Variability in Nutraceutical Lipid Content of Selected Rice (Oryza sativa L. spp. indica) Germplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Rice Germplasm Description and Experimental Design
2.3. Color Parameters
2.4. Analysis of α-tocopherol and γ-oryzanol
2.5. Analysis of Campesterol, β-sitosterol, Octacosanol, and Squalene
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Variance
3.2. Multivariate Analysis
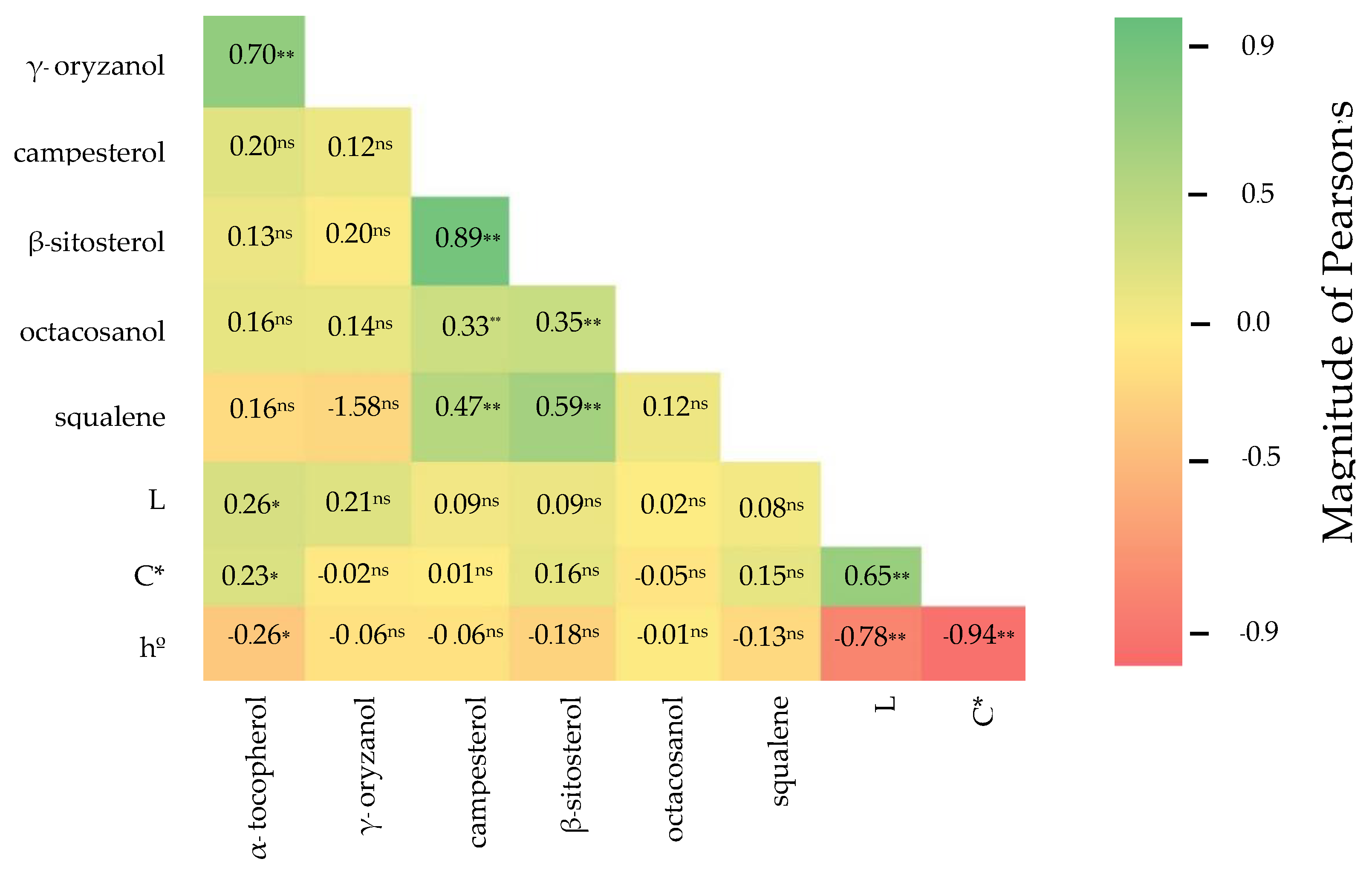
3.3. Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeates, K.; Lohfeld, L.; Sleeth, J.; Morales, F.; Rajkotia, J.; Ogedegbe, O. A global perspective on cardiovascular disease in vulnerable populations. Can. J. Cardiol. 2015, 31, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.B.; Shanmugavelan, P.; Kim, S.N.; Cho, Y.S.; Kim, H.R.; Lee, J.T.; Jeon, W.T.; Lee, D.J. Evaluation of Î3-oryzanol content and composition from the grains of pigmented rice-germplasms by LC-DAD-ESI/MS. BMC Res. Notes 2013, 6, 149. [Google Scholar] [CrossRef]
- Kim, N.H.; Kwak, J.; Bail, J.Y.; Yoon, M.R.; Lee, J.S.; Yoon, S.W.; Kim, I.H. Changes in lipid substances in rice during grain development. Phytochemistry 2015, 116, 170–179. [Google Scholar] [CrossRef]
- Pitija, K.; Nakornriab, M.; Sriseadka, T.; Vanavichit, A.; Wongpornchai, S. Anthocyanin content and antioxidant capacity in bran extracts of some Thai black rice varieties. Int. J. Food Sci. Technol. 2013, 48, 300–308. [Google Scholar] [CrossRef]
