Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory
Abstract
1. Introduction
2. Materials and Methods
2.1. Propagation of Unrooted Runner Plants
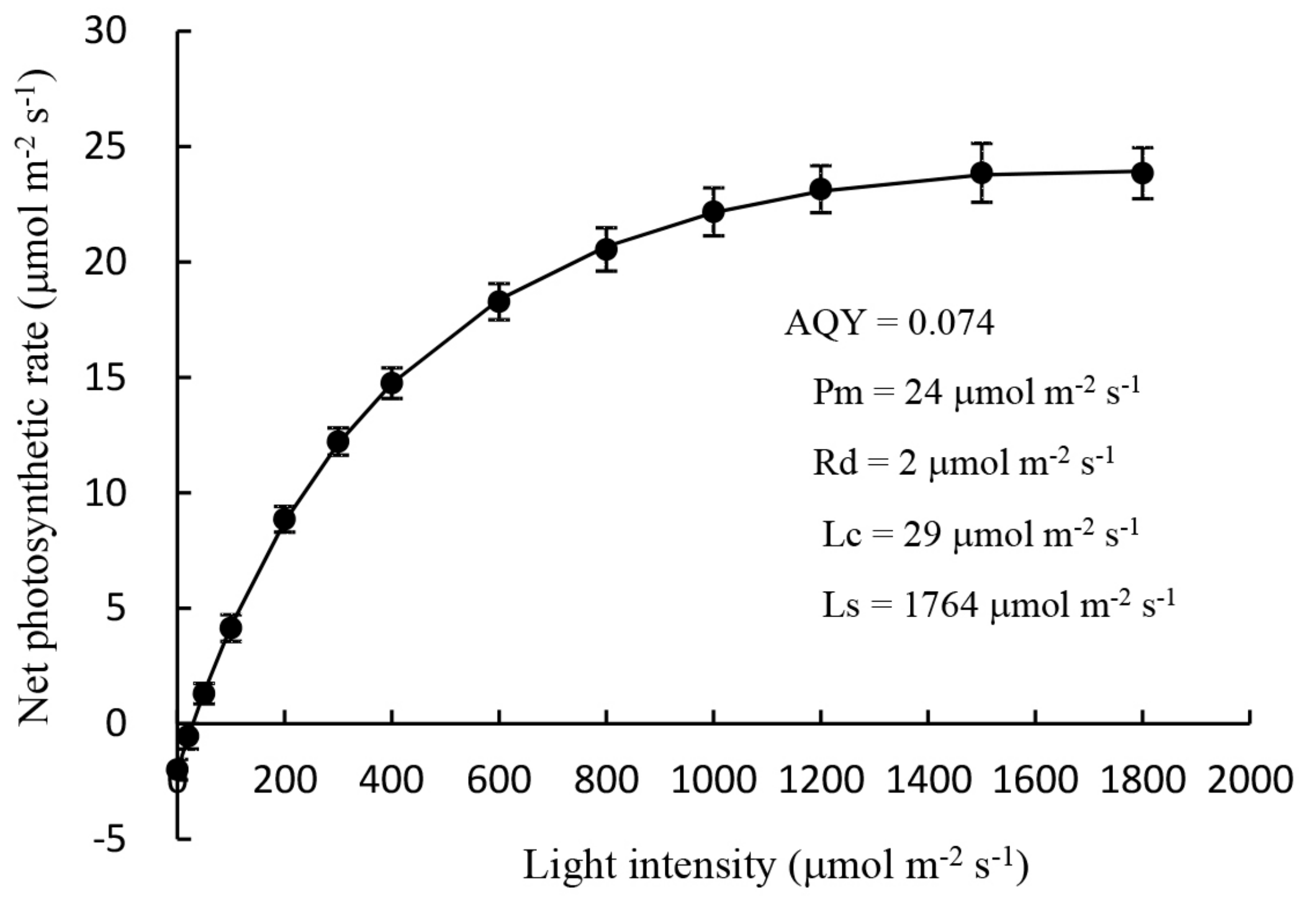
2.1.1. Measurement of the Light Response Curve of Mother Plants
2.1.2. Harvest of Unrooted Runner Plants
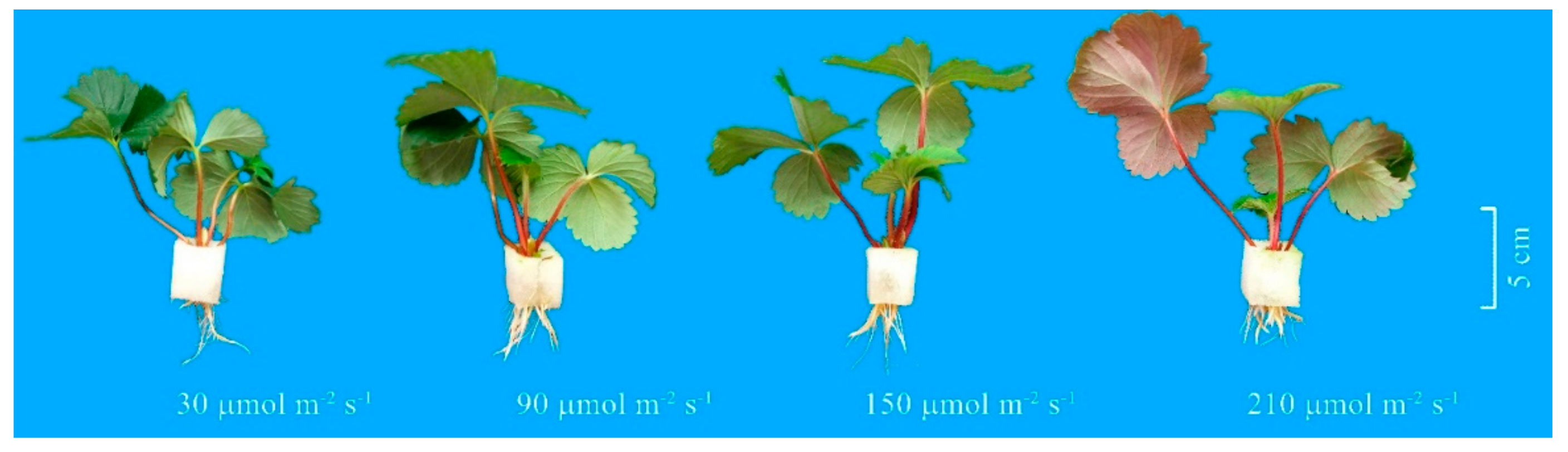
2.2. Effect of Light Intensity on Rooting of Unrooted Runner Plants at the Rooting Stage
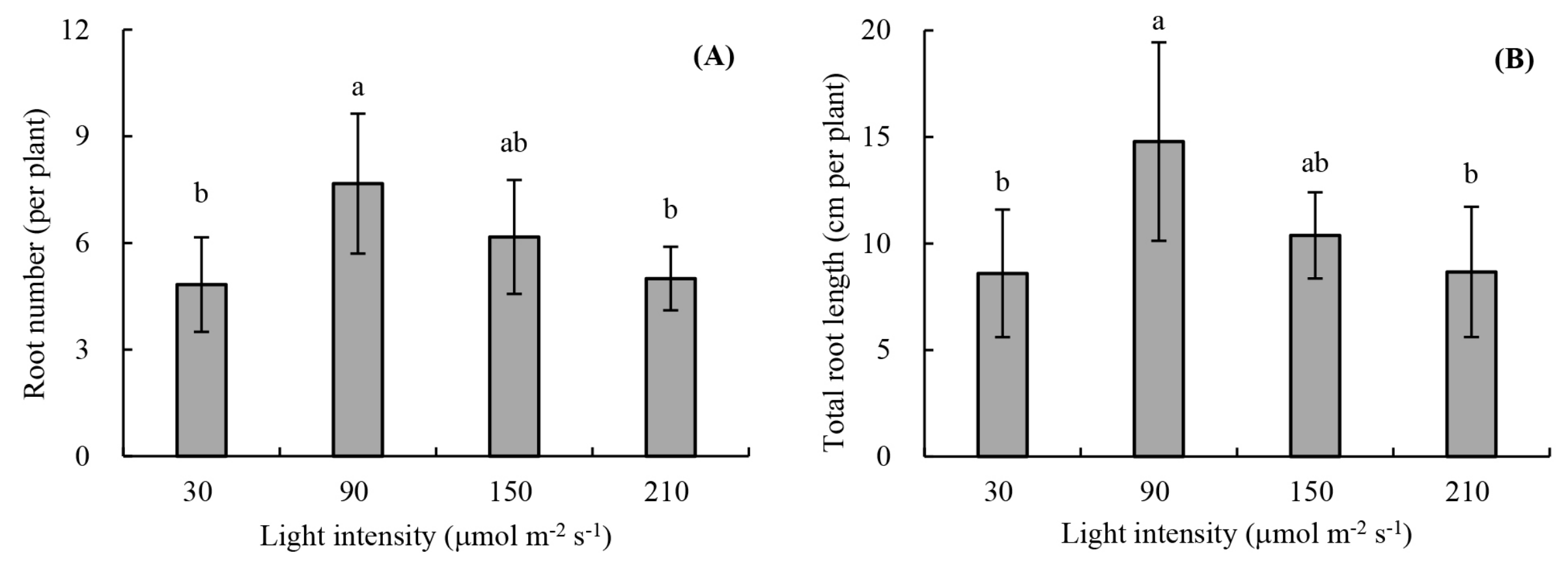
2.2.1. Root Development
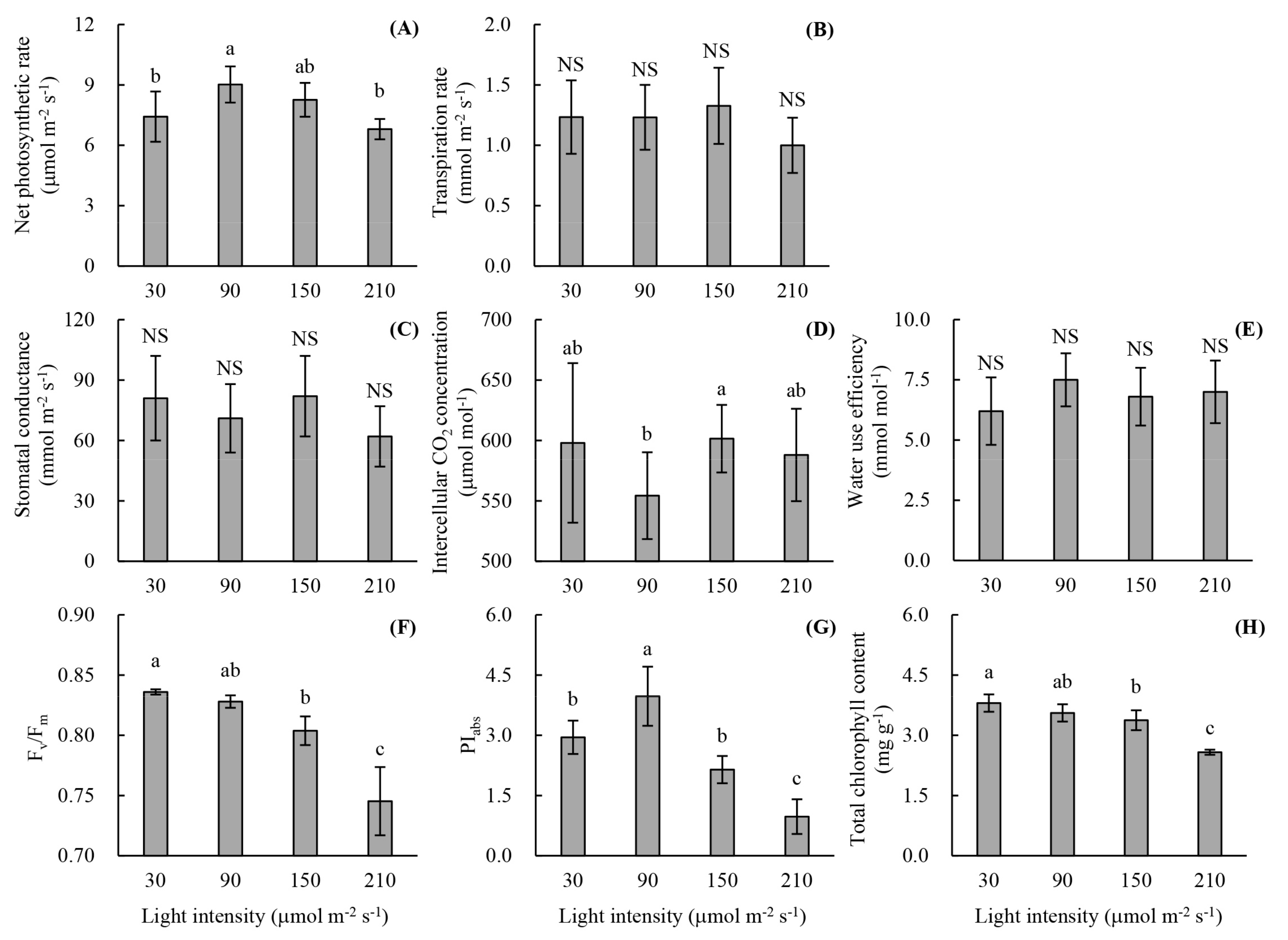
2.2.2. Photosynthetic Parameters
2.2.3. Chlorophyll Fluorescence Parameters
2.2.4. Chlorophyll Content
2.3. Effect of Light Intensity on Growth of Rooted Runner Plants at the Seedling Stage
2.3.1. Growth Characteristics
2.3.2. Photosynthetic Parameters, Chlorophyll Fluorescence Parameters, and Chlorophyll Content
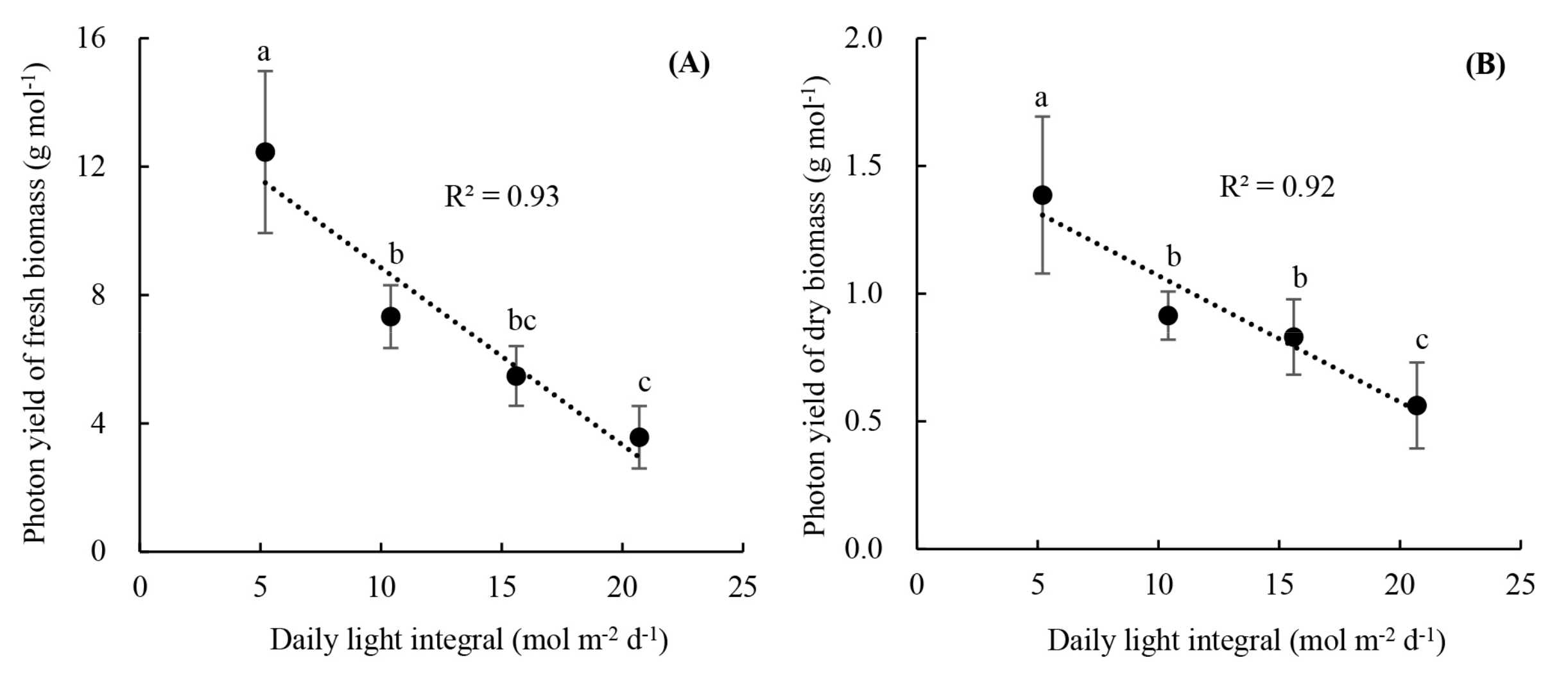
2.3.3. Photon Yields of Fresh and Dry Biomass
2.4. Statistical Analysis
3. Results and Discussion
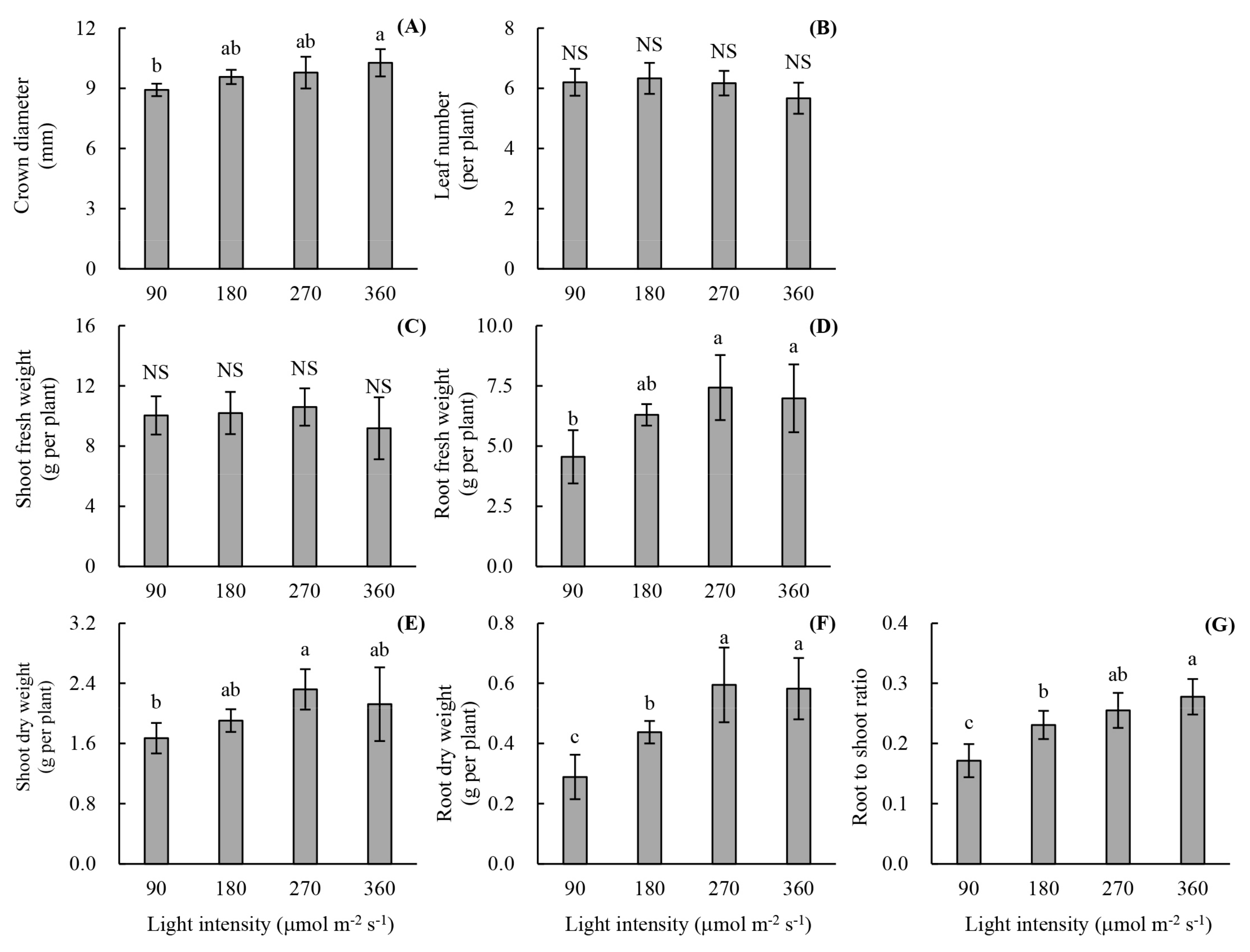
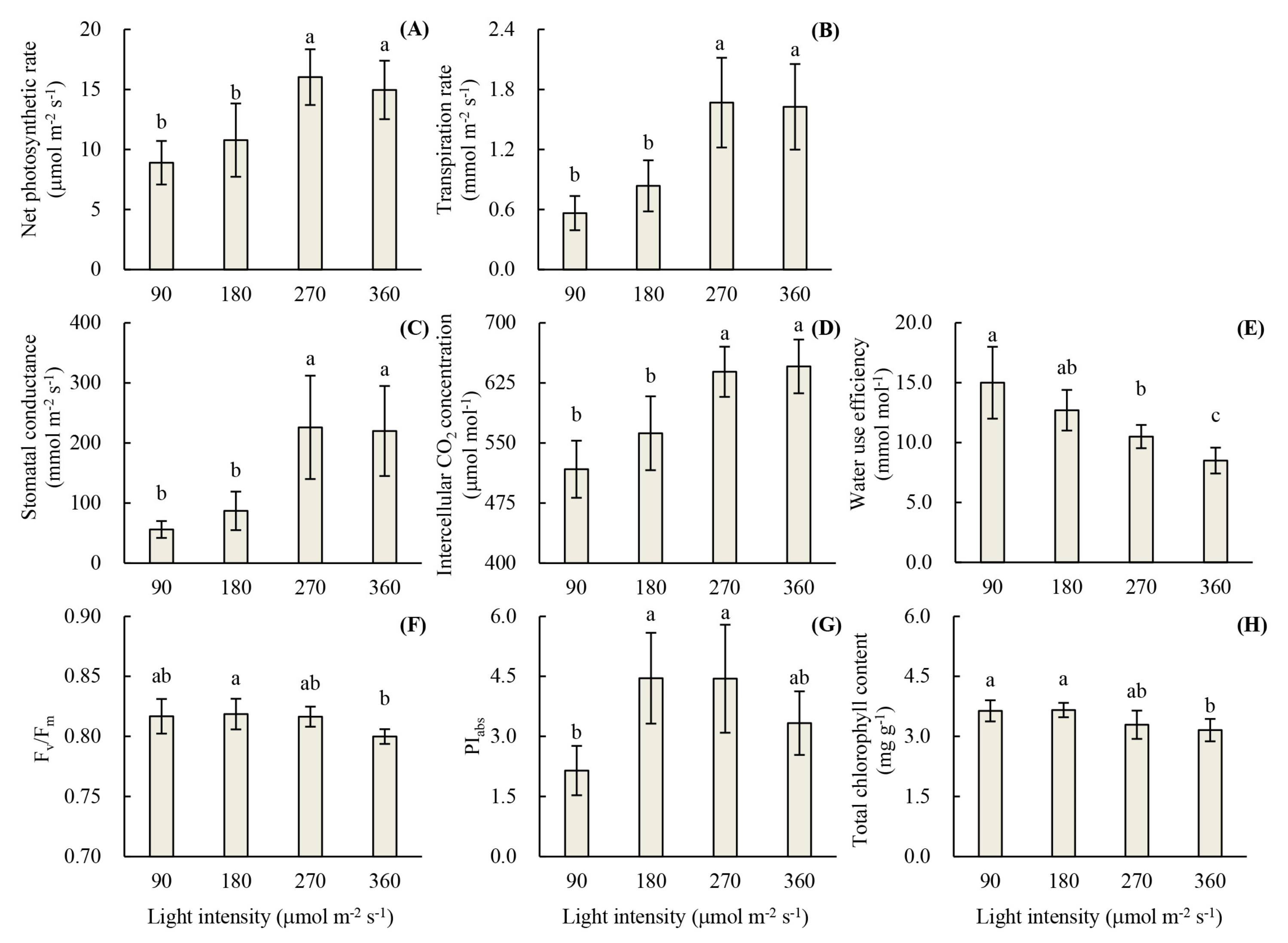
