Weed Suppression in Only-Legume Cover Crop Mixtures
Abstract
1. Introduction
2. Materials and Methods
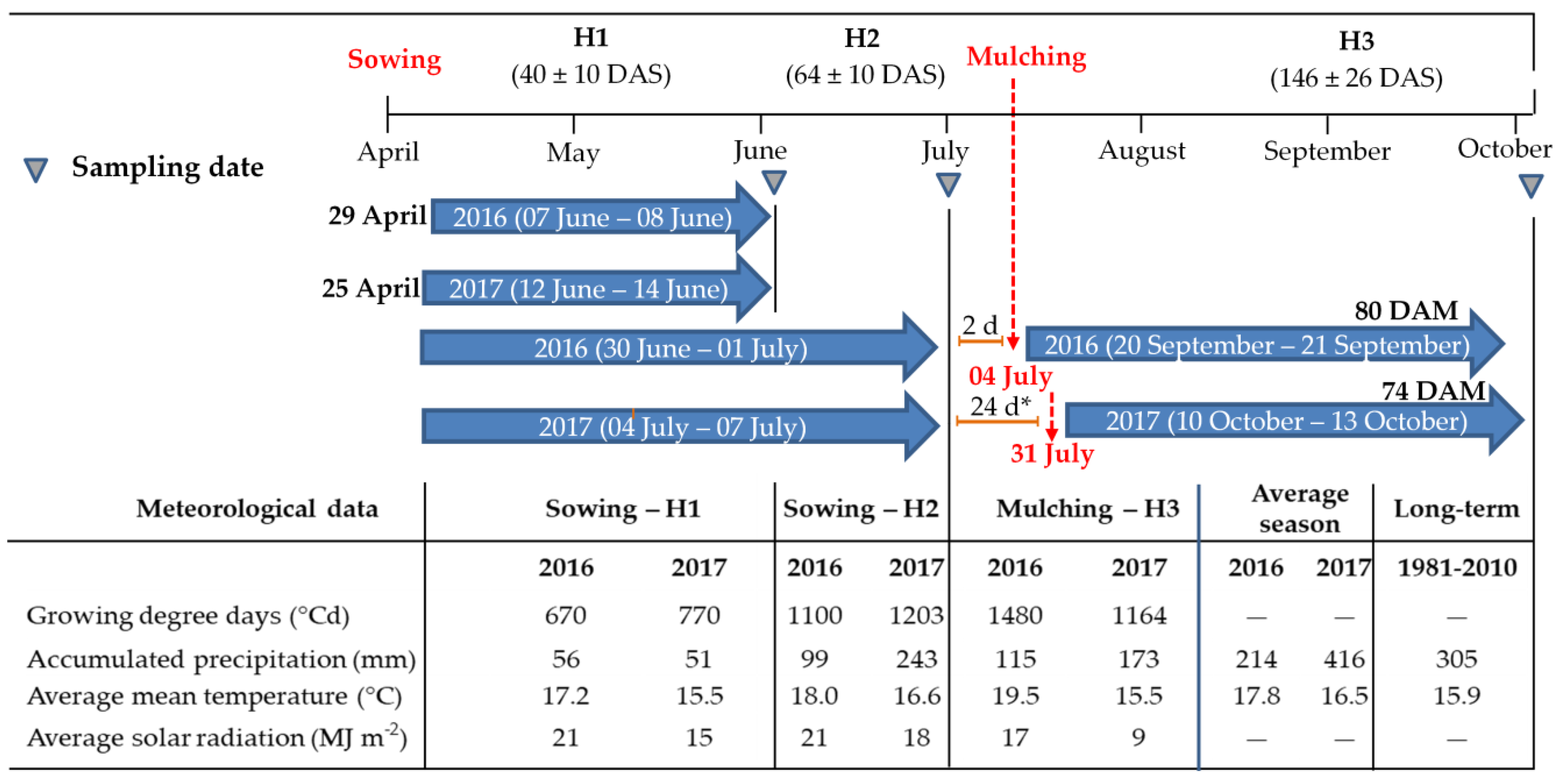
2.1. Study Sites