- Sompong, R.; Siebeinhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Zubair, M.; Anwar, F.; Ashraff, M.; Uddin, M.K. Characterization of high-value bioactives in some selected varieties of Pakistani rice (Oryza sativa L.). Int. J. Mol. Sci. 2012, 13, 4608–4622. [Google Scholar] [CrossRef]
- Chotimarkorn, C.; Benjakul, S.; Silalai, N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008, 111, 636–641. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Factors influencing antioxidant compounds in rice. Crit. Rev. Food Sci. Nutr. 2017, 57, 893–922. [Google Scholar] [CrossRef]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Nagendra Prasad, M.N.; Sanjay, K.R.; Shravya Khatokar, M.; Vismaya, M.N.; Nanjunda Swamy, S. Health benefits of rice bran-A review. J. Nutr. Food Sci. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Taylor, J.C.; Rapport, L.; Lockwood, G.B. Octacosanol in human health. Nutrition 2003, 19, 192–195. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, J.; Pyo, Y.G.; Oh, S.W.; Lee, J.S.; Kim, I.H. Nutraceutical lipid substances in Korean rice cultivars. J. Food Nutr. Res. 2014, 2, 40–46. [Google Scholar]
- Nurmi, T.; Lampi, A.M.; Nystro, L.; Piironen, V. Effects of environment and genotype on phytosterols in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9814–9823. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Engel, K.H. Content of γ-oryzanol and composition of steryl ferulates in brown rice (Oryza sativa L.) of European origin. J. Agric. Food Chem. 2006, 54, 8127–8133. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Ng, L.T. Quantification of tocopherols, tocotrienols, and γ-oryzanol contents and their distribution in some commercial rice varieties in Taiwan. J. Agric. Food Chem. 2011, 59, 11150–11159. [Google Scholar] [CrossRef]
