1. Introduction
Weed management programs relying primarily on a single herbicide with the same mechanism of action, promote the evolution of herbicide-resistant weeds [
1]. The continuous and widespread use of glyphosate, for example, since the introduction of glyphosate-resistant (GR) crops in the early ’90s has contributed greatly to the evolution of GR weeds [
2,
3,
4]. Glyphosate [
N-(phosphonomethyl)glycine] is a foliar-applied, non-selective, systemic herbicide that has been used extensively throughout the world over the past thirty years [
5]. It inhibits the biosynthesis of aromatic amino acids, i.e., phenylalanine, tryptophan and tyrosine, which in turn leads to plant metabolic disruptions through the inhibition of protein and secondary product biosynthesis [
6,
7].
Glyphosate, following the use of residual herbicides, enabled the exponential increase of no-till systems, which improve soil and water conservation in extensive agricultural production systems worldwide [
8]. In these systems, glyphosate is used to knock down vegetation during the fallow period or prior to crop planting and it is usually tank-mixed with a soil-active herbicide such as metsulfuron-methyl, atrazine or other preemergence herbicides for residual weed control.
The adoption of transgenic crops worldwide was reported at 185 million ha in 2016, of which more than 80% of the fields has been planted with herbicide-resistant crops; mostly cotton (
Gossypium hirsutum L.), maize (
Zea mays L.), soybean (
Glycine max (L.) Merr.) and canola (
Brassica spp.), almost all being GR [
9,
10]. USA, Argentina, Brazil, Canada, China and Paraguay are the countries that have the highest adoption of GR technology [
9,
10]. Despite the significant economic [
11] and environmental benefits to agriculture such as reductions in soil disturbance, environmental pollution and cost of food production [
12,
13] the overuse of glyphosate has exerted unprecedented selection pressure on weed populations, resulting in the evolution of GR weeds [
4]. Nevertheless, GR weeds are not yet a problem in many parts of the world, including Argentina where this research was conducted, and actions can be taken to achieve the economic, agronomic and environmental sustainability of the glyphosate in these areas [
3,
4].
Regarding glyphosate sustainable use one way that it can be achieved is by establishing criteria to improve the accuracy of how glyphosate efficacy is evaluated; usually through the development of dose-response curves that enable the determination of the optimum rate for individual weed species [
14,
15,
16]. Dose-response experiments are widely used to evaluate herbicide effectiveness [
14,
17,
18]. Values describing the growth response of a weed to an herbicide are often estimated from the dose-response curves, and these are used to compare differential susceptibility across weed populations or species. A reference value most commonly used is ED
50, the herbicide rate that reduces plant growth by 50%. Sandral et al. [
19] used log-logistic dose-response curves to estimate and compare ED
50 values of five herbicides applied to subterranean clover (
Trifolium subterraneum L.). Carlson and Burnside [
20] also compared ED
50 estimates to determine the relative sensitivity of several species to the application of four herbicides including glyphosate.
The effectiveness of an herbicide based on assessment of visible control, compared to a nontreated control, allows for a qualitative evaluation of herbicide efficacy [
21]. Nevertheless, little information is provided on potential weed fecundity associated with the herbicide treatment [
22]. The long-term impact of an herbicide program on weed population dynamics is related to the reduction in weed seed production potential associated with the herbicide treatments [
22]. Recurrent selection, especially with reduced herbicide rates, results in high risk of evolution of herbicide resistance [
23]. Most common weed species have high fecundity outputs coupled with viable and dormant seeds [
24] and the use of low herbicide rates could cause a fast buildup of the seedbank [
25].
Glyphosate and GR crops have significantly facilitated the improvement of weed management and production practices through higher efficacy and simplification of weed control [
26]. However, glyphosate is no longer broadly effective as it once was, hence the renewal of weed management approaches is required [
26]. Integrated weed management approaches, for example, are needed that will allow the continued use of glyphosate as a tool for weed management. Glyphosate use is valuable because abandoning glyphosate would reverse the progress that has been made toward practicing conservation agriculture. It is therefore imperative to develop means of preserving the remaining utility of glyphosate and to delay the development of herbicide resistance on new herbicide resistance technologies involving glufosinate, 2,-D and dicamba.
Glyphosate at the recommended rate is effective on the majority of broadleaf and grass weeds species when applied at 1–2 tillers for grass weeds or 5–7 cm for broadleaf species, but we hypothesized that a higher rate may be required to control plants at later growth stages depending on the weed species. Furthermore, including weed fecundity assessment is vital in evaluating the efficacy of herbicide treatments. It has been reported that herbicide rotation alone cannot prevent the development of herbicide resistant weeds [
4,
26]. Complementary measures such as fecundity-avoidance biomass threshold, i.e., the maximum allowable weed biomass for herbicide application in order to prevent weed seed production and dispersal, need to be included in the herbicide efficacy evaluation procedures and weed management programs.
