Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea americana Mill.) Trees
Abstract
1. Introduction
2. Materials and Methods
Data Analyses
3. Results
3.1. Plant Dry Weight and Nutrient Content
3.2. Plant Water Status and Net CO2 Assimilation
3.3. Principal Components Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreyra, R.; Maldonado, P.; Celedón, J.; Gil, P.M.; Torres, A.; Sellés, G. Soil air content effects on the water status of avocado trees. Acta Hort. 2008, 72, 291–296. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Maldonado, P.; Celedón, J.; Gil, P.M. Efecto del clima, de las características de la hoja y de la metodología de medición en el potencial hídrico xilemático en palto (Persea americana Mill.). Agricultura Técnica 2007, 67, 182–188. [Google Scholar] [CrossRef]
- CIREN. Descripciones de Suelos; Estudio Agrologico V Región: Santiago, Chile, 2009; Centro de Información de Recursos naturales, Publicación Ciren N° 116, Propiedad Intelectual N° 101.789; p. 279. [Google Scholar]
- Gil, P.M.; Bonomelli, C.; Schaffer, B.; Ferreyra, R.; Gentina, C. Effect of soil water-to-air ratio on biomass and mineral nutrition of avocado trees. J. Soil Sci. Plant Nutr. 2012, 12, 609–630. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Press: Norwell, MA, USA, 2002. [Google Scholar]
- Bonomelli, C.; Ruiz, R. Effects of foliar and soil Calcium application on yield and quality in table grape cv. Thompson seedless. J. Plant Nutr. 2010, 33, 299–314. [Google Scholar] [CrossRef]
- Martínez, J.P.; Muena, V.; Ruiz, R. Nutrición y Fertilidad en Paltos; Boletín INIA N° 283; Instituto de Investigaciones Agropecuarias: La Cruz, Chile, 2014; p. 74. [Google Scholar]