- Goffman, F.D.; Pinson, S.; Bergman, C. Genetic diversity for lipid content and fatty acid profile in rice bran. J. Am. Oil Chem. Soc. 2003, 80, 485–490. [Google Scholar] [CrossRef]
- Moonsap, P.; LaksanavilatSittipun, N.; Tasanasuwan, S.; Kate-Ngam, S.; Jantasuriyarat, C. Genetic diversity of Indo-China rice varieties using ISSR, SRAP and InDel markers. J. Genet. 2019, 98, 80. [Google Scholar] [CrossRef]
- Pasudee, T.; Jamjod, S.; Chiang, Y.C.; Rerkasem, B.; Schaal, B.A. Genetic structure and isolation by distance in a landrace of Thai rice. Proc. Natl. Acad. Sci. USA 2009, 106, 13880–13885. [Google Scholar] [CrossRef]
- Rerkasem, B.; Rerkasem, K. Agrodiversity for in situ conservation of Thailand’s native rice germplasm. CMU J. 2002, 1, 129–148. [Google Scholar]
- Butsat, S.; Siriramornpun, S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 217, 630–638. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Tangkhawanit, E.; Kaewseejan, N. Reducing retrogradation and lipid oxidation of normal and glutinous rice flours by adding mango peel powder. Food Chem. 2016, 201, 160–167. [Google Scholar] [CrossRef]
- Renuka, N.; Mathure, S.V.; Thengaue, R.J.; Nadaf, A.B. Determination of some minerals and β-carotene contents in aromatic indica rice (Oryza sativa L.) germplasm. Food Chem. 2016, 191, 2–6. [Google Scholar] [CrossRef]