Therefore, our objective, using glyphosate dose-response curves, was to re-assess the evaluation process of herbicide efficacy. In addition, based on these assessments, to recommend ways to optimize application rate margins based on key weed species, selected growth stages, and fecundity-avoidance biomass threshold. This approach could assist in preserving the effectiveness of successful herbicides such as glyphosate on sensitive weed species, an issue that is still of great importance. In addition, this approach can be applied on automated weed control and robotics technology for targeted rate-species specific applications.
2. Materials and Methods
2.1. Experimental Set-Up
Seeds of
Anoda cristata, (L.) Schltdl. (crested anoda),
Chenopodium album L. (lambsquarters),
Digitaria sanguinalis (L.) Scop. (large crabgrass)
, Eleusine indica (L.) Gaerth (goosegrass) and
Portulaca oleracea (L.; purslane) were collected from local weed populations from GR soybean fields nearby university experimental station, located in Zavalla, Pampa, Argentina (33°1′ S, 60°53′ W). Based on previous surveys at 5000 points-quadrats in more than 300 fields these species were the most frequent [
27,
28]. Seed samples before the initiation of the experiments were stored in sealed vials at 5 °C and were sown in a 3 L plastic pot containing a commercial potting mix. Once established, weed seedlings were thinned to three per pot and were grown outdoors under a temperature range of 18–32 °C. The plants were watered as needed.
2.2. Herbicide Application
Glyphosate as isopropylamine salt (600 g ae L
−1) was applied at 0, 0.24, 0.48, 0.96, 1.92 and 3.84 kg ae ha