- Schaffer, B.; Andersen, P.C.; Ploetz, R.C. Responses of fruit crops to flooding. In Hort Reviews; Janick, J., Ed.; AVI Publishing Co., Inc.: Westport, CT, USA, 1992; pp. 257–313. [Google Scholar]
- Gil, P.M.; Schaffer, B.; Gutierrez, M.; Li, C. Effect of Waterlogging on plant water status, leaf gas exchange and biomass of avocado (Persea americana Mill.). In Proceedings of the VI International Avocado Congress, Viña del Mar, Chile, 12–16 November 2007; Section 3C, paper 110. p. 10. [Google Scholar]
- Sanclemente, M.A.; Schaffer, B.; Gil, P.M. Pruning after flooding hastens recovery of flood-stressed avocado (Persea americana Mill.) trees. Sci. Hortic. 2014, 169, 27–35. [Google Scholar] [CrossRef]
- Doupis, G.; Kavroulakis, N.; Psarras, G.; Papadakis, I.E. Growth, photosynthetic performance and antioxidative response of ‘Hass’ and ‘Fuerte’ avocado (Persea americana Mill.) plants grown under high soil moisture. Phtosynthetica 2017, 55, 655–663. [Google Scholar] [CrossRef]
- Valoras, N.; Letey, J.; Stolzy, L.H.; Frolich, F. The oxygen requirements for root growth of three avocado varieties. Amer. Soc. Hort. Sci. 1964, 85, 172–178. [Google Scholar]
- Tzatzani, T.T.; Kavroulakis, N.; Doupis, G.; Psarras, G.; Papadakis, I.E. Nutritional status of ‘Hass’ and ‘Fuerte’ avocado (Persea americana Mill.) plants subjected to high soil moisture. J. Plant Nutr. 2019. [Google Scholar] [CrossRef]
- Atwell, B.J. Response of roots to mechanical impedance. Environ. Exper. Bot. 1993, 33, 27–40. [Google Scholar] [CrossRef]
- Kepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hort. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- De Freitas, T.; Mitcham, E.J. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- Voogt, W. The growth of beefsteak tomato as affected bay K/Ca ratios in the nutrient solution. Acta Hortic. 1987, 22, 155–165. [Google Scholar]