- Berman, C.J.; Xu, Z. Genotype and environment effects on tocopherol, tocotrienol, and γ-oryzanol contents of Southern U.S. rice. Cereal Chem. 2003, 80, 446–449. [Google Scholar] [CrossRef]
- Ali, A.; Sadaqat, H.A.; Kashif, M.; Wahid, M.A. Exploration of breeding potential for genetic biofortification in spring wheat. Pak. J. Agric. Sci. 2018, 55, 793–799. [Google Scholar]
- Maione, C.; Berbosa, R.M. Recent applications of multivariate data analysis methods in the authentication of rice and the most analyzed parameters: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1868–1879. [Google Scholar] [CrossRef]
- Sanni, K.A.; Fawole, S.A.; Ogunbayp, D.D.; Tia, E.A.; Somado, K.; Futakuchi, M.S.; Nwilene, F.E.; Guei, R.G. Multivariate analysis of diversity of landrace rice germplasm. Crop Sci. 2012, 52, 494–504. [Google Scholar] [CrossRef]
- Madhulatha, T.S. An overview on clustering methods. IOSR-JEN 2012, 2, 719–725. [Google Scholar] [CrossRef]
- Duangpapeng, P.; Lertrat, K.; Lomthaisong, K.; Scott, M.P.; Suriharn, B. Variability in anthocyanins, phenolic compounds and antioxidant capacity in the tassels of collected waxy corn germplasm. Agronomy 2019, 9, 158. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Scott, M.P.; Lertrat, K. Genotypic variability in anthocyanins, total phenolics, and antioxidant activity among diverse waxy corn germplasm. Euphytica 2014, 203, 237–248. [Google Scholar] [CrossRef]
- Ha, T.Y.; Ko, S.N.; Lee, S.M.; Kim, H.R.; Chung, S.H.; Kim, S.R.; Yoon, H.H.; Kim, I.H. Changes in nutraceutical lipid components of rice at different degrees of milling. Eur. J. Lipid Sci. Technol. 2006, 108, 175–181. [Google Scholar] [CrossRef]
