Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Seed Germination Percentage
3.2. Germination Speed
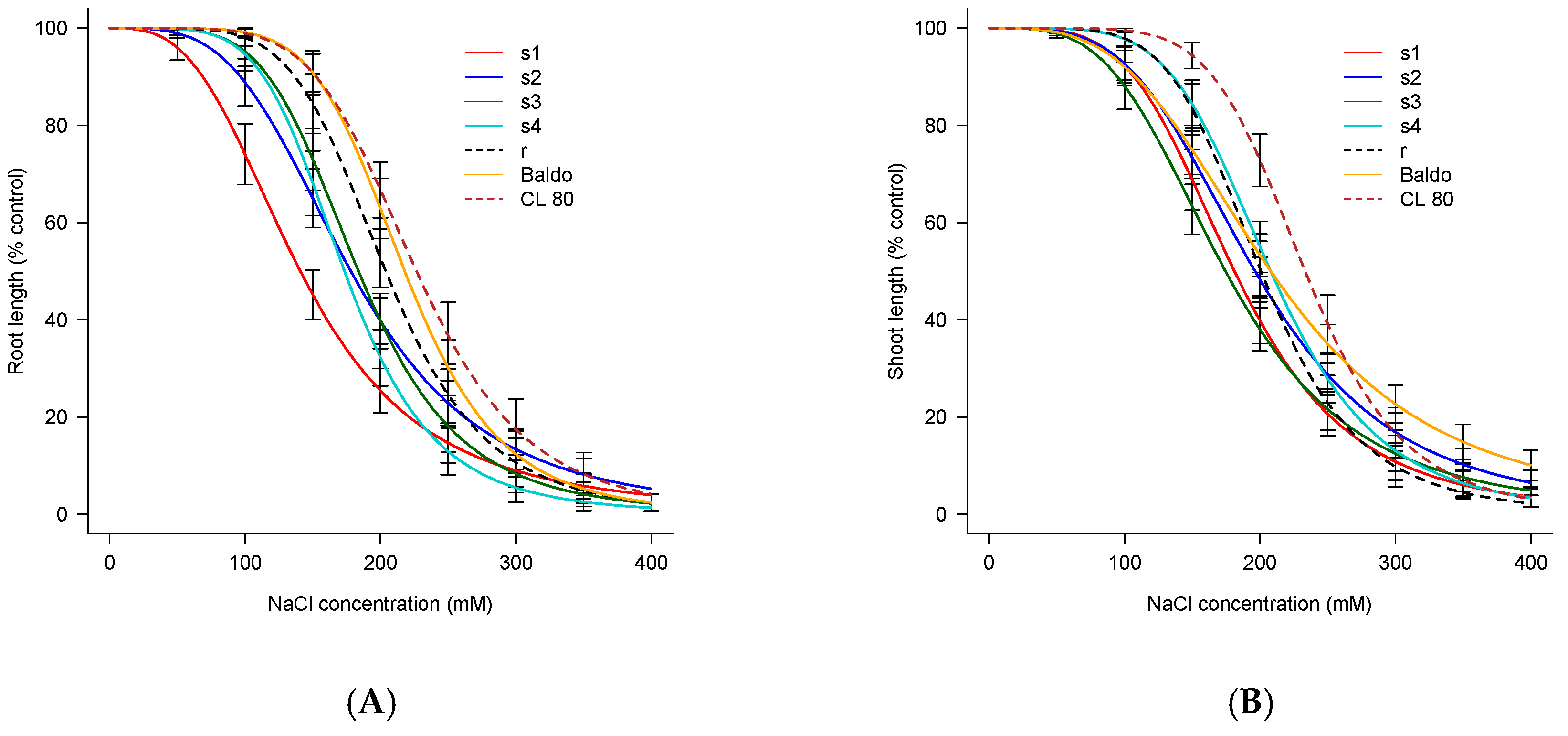
3.3. Root and Shoot Length
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Chapter Three-Weedy (Red) rice: An emerging constraint to global rice production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 181–228. [Google Scholar]
- United Nations. World Population Prospects 2019: Data Booklet; Statistical Papers-United Nations (Ser. A), Population and Vital Statistics Report; UN: New York, NY, USA, 2019; ISBN 978-92-1-004247-5. Available online: https://www.un-ilibrary.org/population-and-demography/world-population-prospects-2019-data-booklet_3e9d869f-en (accessed on 13 August 2019).