- Nguyen, H.; Maneepong, S.; Suraninpong, P. Effects of potassium, calcium and magnesium ratios in soil on their uptake and fruit quality of pummelo. J. Agric. Sci. 2017, 9, 110–121. [Google Scholar] [CrossRef]
- Kopsell, D.E.; Kopsell, D.A.; Sams, C.E.; Barickman, T.C. Ratio of calcium to magnesium influences biomass elemental accumulations and pigment concentrations in kale. J. Plant Nutr. 2013, 36, 2154–2165. [Google Scholar] [CrossRef]
- Rato, A.E.; Agulheiro, A.C.; Barroso, J.M.; Riquelme, F. Effect of different calcium fruit content in physical and chemical properties of european plum. J. Plant Nutr. 2010, 33, 301–404. [Google Scholar] [CrossRef]
- Silva, H.; Rodríguez, J. Fertilización de Plantas Frutales; Pontificia Universidad Católica de Chile, Facultad de Agronomía: Santiago, Chile, 1995; p. 519. [Google Scholar]
- Salazar-García, S. Nutrición del Aguacate: Principios y Aplicaciones; Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias: Santiago Ixcuintla, Mexico, 2002; p. 165. [Google Scholar]
- Privé, J.P.; Janes, D. Evaluation of plant and soil moisture sensors for the detection of drought stress in raspberry. Acta Hortic. 2003, 618, 391–396. [Google Scholar] [CrossRef]
- Raviv, M.J.; Lieth, J.H.; Burger, D.W.; Wallach, R. Optimization of transpiration and potential growth rates of ‘Kardinal’ rose with respect to root-zone physical properties. J. Am. Soc. Hort. Sci. 2001, 126, 638–643. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 2012. [Google Scholar]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Martiz, J. Salt Stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 2018, 8, 64. [Google Scholar]