- Mingyai, S.; Srikaeo, K.; Kettawan, A.; Singanusong, R. Effects of extraction methods on phytochemicals of rice bran oils produced from colored rice. J. Oleo Sci. 2018, 67, 135–142. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, A.F.; Melendez-Martinez, A.J.; Gonzalez-Miret, M.L.; Heredia, F. Multivariate statistical analysis of the color-anthocyanin relationships in different soilless-grown strawberry genotypes. J. Agric. Food Chem. 2008, 56, 2735–2741. [Google Scholar] [CrossRef]
- Min, B.; McClung, A.M.; Chen, M.H. Phytochemicals and antioxidant capacities in rice brans of different color. J. Food Sci. 2011, 76, 117–126. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Thaiwai, C.; Sukonthamut, S.; Nokkoul, R.; Tadtong, S. Evaluation of nutrient content and antioxidant, neuritogenic, and neuroprotective activities of upland rice bran oil. ScienceAsia 2018, 44, 257–267. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Xu, Z.; Godber, J.S.; Lanfer-Marquez, U.M. Tocopherols, tocotrienols, and γ-oryzanol contents in Japonica and Indica subspecies of rice (Oryza sativa L.) cultivated in Brazil. Cereal Chem. 2008, 85, 243–247. [Google Scholar] [CrossRef]
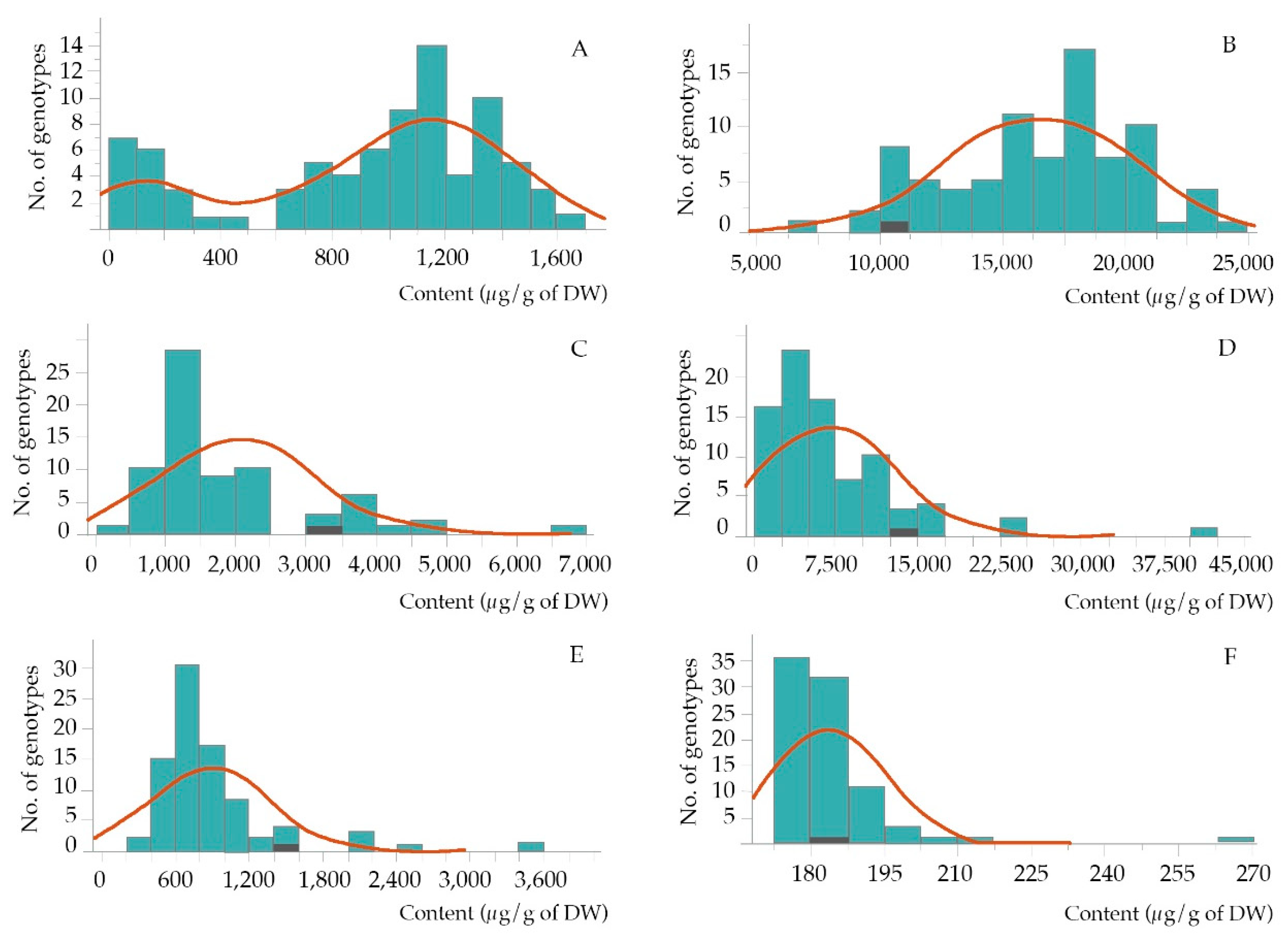
| Compounds | Mean ± SD | Min. | Max. | CV | F-Value |
|---|---|---|---|---|---|
| α -tocopherol | 920 ± 458 | 78 | 1,673 | 49.8 | 48.1 ** |
| γ -oryzanol | 16,501 ± 3,860 | 7,477 | 24,613 | 23.3 | 39.0 ** |
| campesterol | 2,016 ± 1,106 | 495 | 6,699 | 54.8 | 186.6 ** |