- Shaner, D.L. Lessons learned from the history of herbicide resistance. Weed Sci. 2014, 62, 427–431. [Google Scholar] [CrossRef]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Germination of weedy rice in response to field conditions during winter. Weed Technol. 2011, 25, 252–261. [Google Scholar] [CrossRef]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Growth variability of Italian weedy rice populations grown with or without cultivated Rice. Crop Sci. 2015, 55, 394. [Google Scholar] [CrossRef]
- Kraehmer, H.; Jabran, K.; Mennan, H.; Chauhan, B.S. Global distribution of rice weeds—A review. Crop Prot. 2016, 80, 73–86. [Google Scholar] [CrossRef]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Susceptibility to imazamox in Italian weedy rice populations and Clearfield® rice varieties. Weed Res. 2014, 54, 492–500. [Google Scholar] [CrossRef]
- Scarabel, L.; Cenghialta, C.; Manuello, D.; Sattin, M. Monitoring and management of Imidazolinone-resistant red rice (Oryza sativa L., var. sylvatica) in Clearfield® Italian paddy rice. Agronomy 2012, 2, 371–383. [Google Scholar] [CrossRef]
- Busi, R.; Nguyen, N.K.; Chauhan, B.S.; Vidotto, F.; Tabacchi, M.; Powles, S.B. Can herbicide safeners allow selective control of weedy rice infesting rice crops?: Can herbicide safeners allow selective control of weedy rice. Pest Manag. Sci. 2017, 73, 71–77. [Google Scholar] [CrossRef]
- Sudianto, E.; Beng-Kah, S.; Ting-Xiang, N.; Saldain, N.E.; Scott, R.C.; Burgos, N.R. Clearfield® rice: Its development, success, and key challenges on a global perspective. Crop Prot. 2013, 49, 40–51. [Google Scholar] [CrossRef]
- Kaloumenos, N.S.; Capote, N.; Aguado, A.; Eleftherohorinos, I.G. Red rice (Oryza sativa) cross-resistance to imidazolinone herbicides used in resistant rice cultivars grown in northern Greece. Pestic. Biochem. Physiol. 2013, 105, 177–183. [Google Scholar] [CrossRef]
- Dos Reis Goulart, I.C.G.; Pacheco, M.T.; Nunes, A.L.; Merotto, A. Identification of origin and analysis of population structure of field-selected imidazolinone-herbicide resistant red rice (Oryza sativa). Euphytica 2012, 187, 437–447. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Sales, M.A.; Mauromoustakos, A.; Gealy, D.R.; Smith, K.L.; Black, H.L.; Jia, M. Factors affecting the outcrossing rate between ClearfieldTM rice and red rice (Oryza sativa). Weed Sci. 2009, 57, 394–403. [Google Scholar] [CrossRef]
- Busconi, M.; Rossi, D.; Lorenzoni, C.; Baldi, G.; Fogher, C. Spread of herbicide-resistant weedy rice (red rice, Oryza sativa L.) after 5 years of Clearfield rice cultivation in Italy: Herbicide-resistant red rice in Italy. Plant Biol. 2012, 14, 751–759. [Google Scholar] [CrossRef] [PubMed]
- GIRE Gruppo Italiano Resistenza Erbicidi. Available online: http://gire.mlib.cnr.it/index.php?sel=lineeGuida (accessed on 9 January 2019).