- Clark, L.J.; Whalley, W.R.; Barraclough, P.B. How do roots penetrate strong soil? Plant Soil 2003, 255, 93–104. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, S. Citrus decline: Soil fertility and plant nutrition. J. Plant Nutr. 2009, 32, 197–245. [Google Scholar] [CrossRef]
- Oliveira, M.W.; Trivelin, P.C.O.; Boaretto, A.E.; Muraoka, T.; Mortatti, J. Leaching of nitrogen, potassium, calcium and magnesium in a sandy soil cultivated with sugarcane. Pesq. Agropec. Bras. 2002, 37, 861–868. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Sgariboldi, T.; Arroyo-García, R.; Calonego, J.C. Potassium leaching as affected by soil texture and residual fertilization in tropical soils. Commun. Soil Sci. Plant Anal. 2010, 41, 1934–1943. [Google Scholar] [CrossRef]
- Zhao, C.X.; Jia, L.H.; Wang, I.F.; Wang, M.L.; Mcgiffen, M.E. Effects of different soil texture on peanut growth and development. Commun. Soil Sci. Plant Anal. 2015, 46, 2249–2257. [Google Scholar] [CrossRef]
- Storey, R.; Jones, G.; Schachtman, P.; Treeby, M. Calcium-accumulating cells in the meristematic region of grapevine root apices. Funct. Plant Biol. 2003, 30, 719–727. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; Soltis, P.S.; Wolstenholme, B.N. Taxonomy and botany. In The Avocado: Botany, Production and Uses, 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 168–199. [Google Scholar]
- Lauchli, A. Translocation or inorganic solutes. Annu. Rtv. Plant Physiol. 1972, 3, 197–218. [Google Scholar] [CrossRef]
- Malazian, G. Histoautoradiographic Studies of Calcium Uptake and Transport across Grape (Vitis vinifera L.) Root Cortex in Relation to Transpiration; University of California, Davis: Davis, CA, USA, 1965. [Google Scholar]