3.1. Root Development and Leaf Photosynthetic Activity of Unrooted Runner Plants as Affected by Light Intensity at the Rooting Stage
3.2. Plant Growth and Photon Yield of Biomass of Rooted Runner Plants as Affected by Light Intensity at the Seedling Stage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jun, H.J.; Jeon, E.H.; Kang, S.I.; Bae, G.H. Optimum nutrient solution strength for Korean strawberry cultivar ‘Daewang’ during seedling period. Korean J. Hortic. Sci. Technol. 2014, 32, 812–818. [Google Scholar] [CrossRef]
- Paulus, A.O. Fungal diseases of strawberry. HortScience 1990, 25, 885–889. [Google Scholar] [CrossRef]
- Xu, X.; Wedgwood, E.; Berrie, A.M.; Allen, J.; O’Neill, T.M. Management of raspberry and strawberry grey mould in open field and under protection. A review. Agron. Sustain. Dev. 2011, 32, 531–543. [Google Scholar] [CrossRef]
- Kim, S.K.; Jeong, M.S.; Park, S.W.; Kim, M.J.; Na, H.Y.; Chun, C. Improvement of runner plant production by increasing photosynthetic photon flux during strawberry transplant propagation in a closed transplant production system. Korean J. Hortic. Sci. Technol. 2010, 28, 535–539. [Google Scholar]