2.2. Experimental Design
2.3. Weed Biomass and Cover Crop Biomass
2.4. Statistical Analysis
3. Results
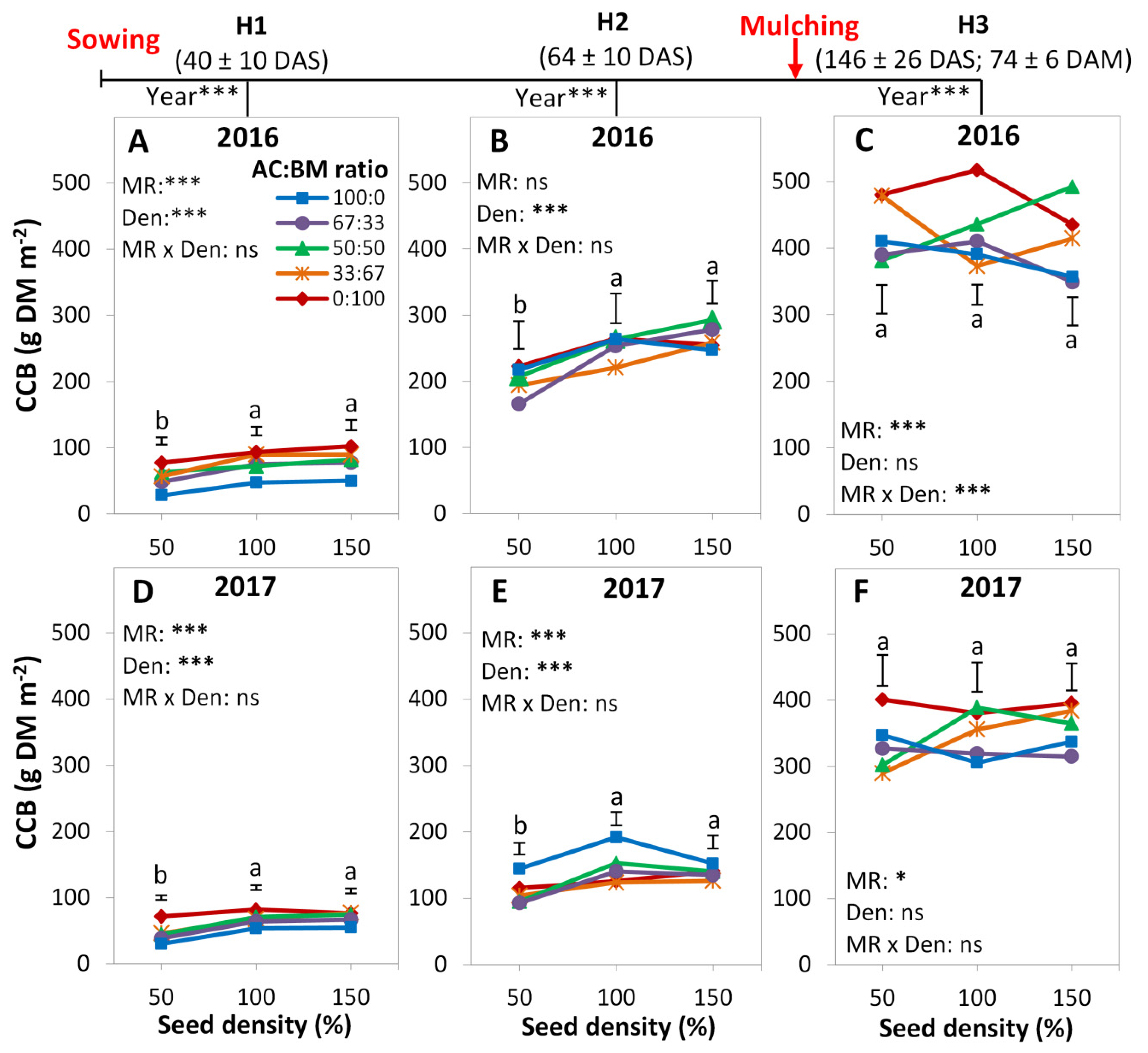
3.1. Cover Crop Biomass
