Energy 2014, 66, 550–558. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Gibert, O.; Moreno, N.; Querol, X.; Batis, N.H.; Cortina, J.L. Recovery of nutrients (N-P-K) from potassium-rich sludge anaerobic digestion side-streams by integration of a hybrid sorption-membrane ultrafiltration process: Use of powder reactive sorbents as nutrient carriers. Sci. Total Environ. 2017, 599–600, 422–430. [Google Scholar]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2012, 1, 137–144. [Google Scholar]
- Kucic, S.; Cosic, I.; Vukovic, M.; Briski, F. Sorption kinetic studies of ammonium from aqueous solution on different inorganic and organic media. Acta Chim. Slov. 2013, 60, 109–119. [Google Scholar] [PubMed]
- Mazeikiene, A.; Valentukrvicirne, M. Removal of ammonium ions from digested sludge fugate by using natual zeolite. J. Environ. Eng. Landsc. Manag. 2016, 24, 176–184. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphates and ammonium sorption of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. A wood based low temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 2012, 353, 73–84. [Google Scholar] [CrossRef]
- Arcoya, A.; Gonzales, J.A.; Travisio, N.; Seoane, X.L. Physiochemical and catalytic properties of a modified natural zeolite. Clay Min. 1994, 29, 123–131. [Google Scholar] [CrossRef]
- Chu, P. The deammoniation reaction of ammonium Y zeolite. J. Catal. 1976, 43, 346–352. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kamman, C.; Muller, C. A review of Biochar and Soil Nitrogen Dynamics. Agron 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Knowles, O.A.; Robinson, B.H.; Contangelo, A.; Clucas, L. Biochar for mitigation of nitrate leaching from soil amended with biosolids. Sci. Total Environ. 2011, 409, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a columbian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact Beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thiesa, J.; Luizãoc, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Zheng, J.; Stewart, C.E.; Cotrufo, F. Biochar and nitrogen fertilizer alters nitrogen dynamics and greenhouse gas fluxes from two temperate soils. J. Environ. Qual. 2012, 41, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Szegi, T.A.; Czibulya, Z.; Makadi, M.; Gal, A.; Tombacz, E. Improvement of physical and chemical properties of Hungarian sandy soils by adding organic and inorganic amendments. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 10–13. [Google Scholar]
- Makádi, M.; Henzsel, I.; Lazányi, J. Application of bentonite in agriculture. In Proceedings of the Conference of “Agrárgazdaság, vidékfejlesztés és agrárinformatika az évezred küszöbén (AVA)”, Debrecen, Hungary, 1–2 April 2003. (In Hungarian with English summary). [Google Scholar]
- Makadi, M.; Tomocsik, A.; Oroz, V.; Bogdanyi, Z.; Biro, B. Effect of biogas-digestate and bentonite on some enzyme activities of the amended soil. Cereal Res. Commun. 2007, 35, 741–744. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Sauras, T.; Cortina, J.L. Valorisation of N and P from waste water by using natural reactive hybrid sorbents: Nutrients (N,P,K) release evaluation in amended soils by dynamic experiments. Sci. Total Environ. 2018, 612, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wee, C.; Sohn, B. Effect of ammonium- and potassium-loaded zeolite on kale (Brassica alboglabra) growth and soil properties. Am. J. Plant Sci. 2013, 4, 1976–1982. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, Y. Zeolite as slow release fertilizer on spinach yields and quality in a greenhouse test. J. Plant Nutr. 2013, 36, 1496–1505. [Google Scholar] [CrossRef]
- Perrin, T.S.; Drost, D.T.; Boettinger, J.L.; Norton, J.M. Ammonium-loaded Clinoptilolite: A slow-release nitrogen fertilizer for sweet corn. J. Plant Nutr. 1998, 21, 515–530. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- NIBIO. Gjødslingshåndbok (Fertilizer Handbook). Norwegian Institute for Bioeconomy Reseach. 2016. Available online: http://www.bioforsk.no/ikbViewer/page/prosjekt/lenker?p_dimension_id=19190&p_dim2=15837 (accessed on 8 January 2017).
- Ericsson, T. Growth and shoot:root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 168/169, 205–214. [Google Scholar] [CrossRef]
- Hilbert, D.W. Optimization of plant root: Shoot ratios and internal nitrogen concentration. Ann. Bot. 1990, 66, 91–99. [Google Scholar] [CrossRef]
- Ågren, I.G.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P. The soil microbial biomass: Concept, Measurement and applications in soil ecosystem research. Microb. Environ. 2001, 16, 131–140. [Google Scholar] [CrossRef]
- He, Z.L.; Calvert, D.V.; Alva, A.K.; Li, Y.C.; Banks, D.J. Clinoptilolite zeolite and cellulose amendments to reduce ammonia volatilization in a calcareous sandy soil. Plant Soil 2002, 247, 253–260. [Google Scholar] [CrossRef]
| N | N + zeo | Nsorb | |
|---|---|---|---|
| 15N recovery | |||
| Chernozem | 54.8 (2.28) | 59.4 (2.20) | 24.8 (2.16) |
| Sandy soil | 47.6 (0.72) | 56.4 (1.01) | 26.2 (0.51) |
| MFE | |||
| Chernozem | 108.3 | 45.3 | |
| Sandy soil | 118.5 | 55.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foereid, B.; Alvarenga, E.; Szocs, J.; Makadi, M. Ammonium Sorbed to Zeolite Is Partly Available to Wheat in the First Growth Cycle. Agronomy 2019, 9, 122. https://doi.org/10.3390/agronomy9030122
Foereid B, Alvarenga E, Szocs J, Makadi M. Ammonium Sorbed to Zeolite Is Partly Available to Wheat in the First Growth Cycle. Agronomy. 2019; 9(3):122. https://doi.org/10.3390/agronomy9030122
Chicago/Turabian StyleFoereid, Bente, Emilio Alvarenga, Julia Szocs, and Marianna Makadi. 2019. "Ammonium Sorbed to Zeolite Is Partly Available to Wheat in the First Growth Cycle" Agronomy 9, no. 3: 122. https://doi.org/10.3390/agronomy9030122
APA StyleFoereid, B., Alvarenga, E., Szocs, J., & Makadi, M. (2019). Ammonium Sorbed to Zeolite Is Partly Available to Wheat in the First Growth Cycle. Agronomy, 9(3), 122. https://doi.org/10.3390/agronomy9030122