- Wu, C.C.; Hsu, S.T.; Chang, M.Y.; Fang, W. Effect of light environment on runner plant propagation of strawberry. Acta Hortic. 2011, 907, 297–302. [Google Scholar] [CrossRef]
- Park, S.W. Establishment of a Propagation System for Strawberry Using a Plant Factory with Artificial Lighting. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2018. [Google Scholar]
- Xu, X. Optimizing Environmental Parameters for Precision Indoor Propagation of Day-Neutral Strawberry. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2019. [Google Scholar]
- Durner, E.F.; Poling, E.B.; Maas, J.L. Recent advances in strawberry plug transplant technology. HortTechnology 2002, 12, 545–550. [Google Scholar] [CrossRef]
- Saito, Y.; Imagawa, M.; Yabe, K.; Bantog, N.; Yamada, K.; Yamaki, S. Stimulation of rooting by exposing cuttings of runner plants to low temperature to allow the raising of strawberry seedlings during summer. J. Jpn. Soc. Hortic. Sci. 2008, 77, 180–185. [Google Scholar] [CrossRef]
- He, D.X.; Zheng, J.F.; Du, W.F. A New Method for Harvesting Unrooted Runner Plantlets in Strawberry Plug Plant Production. China Patent 201810474655.0 2018. [Google Scholar]