| β-sitosterol | 7,024 ± 6,107 | 470 | 40,035 | 86.9 | 8,451.8 ** |
| octacosanol | 892 ± 484 | 352 | 3,522 | 54.3 | 80.4 ** |
| squalene | 183.7 ± 11.3 | 175 | 266 | 6.2 | 61.7 ** |
| First | Second | Third | Fourth | |
|---|---|---|---|---|
| Eigenvalues | 2.52 | 1.78 | 0.87 | 0.49 |
| Percent variances | 42.04 | 29.58 | 13.67 | 8.16 |
| Cumulative % total variance | 42.04 | 71.62 | 85.30 | 93.47 |
| Coefficient vector | ||||
| Compounds | ||||
| α-tocopherol | 0.192 | 0.637 | −0.194 | 0.07 |
| γ-oryzanol | 0.139 | 0.657 | −0.167 | 0.21 |
| campesterol | 0.575 | −0.053 | −0.110 | 0.23 |
| β-sitosterol | 0.586 | −0.139 | −0.091 | 0.78 |
| octacosanol | 0.329 | 0.120 | 0.907 | −0.46 |
| squalene | 0.401 | −0.140 | −0.091 | −0.27 |
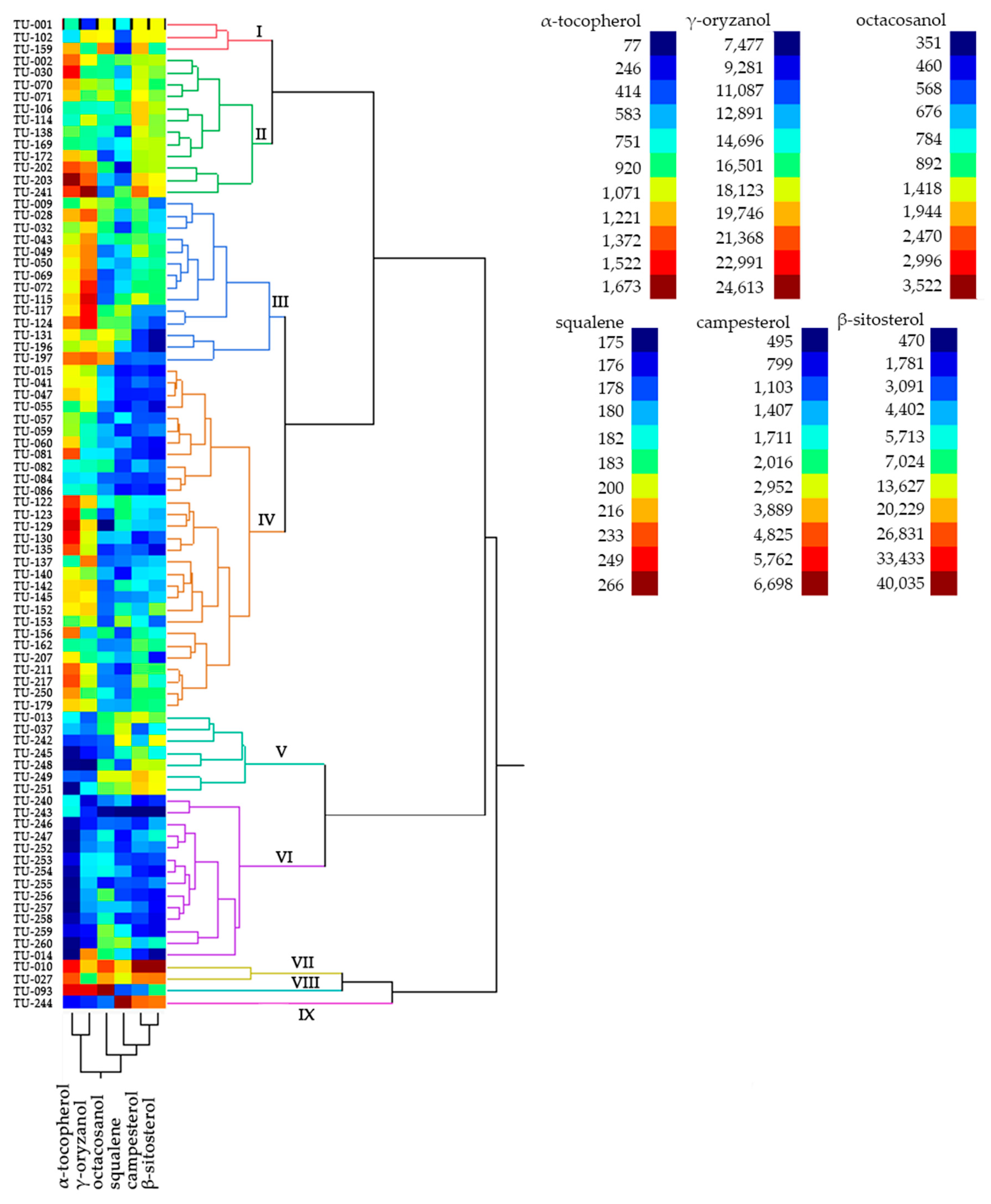
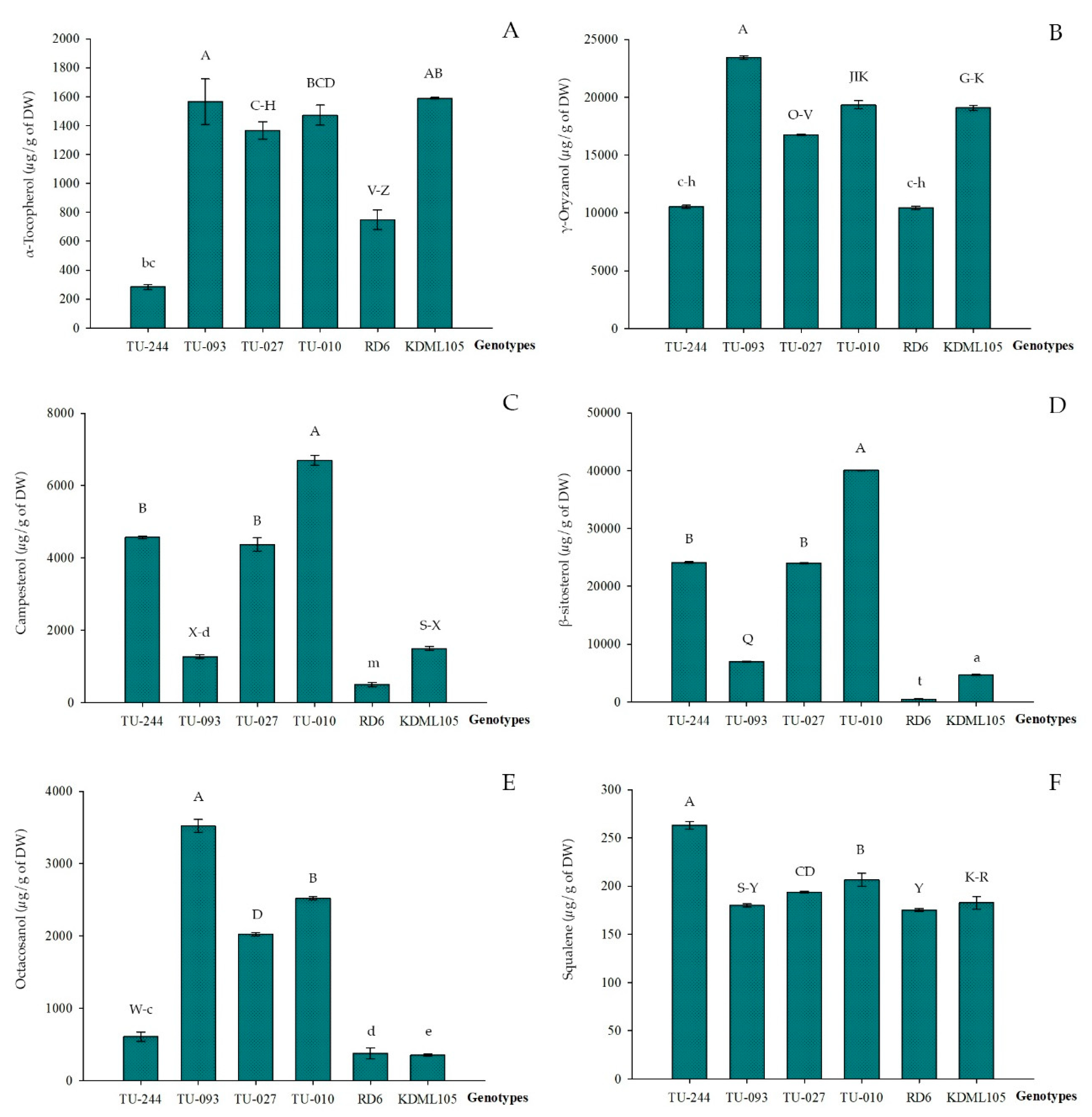
| Clusters | n | Lipid Compounds (µg/g of Dry Weight) | |||||
|---|---|---|---|---|---|---|---|
| α-tocopherol | γ-oryzanol | Campesterol | β-sitosterol | Octacosanol | Squalene | ||
| I | 3 | 910.4 ± 281.6 | 15,108.3 ± 4,276.4 | 3,393.5 ± 399.4 | 12,100.5 ± 4,841.9 | 1,737.0 ± 387.6 | 179.27 ± 2.2 |
| II | 12 | 1,163.5 ± 235.3 | 17,788.9 ± 2,600.0 | 3,036.0 ± 656.4 | 10,662.4 ± 3,237.9 | 850.94 ± 200.3 | 182.7 ± 4.0 |
| III | 14 | 1,127.8 ± 111.8 | 20,644.7 ± 1,826.9 | 1,870.7 ± 612.4 | 4899.7 ± 2,352.6 | 989.4 ± 420.3 | 183.5 ± 4.8 |
| IV | 29 | 1,164.3 ± 255.7 | 17,357.5 ± 1,964.4 | 1,536.8 ± 595.4 | 4698.9 ± 288.7 | 656.2 ± 105.9 | 180.2 ± 3.2 |
| V | 7 | 350.9 ± 259.2 | 11,044.0 ± 2,020.6 | 2,603.0 ± 995.3 | 11,116.7 ± 4,328.5 | 903.2 ± 283.1 | 193.0 ± 9.4 |