- Huang, J.W.; Grunes, J.E.; Kochian, D.I. Alumminum effects on the kinetics of calcium uptake into cells of the wheat roots apex. Planta 1992, 188, 414–421. [Google Scholar] [CrossRef]
- Chino, M. Species differences in calcium and potassium within plant roots. Soil Sci. Plant Nutr. 1981, 27, 487–503. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.; Xu, B.; Conn, S.; Kaiser, N.; Leigh, R.; Tyerman, S. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Ruiz, L.P.; Mansfield, T.A. Calcium in xylem sap and the regulation of its delivery to the shoot. J. Exp. Bot. 1992, 43, 1315–1324. [Google Scholar] [CrossRef]
- Bell, C.W.; Biddulph, O. Translocation of calcium. Exchange versus mass flow. Plant Physiol. 1963, 38, 610–614. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; p. 672. [Google Scholar]
- Kass, C.L. Fertilidad de Suelos; Euned: San José, Costa Rica, 1998; p. 233. [Google Scholar]
- Bonomelli, C.; Artacho, P. Nitrogen application to non-bearing “Bing” sweet cherry trees on Gisela®6 rootstock: Effects on accumulation and partitioning of biomass and nitrogen. Sci. Hort. 2013, 162, 293–304. [Google Scholar] [CrossRef]

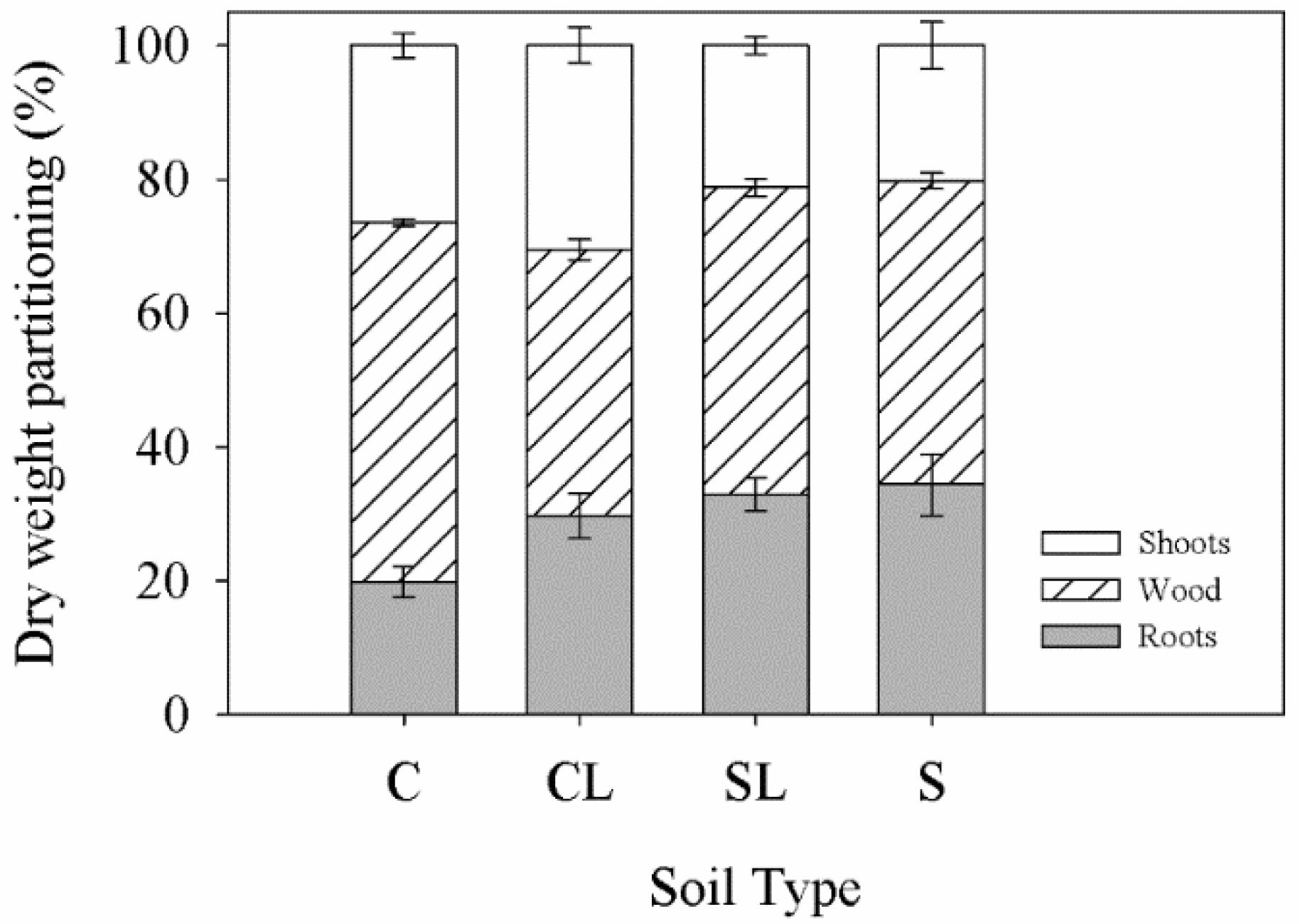
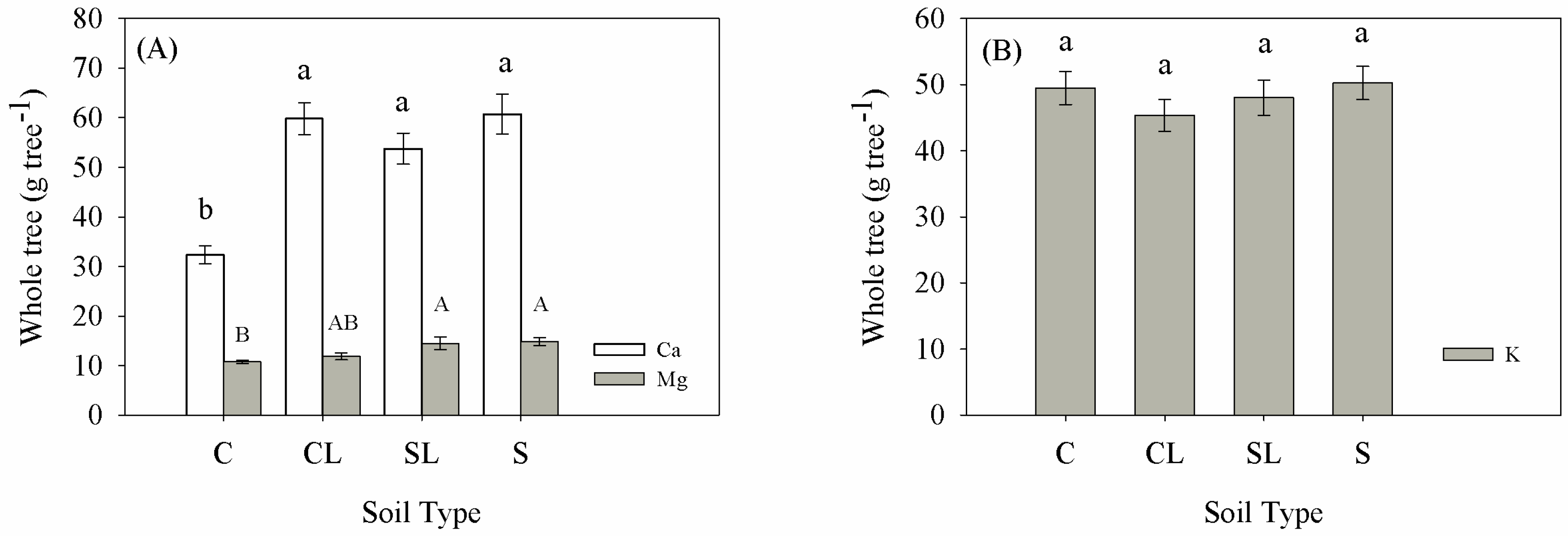
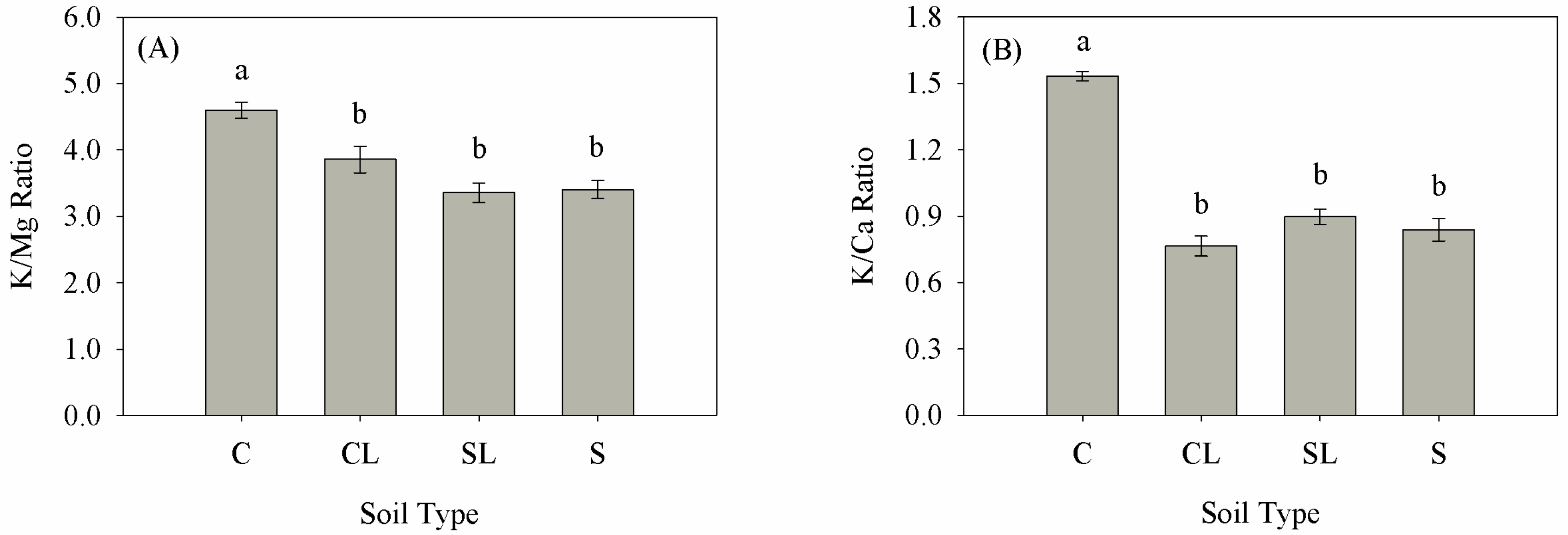
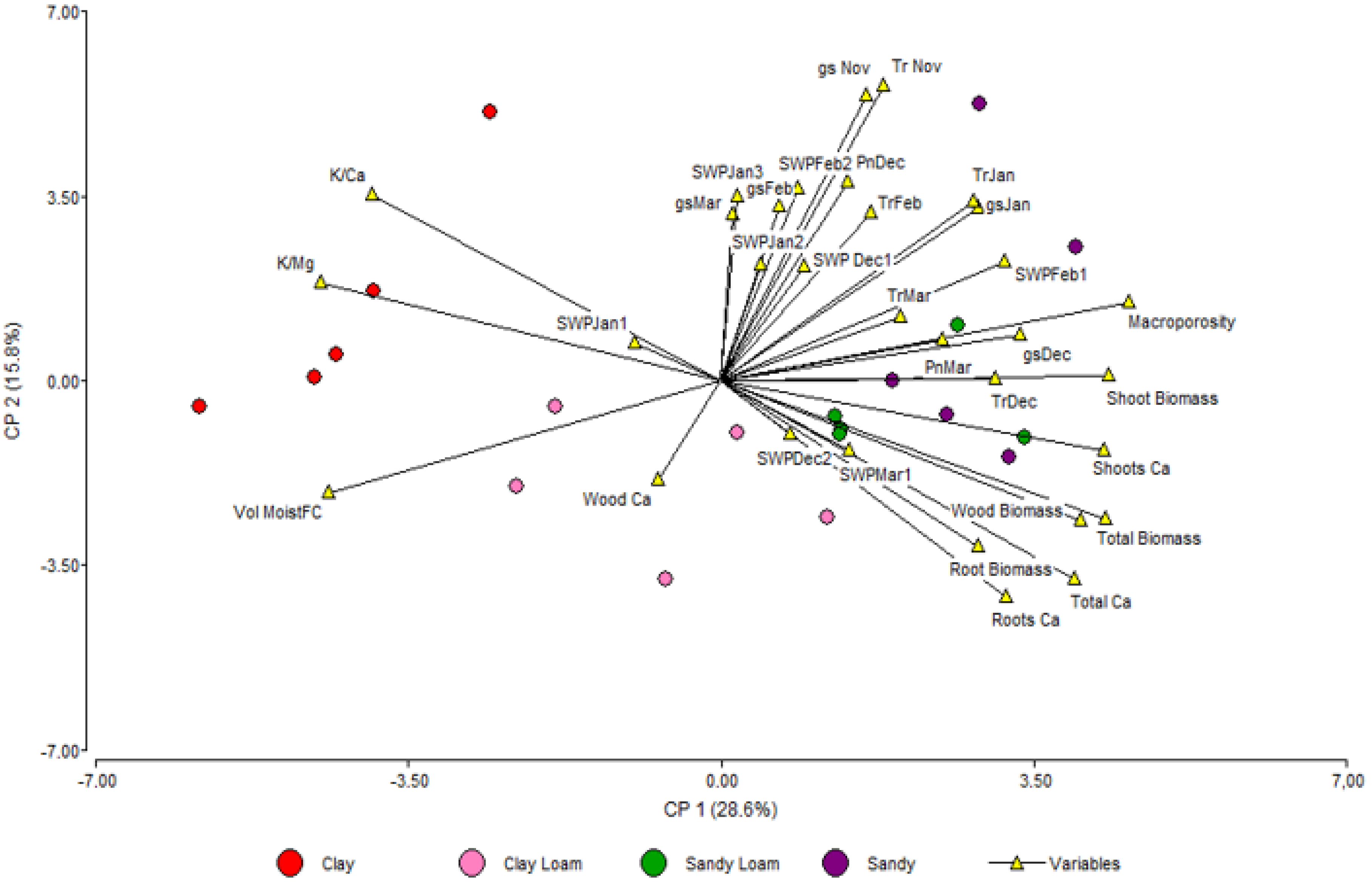
| Treatment | Clay | BD | P | MP | pH | OM | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | g cm−3 | % | % | % | mg kg−1 | ||||||
| C | 39.5 | 1.43 | 46.0 | 17.4 | 6.1 | 1.1 | 78 | 61 | 291 | 2020 | 172 |
| CL | 34.1 | 1.14 | 57.0 | 33.2 | 7.1 | 4.2 | 84 | 64 | 394 | 2160 | 194 |
| SL | 10.1 | 1.45 | 45.3 | 34.8 | 6.1 | 0.6 | 39 | 39 | 171 | 1320 | 145 |
| S | 7.5 | 1.38 | 47.9 | 31.4 | 6.6 | 0.3 | 55 | 30 | 183 | 1020 | 122 |