- Savini, G.; Giorgi, V.; Scarano, E.; Neri, D. Strawberry plant relationship through the stolon. Physiol. Plant. 2008, 134, 421–429. [Google Scholar] [CrossRef]
- Tombesi, S.; Palliotti, A.; Poni, S.; Farinelli, D. Influence of light and shoot development stage on leaf photosynthesis and carbohydrate status during the adventitious root formation in cuttings of Corylus avellana L. Front. Plant. Sci. 2015, 6, 973–985. [Google Scholar] [CrossRef]
- Costa, J.; Challa, H. The effect of the original leaf area on growth of softwood cuttings and planting material of rose. Sci. Hortic. 2002, 95, 111–121. [Google Scholar] [CrossRef]
- Olschowski, S.; Geiger, E.M.; Herrmann, J.V.; Sander, G.; Grüneberg, H. Effects of red, blue, and white LED irradiation on root and shoot development of Calibrachoa cuttings in comparison to high pressure sodium lamps. Acta Hort. 2016, 1134, 245–250. [Google Scholar] [CrossRef]
- Kepenek, K. Photosynthetic effects of light-emitting diode (LED) on in vitro-derived strawberry (Fragaria × ananassa cv. Festival) plants under in vitro conditions. Erwerbs-Obstbau 2019, 61, 179–187. [Google Scholar] [CrossRef]