−1; with each rate logarithmically increased from a rate that caused no observable injury to a rate that caused severe damage or death as preliminary data had indicated (results not shown). Glyphosate was applied using a RDS knap-sack sprayer equipped with a hand-held boom with four TeeJet 8002 flat fan nozzles (TeeJet Technologies, Springfield, IL, USA) calibrated to deliver 140 L ha
−1 at an operating pressure of 30 kPa at three weed growth stages (
Table 1a,b). Average air temperature during herbicide application was 18.1 (±0.4 °C), 21.0 °C (±1.5 °C), 20.7 °C (±0.1 °C) and 19.0 °C (±1.8 °C) and relative humidity was 78.1% (±8.3%), 86.7% (±5.0%), 85.4% (±0.5%) and 86.4% (±6.2%) for the four trials, respectively. Wind speed was less than 1 km h
−1 or 0 force in Beaufort wind scale.
2.3. Experimental Design and Data Collection
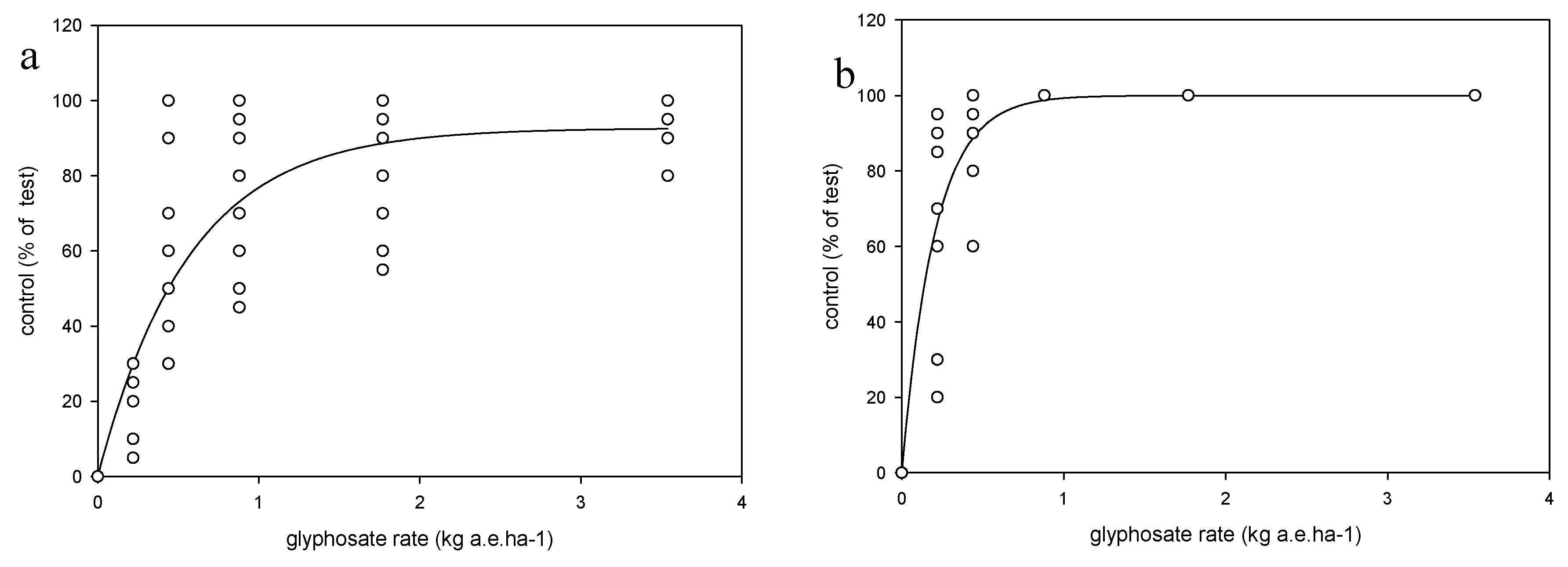
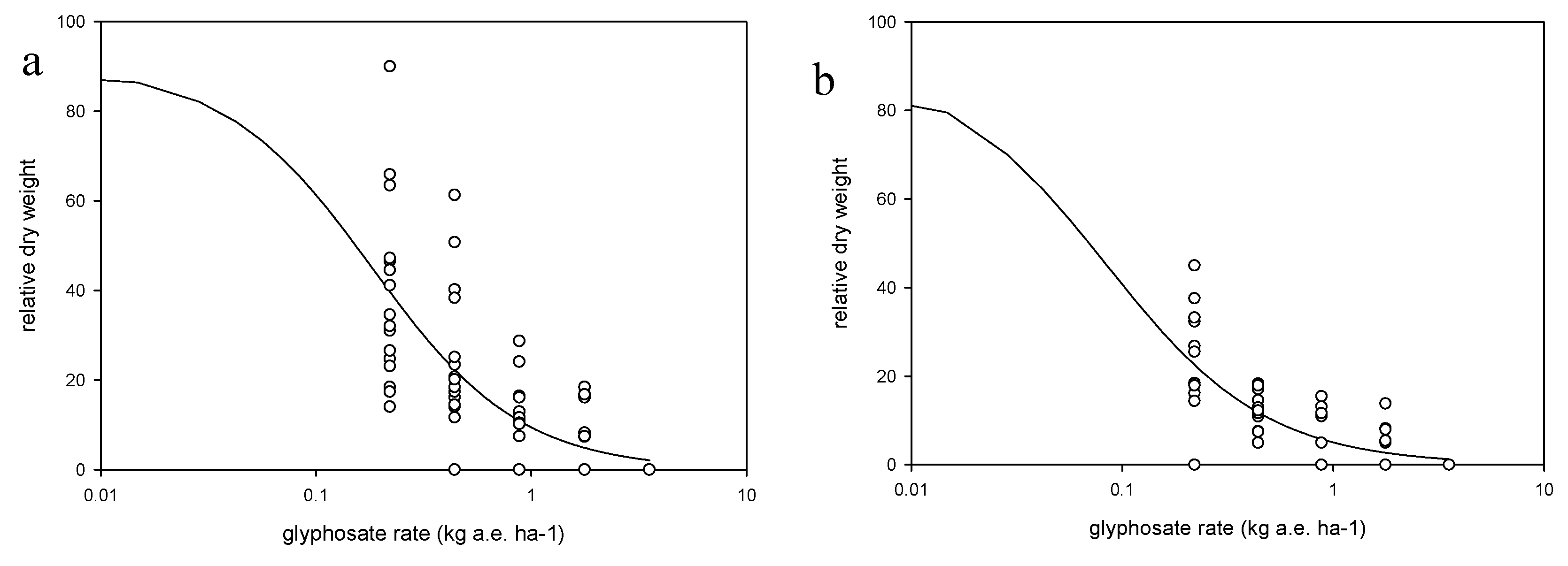
Each weed species was treated as an independent trial in a two-factor factorial design with the factors being the growth stage (i.e., three growth stages were defined for herbicide application) and application rate, in a randomized complete block design with four replications. Each species/trial was conducted four times (runs) across two growing seasons (a and b). Runs 1a and 2a were conducted in late spring, whereas runs 1b and 2b in late summer. The shoot tissue of two plants per pot were harvested 14 days after treatment (DAT), after the evaluation of weed control, which was conducted using a 0–100 scale. The shoot tissues were oven-dried at 110 °C for 72 h. and weighed. Weed response to glyphosate was expressed as dry weight relative to the nontreated plants and as % control relative to the nontreated plants.