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Morphological characterisation of Italian weedy rice (Oryza sativa) populations. Weed Res. 2012, 52, 60–69. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Othman, Y.; Al-Karaki, G.; Al-Tawaha, A.R.; Al-Horani, A. Variation in germination and ion uptake in barley genotypes under salinity conditions. World J. Agric. Sci. 2006, 2, 11–15. [Google Scholar]
- Rozema, J.; Flowers, T. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Velmurugan, A.; Swarnam, T.P.; Ambast, S.K.; Kumar, N. Managing waterlogging and soil salinity with a permanent raised bed and furrow system in coastal lowlands of humid tropics. Agric. Water Manag. 2016, 168, 56–67. [Google Scholar] [CrossRef]
- Ghiglieri, G.; Carletti, A.; Pittalis, D. Analysis of salinization processes in the coastal carbonate aquifer of Porto Torres (NW Sardinia, Italy). J. Hydrol. 2012, 432, 43–51. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Fagnano, M.; Barbieri, G. Saline agriculture in Mediterranean environments. Ital. J. Agron. 2011, 6, 7. [Google Scholar] [CrossRef]
- Capaccioni, B.; Didero, M.; Paletta, C.; Didero, L. Saline intrusion and refreshening in a multilayer coastal aquifer in the Catania Plain (Sicily, Southern Italy): Dynamics of degradation processes according to the hydrochemical characteristics of groundwaters. J. Hydrol. 2005, 307, 1–16. [Google Scholar] [CrossRef]
- Antonellini, M.; Mollema, P.; Giambastiani, B.; Bishop, K.; Caruso, L.; Minchio, A.; Pellegrini, L.; Sabia, M.; Ulazzi, E.; Gabbianelli, G. Salt water intrusion in the coastal aquifer of the southern Po Plain, Italy. Hydrogeol. J. 2008, 16, 1541–1556. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, B.; He, Y.; Zhan, C.; Cheng, Y.; Zhang, J.; Zhang, H.; Cheng, J.; Wang, Z. A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 2019, 97, 1089–1104. [Google Scholar] [CrossRef]
- Thiam, M.; Champion, A.; Diouf, D.; Ourèye SY, M. NaCl effects on in vitro germination and growth of some Senegalese cowpea (Vigna unguiculata (L.) Walp.) cultivars. ISRN Biotechnol. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Bao, Y.; Wu, Y.; Zhang, H. Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 2011, 178, 297–307. [Google Scholar] [CrossRef]
- Pearson, G.A.; Ayers, A.D.; Eberhard, D.L. Relative salt tolerance of rice during germination and early seedling development. Soil Sci. 1966, 102, 151. [Google Scholar] [CrossRef]
- Mondal, S.; Borromeo, T.H. Screening of salinity tolerance of rice at early seedling stage. J. Biosci. Agric. Res. 2016, 10, 843–847. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef]
- Hakim, M.A.; Juraimi, A.S.; Hanafi, M.M.; Selamat, A.; Ismail, M.R.; Karim, S.M.R. Studies on seed germination and growth in weed species of rice field under salinity stress. J. Environ. Biol. 2011, 32, 529–536. [Google Scholar] [PubMed]
- ENR Database Superfici -Ente Nazionale Risi. Available online: http://www.enterisi.it/servizi/Menu/dinamica.aspx?idSezione=17298&idArea=19887&idCat=19887&ID=19887&TipoElemento=area (accessed on 27 September 2019).
- Formentin, E.; Sudiro, C.; Perin, G.; Riccadonna, S.; Barizza, E.; Baldoni, E.; Lavezzo, E.; Stevanato, P.; Sacchi, G.A.; Fontana, P.; et al. Transcriptome and cell physiological analyses in different rice cultivars provide new insights into adaptive and salinity stress responses. Front. Plant Sci. 2018, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Barrocu, G. Seawater intrusion in coastal aquifers of Italy. In Coastal Aquifers Intrusion Technology: Mediterranean Countries; López-Geta, J.A., De Dios Gómez, J., de la Orden, J.A., Ramos, G., Rodriguez, L., Eds.; Ministerio de Ciencia y Tecnologia: Madrid, Spain, 2003; pp. 207–223. [Google Scholar]
- Chauhan, B.S.; Johnson, D.E. Seed germination ecology of Junglerice (Echinochloa colona): A major weed of rice. Weed Sci. 2009, 57, 235–240. [Google Scholar] [CrossRef]
- Sadeghloo, A.; Asghari, J.; Ghaderi-Far, F. Seed germination and seedling emergence of Velvetleaf (Abutilon theophrasti) and Barnyardgrass (Echinochloa crus-galli). Planta Daninha 2013, 31, 259–266. [Google Scholar] [CrossRef]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Effects of winter flooding on weedy rice (Oryza sativa L.). Crop Prot. 2010, 29, 1232–1240. [Google Scholar] [CrossRef]
- Forcella, F.; Wilson, R.G.; Renner, K.A.; Dekker, J.; Harvey, R.G.; Alm, D.A.; Buhler, D.D.; Cardina, J. Weed seedbanks of the U.S. corn belt: Magnitude, variation, emergence, and application. Weed Sci. 1992, 40, 636–644. [Google Scholar] [CrossRef]
- RCoreTeam. A Language and Environment for Statistical Computing; R Foundation for Statistical; Computing: Vienna, Austria, 2017. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Bai, J.; Huang, L.; Gao, Z.; Lu, Q.; Wang, J.; Zhao, Q. Soil seed banks and their germination responses to cadmium and salinity stresses in coastal wetlands affected by reclamation and urbanization based on indoor and outdoor experiments. J. Hazard. Mater. 2014, 280, 295–303. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Liu, J.G.; Mahoney, K.J.; Sikkema, P.H.; Swanton, C.J. The importance of light quality in crop-weed competition: Light quality and crop competition. Weed Res. 2009, 49, 217–224. [Google Scholar] [CrossRef]
- Patade, V.Y.; Maya, K.; Zakwan, A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res. J. Seed Sci. 2011, 4, 125–136. [Google Scholar]
- IRRI Stress and Disease Tolerance. Available online: http://www.knowledgebank.irri.org/ricebreedingcourse/Breeding_for_salt_tolerance.htm (accessed on 22 July 2019).