- Lovell, P.H.; Illsley, A.; Moore, K.G. The effects of light intensity and sucrose on root formation, photosynthetic ability, and senescence in detached cotyledons of Sinapis alba L. and Raphanus sativus L. Ann. Bot. 1972, 36, 123–134. [Google Scholar] [CrossRef]
- Loach, K.; Gay, A.P. The light requirement for propagating hardy ornamental species from leafy cuttings. Sci. Hortic. 1979, 10, 217–230. [Google Scholar] [CrossRef]
- Graper, D.F.; Healy, W. High pressure sodium irradiation and infrared radiation accelerate petunia seedling growth. J. Am. Soc. Hortic. Sci. 1991, 116, 35–438. [Google Scholar] [CrossRef]
- Pramuk, L.A.; Runkle, E.S. Photosynthetic daily light integral during the seedling stage influences subsequent growth and flowering of celosia, impatiens, salvia, tagetes, and viola. HortScience 2005, 40, 1336–1339. [Google Scholar] [CrossRef]
- Currey, C.J.; Hutchinson, V.A.; Lopez, R.G. Growth, morphology, and quality of rooted cuttings of several herbaceous annual bedding plants are influenced by photosynthetic daily light integral during root development. HortScience 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Biomass accumulation and allocation, photosynthesis, and carbohydrate status of new guinea impatiens, geranium, and petunia cuttings are affected by photosynthetic daily light integral during root development. J. Am. Soc. Hortic. Sci. 2015, 140, 542–549. [Google Scholar] [CrossRef]
- Park, S.W.; Kwack, Y.; Chun, C. Growth of runner plants grown in a plant factory as affected by light intensity and container volume. Hortic. Sci. Technol. 2017, 35, 439–445. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Hikosaka, S.; Goto, E.; Aoki, T. Effects of light conditions and air temperature on the growth of everbearing strawberry during the vegetative stage. Acta Hortic. 2009, 842, 817–820. [Google Scholar] [CrossRef]
- Yamazaki, K. Nutrient Solution Culture; Pak-kyo Co.: Tokyo, Japan, 1982; p. 41. [Google Scholar]
- Ye, Z.P. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Polley, H.W. Implications of atmospheric and climatic change for crop yield and water use efficiency. Crop Sci. 2002, 42, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Strasser, R.J.; Govindjee. Greening of peas: Parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts, phytophenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E.S. Daily light integral influences rooting and quality of petunia cuttings. Acta Hortic. 2006, 711, 369–374. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Na, Y.W.; Jeong, H.J.; Lee, S.Y.; Choi, H.G.; Kim, S.H.; Rho, I.R. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Strasser, R.J.; Krüger, G.H.J. Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol. Plant. 2004, 121, 239–249. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9–20. [Google Scholar] [CrossRef]
- Faust, J.E.; Holcombe, V.; Rajapakse, N.C.; Layne, D.R. The effect of daily light integral on bedding plant growth and flowering. HortScience 2005, 40, 645–649. [Google Scholar] [CrossRef]
- Dou, H.J.; Niu, G.H.; Gu, M.M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.H.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, E.M.F.M.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–104. [Google Scholar] [CrossRef]
- Tarila, A.G.I.; Ormrod, D.P.; Adedipe, N.O. Stomatal responses of the cowpea (Vigna unguiculata L.) to light intensity. Biochem. Physiol. Pflanzen 1978, 172, 541–545. [Google Scholar] [CrossRef]
- Chung, H.Y.; Chang, M.Y.; Wu, C.C.; Fang, W. Quantitative evaluation of electric light recipes for red leaf lettuce cultivation in plant factories. HortTechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Ji, F.; He, D.; Niu, G. Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory. Agronomy 2019, 9, 875. https://doi.org/10.3390/agronomy9120875
Zheng J, Ji F, He D, Niu G. Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory. Agronomy. 2019; 9(12):875. https://doi.org/10.3390/agronomy9120875
Chicago/Turabian StyleZheng, Jianfeng, Fang Ji, Dongxian He, and Genhua Niu. 2019. "Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory" Agronomy 9, no. 12: 875. https://doi.org/10.3390/agronomy9120875
APA StyleZheng, J., Ji, F., He, D., & Niu, G. (2019). Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory. Agronomy, 9(12), 875. https://doi.org/10.3390/agronomy9120875