| VI | 14 | 214.2 ± 227.0 | 12,217.4 ± 2,841.3 | 1,060.6 ± 252.9 | 2,929.2 ± 1,743.9 | 770.9 ± 212.9 | 180.0 ± 4.0 |
| VII | 2 | 1,418.9 ± 75.0 | 18,045.0 ± 1,841.3 | 5,531.2 ± 1,650.7 | 32,007.5 ± 11,352.6 | 2,273.0 ± 352.9 | 206.2 ± 7.3 |
| VIII | 1 | 1,565.8 ± 0.0 | 23,423.0 ± 0.0 | 1,267.9 ± 0.0 | 6,974.8 ± 0.0 | 3,522.0 ± 0.0 | 178.6 ± 0.0 |
| IX | 1 | 283.7 ± 0.0 | 10,521.0 ± 0.0 | 4,571.0 ± 0.0 | 24,102.0 ± 0.0 | 606.7 ± 0.0 | 266.1 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harakotr, B.; Prompoh, K.; Boonyuen, S.; Suriharn, B.; Lertrat, K. Variability in Nutraceutical Lipid Content of Selected Rice (Oryza sativa L. spp. indica) Germplasms. Agronomy 2019, 9, 823. https://doi.org/10.3390/agronomy9120823
Harakotr B, Prompoh K, Boonyuen S, Suriharn B, Lertrat K. Variability in Nutraceutical Lipid Content of Selected Rice (Oryza sativa L. spp. indica) Germplasms. Agronomy. 2019; 9(12):823. https://doi.org/10.3390/agronomy9120823
Chicago/Turabian StyleHarakotr, Bhornchai, Kasidid Prompoh, Supakorn Boonyuen, Bhalang Suriharn, and Kamol Lertrat. 2019. "Variability in Nutraceutical Lipid Content of Selected Rice (Oryza sativa L. spp. indica) Germplasms" Agronomy 9, no. 12: 823. https://doi.org/10.3390/agronomy9120823
APA StyleHarakotr, B., Prompoh, K., Boonyuen, S., Suriharn, B., & Lertrat, K. (2019). Variability in Nutraceutical Lipid Content of Selected Rice (Oryza sativa L. spp. indica) Germplasms. Agronomy, 9(12), 823. https://doi.org/10.3390/agronomy9120823