- Bertazzini, M.; Sacchi, G.A.; Forlani, G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. J. Plant Physiol. 2018, 226, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Goulart, I.C.G.R.; Matzenbacher, F.O.; Merotto, A. Differential germination pattern of rice cultivars resistant to imidazolinone herbicides carrying different acetolactate synthase gene mutations: Differential germination pattern of rice cultivars. Weed Res. 2012, 52, 224–232. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Kianian, S.F.; Foley, M.E. Seed dormancy imposed by covering tissues interrelates to shattering and seed morphological characteristics in weedy rice. Crop Sci. 2005, 45, 948–955. [Google Scholar] [CrossRef]
- Serra, F.; Fogliatto, S.; Vidotto, F. Effect of salinity on Echinochloa crus-galli germination as affected by herbicide resistance. Ital. J. Agron. 2018, 13, 221–228. [Google Scholar] [CrossRef]
| NaClconcentration (mM) | Germination of weedy rice populations | Germination of rice varieties | one-way ANOVA p value | |||||
| s1 | s2 | s3 | s4 | r | Baldo | CL80 | ||
| 0 | 93.33 | 91.67 | 91.67 | 96.67 | 76.67 | 96.67 | 88.33 | 0.055 |
| 50 | 98.33b | 98.33b | 96.67b | 96.67b | 90.00a | 99.00b | 94.33ab | 0.027 |
| 100 | 88.33abc | 94.67bc | 98.33c | 96.67c | 80.00a | 98.33c | 85.00ab | 0.012 |
| 150 | 86.67 | 91.67 | 90.00 | 99.33 | 71.67 | 98.33 | 86.67 | 0.074 |
| 200 | 76.67abc | 83.33c | 81.67bc | 91.67c | 53.33a | 95.00c | 58.33ab | 0.010 |
| 250 | 75.00 | 75.00 | 53.33 | 78.33 | 48.33 | 65.00 | 56.67 | 0.084 |
| 300 | 66.67 | 58.33 | 45.00 | 75.00 | 36.67 | 61.67 | 35.00 | 0.084 |
| 350 | 10.00ab | 33.33bc | 23.33abc | 48.33cd | 0.33a | 63.33d | 15.00ab | 0.002 |
| 400 | 6.67a | 15.00a | 1.67a | 10.00a | 1.67a | 41.67b | 3.33a | 0.001 |
| Germination reduction compared to control (%) | ||||||||
| NaCl concentration (mM) | Germination reduction of weedy rice populations | Germination reduction of rice varieties | one-way ANOVA p value | |||||
| s1 | s2 | s3 | s4 | r | Baldo | CL80 | ||
| 50 | −5.36bc 1 | −7.27b | −5.45bc | 0.00c | −17.39a | −2.41bc | −6.79b | 0.010 |
| 100 | 5.36 | −3.27 | −7.27 | 0.00 | −4.35 | −1.72 | 3.78 | 0.334 |
| 150 | 7.14 | 0.00 | 1.82 | −2.76 | 6.52 | −1.72 | 1.89 | 0.940 |
| 200 | 17.86 | 9.09 | 10.91 | 5.17 | 30.43 | 1.72 | 33.96 | 0.092 |
| 250 | 19.64 | 18.18 | 41.82 | 18.96 | 36.96 | 32.76 | 35.85 | 0.342 |
| 300 | 28.57 | 36.36 | 50.91 | 22.43 | 52.17 | 36.21 | 60.38 | 0.454 |
| 350 | 89.28cd | 63.64bc | 74.54bcd | 50.00ab | 99.56d | 34.48a | 83.02cd | 0.003 |