The third plant was left in the pot until maturity, usually three to four weeks after treatment. At maturity, the plants were harvested, dried, weighed and threshed manually for seed collection. Seeds were separated from debris using meshes of various size-openings and/or a blower. The number of seeds per plant for A. cristata was determined by counting all the cleaned seeds. The total seed production per plant of P. oleracea was estimated using a linear regression model generated with the data for seed number and seed weight of four subsamples containing 10, 50, 100 and 150 seeds each. For D. sanguinalis and E. indica, the total seed production per plant was estimated by counting the seeds from 10 randomly selected spikes of each plant. Seed number per plant was expressed as % of the nontreated control.
2.4. Models Used and Data Analysis
Exponential models (Equation (1)) for each species were fitted to the weed control ratings at 14 DAT:
where
y = percentage control rating;
x = application rate (kg ae ha
−1) and
a and
b = model parameters.
To quantify the response of weed species to glyphosate application rate a log-logistic model was fitted to the dry shoot biomass data (Equation (2)).
where
Y = shoot biomass (g plant
−1);
x = rate of glyphosate application (kg ae ha
−1);
D = the upper limit of the curve asymptote;
b = the rate of change (slope) near the inflexion point denoted by ED
50. The model was reduced from the original model proposed by Streibig and Kudsk [
14] because the lower limit of the logistic function was zero (the original model involved a parameter
C determining the lower boundary of the logistic curve). This is a reasonable assumption, as postulated by Madsen and Jensen [
29], as plants were killed at the highest dose.
For practical weed control, the ED
90 value is more relevant than the ED
50. Therefore, a logistic model can be reparametrized to calculate the ED
90 value (Equation (3))
where
Y = shoot biomass (g plant
−1);
x = glyphosate application rate (kg ae ha
−1);
D = upper limit of the curve asymptote and
b = rate of change (slope) near the inflexion point denoted by ED
90.
The number of seeds per plant was linearly related to seed dry weight in P. oleracea as described in Equation (4) which was used to estimate fecundity.
Linear models were fitted to relative seed number as follows:
where
x is shoot dry weight,
a and
b constants, i.e., slope and seed output increase per unit of plant biomass increase.
The following equation for the estimation of seed production for
C. album was used [
30]:
where
y = estimated seed production and
x = biomass production.
Arcsin transformations were used (e.g., when dependent variable was a proportion or percentage) and transformed data were then used to fit the models described above based on least squares techniques, using SPSS, Statgraphics and SigmaPlot software. Test for constant variance and residuals means square error (RMSE) were carefully observed in order to determine quality of regressions fit to data. Analysis of variance (ANOVA) between experimental runs for the weed control, biomass and ED90 parameters, described in equations 1, 2 and 3, revealed no significant differences (p > 0.05) among the experimental runs, hence the data were pooled, and models fitted to pooled data. For seed production the data were not pooled, so each species × run was analyzed separately. Analyses of variance also were performed on each species for parameters a and b (Equation (1)) and for parameters D, b and ED50 (Equation (2)), and ED90 (Equation (3)) to test differences among experimental runs at p > 0.05.
4. Conclusions and Implications
Under field conditions many interlinked factors affect the efficacy of herbicides (Doll et al. [
52]) and some situations necessitate a full rate. The best agronomic practices (e.g., reducing row spacing, planting fast LAI-development varieties and optimum plant population) are necessary to improve the performance of low herbicide rates. Dose-response parameters are highly sensitive to changes in management and development of the crop weed canopy [
41]. Crop rotation, in combination with reduced tillage, significantly decreases the seed production of grasses and broadleaved weeds regardless of the level of management input applied [
53]. Further, weed species emerged simultaneously in the field; thus, the field rate should be targeted to the most tolerant species. Nevertheless, our findings emphasized that rates were weed- and size-specific and for that matter, effective control of tolerant species was most particularly dependent on weed size. Thus, weed size should be reported precisely, as proposed by Lancashire et al. [
54] and should be linked with fecundity-avoidance biomass threshold for improving evaluations on herbicide efficacy, hence weed management programs. Improving product labeling concerning the precision of herbicide recommendation rates could improve the evaluation of herbicide efficacy. Discrepancies in the glyphosate rates needed to achieve a certain control level and have reported in the literature may be due to considerable variation in weed sizes. Recent research has shown that digitized photographs of very detailed sizes, for example, are a useful tool to overcome this difficulty. In addition, models that predict patterns of weed emergence, coupled with size estimation [
55], are of most importance to refine herbicide rate recommendations for different cropping systems. The development and further refinement of such information is of major importance towards a justified use of herbicides. Future robotic weed control systems will be able to collect data about the presence of weeds, facilitate storage and analysis of those data, support decision making about when and where to control weeds by using image recognition software, execute weed control with variable rate spray technologies, as an improvement of “see and spray” or “spot and spray” technologies, and then gather data about the efficacy of the treatment and thus allow evaluation of decisions [
56].