| Nutrient Content (g) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Treatment | Shoot | Wood | Root | |||||||||
| K | C | 14.5 | ± | 1.0 | b | 29.3 | ± | 2.5 | a | 5.7 | ± | 0.9 | ab |
| CL | 24.2 | ± | 1.6 | a | 15.7 | ± | 0.8 | b | 5.5 | ± | 0.9 | b | |
| SL | 23.6 | ± | 0.9 | a | 13.5 | ± | 1.0 | b | 10.9 | ± | 1.8 | ab | |
| S | 24.3 | ± | 1.2 | a | 14.0 | ± | 0.6 | b | 11.9 | ± | 2.2 | a | |
| Ca | C | 13.5 | ± | 0.6 | b | 10.5 | ± | 1.0 | a | 8.3 | ± | 1.2 | b |
| CL | 23.2 | ± | 2.3 | a | 10.5 | ± | 0.5 | a | 26.1 | ± | 3.6 | a | |
| SL | 22.1 | ± | 0.9 | a | 10.0 | ± | 1.3 | a | 21.7 | ± | 3.1 | a | |
| S | 25.6 | ± | 0.9 | a | 9.9 | ± | 0.6 | a | 25.1 | ± | 3.9 | a | |
| Mg | C | 4.1 | ± | 0.2 | c | 4.1 | ± | 0.2 | a | 2.5 | ± | 0.3 | b |
| CL | 4.6 | ± | 0.4 | bc | 2.4 | ± | 0.2 | b | 4.8 | ± | 0.6 | ab | |
| SL | 5.8 | ± | 0.2 | ab | 2.7 | ± | 0.2 | b | 6.0 | ± | 1.2 | a | |
| S | 6.2 | ± | 0.4 | a | 2.7 | ± | 0.1 | b | 6.0 | ± | 0.9 | a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomelli, C.; Gil, P.M.; Schaffer, B. Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea americana Mill.) Trees. Agronomy 2019, 9, 837. https://doi.org/10.3390/agronomy9120837
Bonomelli C, Gil PM, Schaffer B. Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea americana Mill.) Trees. Agronomy. 2019; 9(12):837. https://doi.org/10.3390/agronomy9120837
Chicago/Turabian StyleBonomelli, Claudia, Pilar M. Gil, and Bruce Schaffer. 2019. "Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea americana Mill.) Trees" Agronomy 9, no. 12: 837. https://doi.org/10.3390/agronomy9120837
APA StyleBonomelli, C., Gil, P. M., & Schaffer, B. (2019). Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea americana Mill.) Trees. Agronomy, 9(12), 837. https://doi.org/10.3390/agronomy9120837