| 400 | 92.86b | 83.64b | 98.18b | 89.65b | 97.83b | 56.90a | 96.23b | 0.001 |
| Weedy Rice Population/Rice Variety | EC50 |
|---|---|
| s1 | 312.80 ± 6.06 c |
| s2 | 310.32 ± 11.27 bc |
| s3 | 270.16 ± 11.06 ab |
| s4 | 338.35 ± 9.47 cd |
| r | 229.70 ± 17.11 a |
| Baldo | 369.63 ± 22.67 d |
| CL80 | 247.27 ± 13.72 a |
| Weedy Rice Population/Rice Variety | |||||||
|---|---|---|---|---|---|---|---|
| NaCl Concentration (mM) | s1 | s2 | s3 | s4 | r | Baldo | CL80 |
| 0 | 2.71 ± 0.06 b | 3.82 ± 0.09 c | 3.44 ± 0.08 c | 3.20 ± 0.08 bc | 1.87 ± 0.11 a | 1.73 ± 0.55 a | 1.57 ± 0.08 a |
| 50 | 3.06 ±0.06 cd | 3.81 ± 0.06 e | 3.23 ± 0.06 d | 2.88 ± 0.04 c | 2.05 ± 0.07 b | 1.69 ± 0.12 a | 2.06 ± 0.07 b |
| 100 | 3.20 ± 0.07 d | 4.24 ± 0.08 f | 3.60 ± 0.07 e | 3.48 ± 0.08 e | 2.59 ± 0.12 c | 2.08 ± 0.04 a | 2.34 ± 0.09 b |
| 150 | 4.29 ± 0.10 c | 4.76 ± 0.12 de | 5.03 ± 0.13 e | 4.38 ± 0.11 cd | 4.19 ± 0.26 c | 2.47 ± 0.08 a | 3.18 ± 0.13 b |
| 200 | 6.17 ± 0.21 bc | 6.04 ± 0.23 bc | 6.46 ± 0.31 bc | 5.73 ± 0.30 ab | 10.16 ± 2.78 c | 3.52 ± 0.18 a | 8.84 ± 1.32 bc |
| 250 | 7.78 ± 0.31 ab | 7.32 ± 0.32 ab | 10.52 ± 1.57 b | 8.36 ± 1.16 ab | - | 4.78 ± 0.55 a | 10.14 ± 2.68 b |
| 300 | 11.25 ± 0.61 ab | 9.60 ± 0.66 a | - | 10.25 ± 1.07 ab | - | 9.01 ± 2.04 a | - |
| 350 | - | - | - | - | - | 6.78±0.66 | - |
| 400 | - | - | - | - | - | - | - |
| Weedy Rice Population/Rice Variety | EC50 Root | EC50 Shoot |
|---|---|---|
| s1 | 140.74 ± 4.99 a | 181.13 ± 4.51 b |
| s2 | 178.13 ± 5.20 c | 196.30 ± 4.97 c |
| s3 | 183.71 ± 4.43 d | 174.54 ± 4.90 a |
| s4 | 173.40 ± 4.11 b | 207.89 ± 4.21 e |
| r | 203.88 ± 4.51 e | 200.72 ± 4.18 d |
| Baldo | 217.92 ± 4.22 f | 207.93 ± 5.37 e |
| CL80 | 226.62 ± 5.26 g | 233.30 ± 4.29 f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogliatto, S.; Serra, F.; Patrucco, L.; Milan, M.; Vidotto, F. Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice. Agronomy 2019, 9, 658. https://doi.org/10.3390/agronomy9100658
Fogliatto S, Serra F, Patrucco L, Milan M, Vidotto F. Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice. Agronomy. 2019; 9(10):658. https://doi.org/10.3390/agronomy9100658
Chicago/Turabian StyleFogliatto, Silvia, Francesca Serra, Lorenzo Patrucco, Marco Milan, and Francesco Vidotto. 2019. "Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice" Agronomy 9, no. 10: 658. https://doi.org/10.3390/agronomy9100658
APA StyleFogliatto, S., Serra, F., Patrucco, L., Milan, M., & Vidotto, F. (2019). Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice. Agronomy, 9(10), 658. https://doi.org/10.3390/agronomy9100658