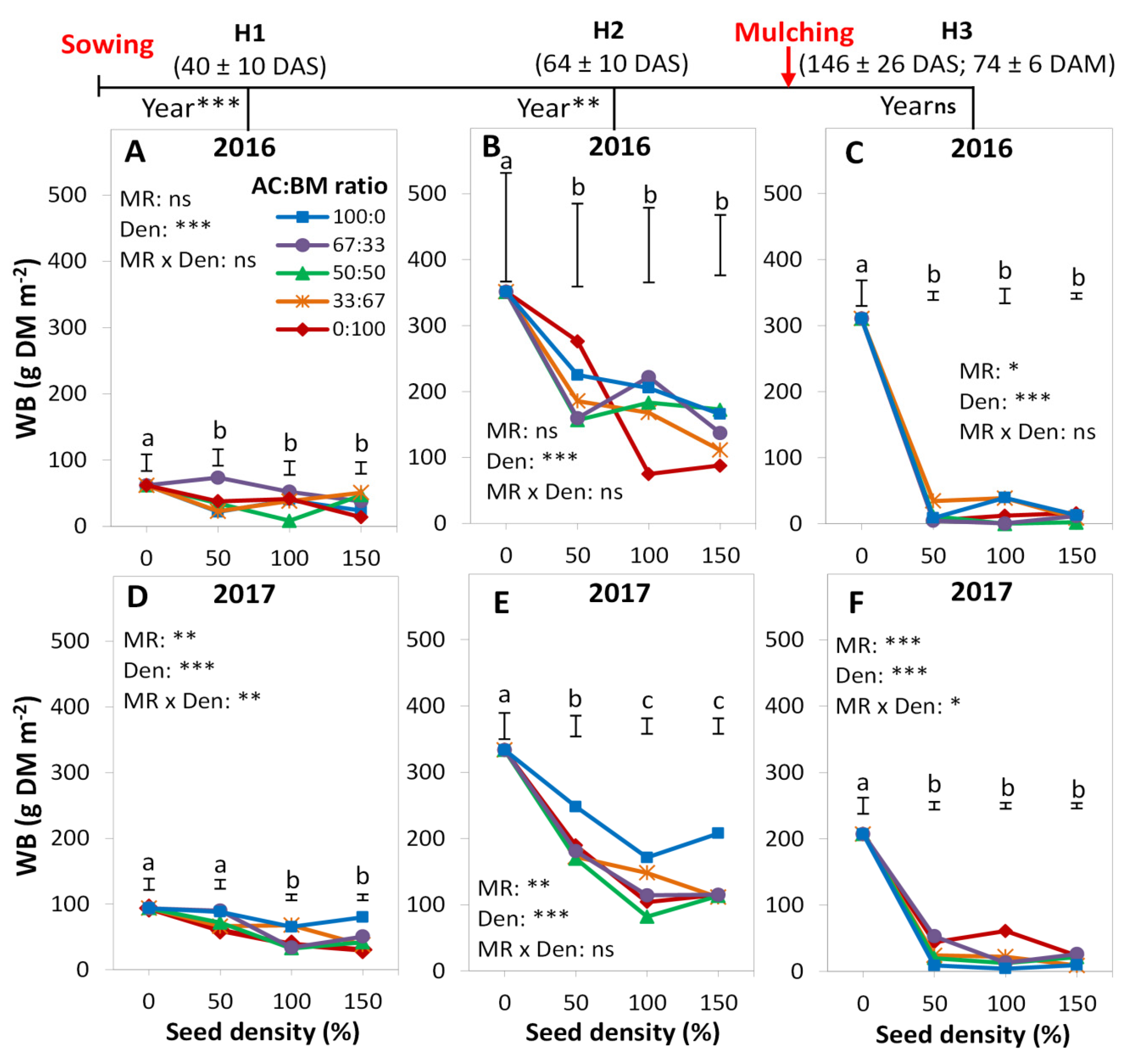
3.2. Weed Biomass
4. Discussion
4.1. Cover Crop Species Effect
4.2. Diversity Effects
4.3. Proportion Effects
4.4. Density Effects
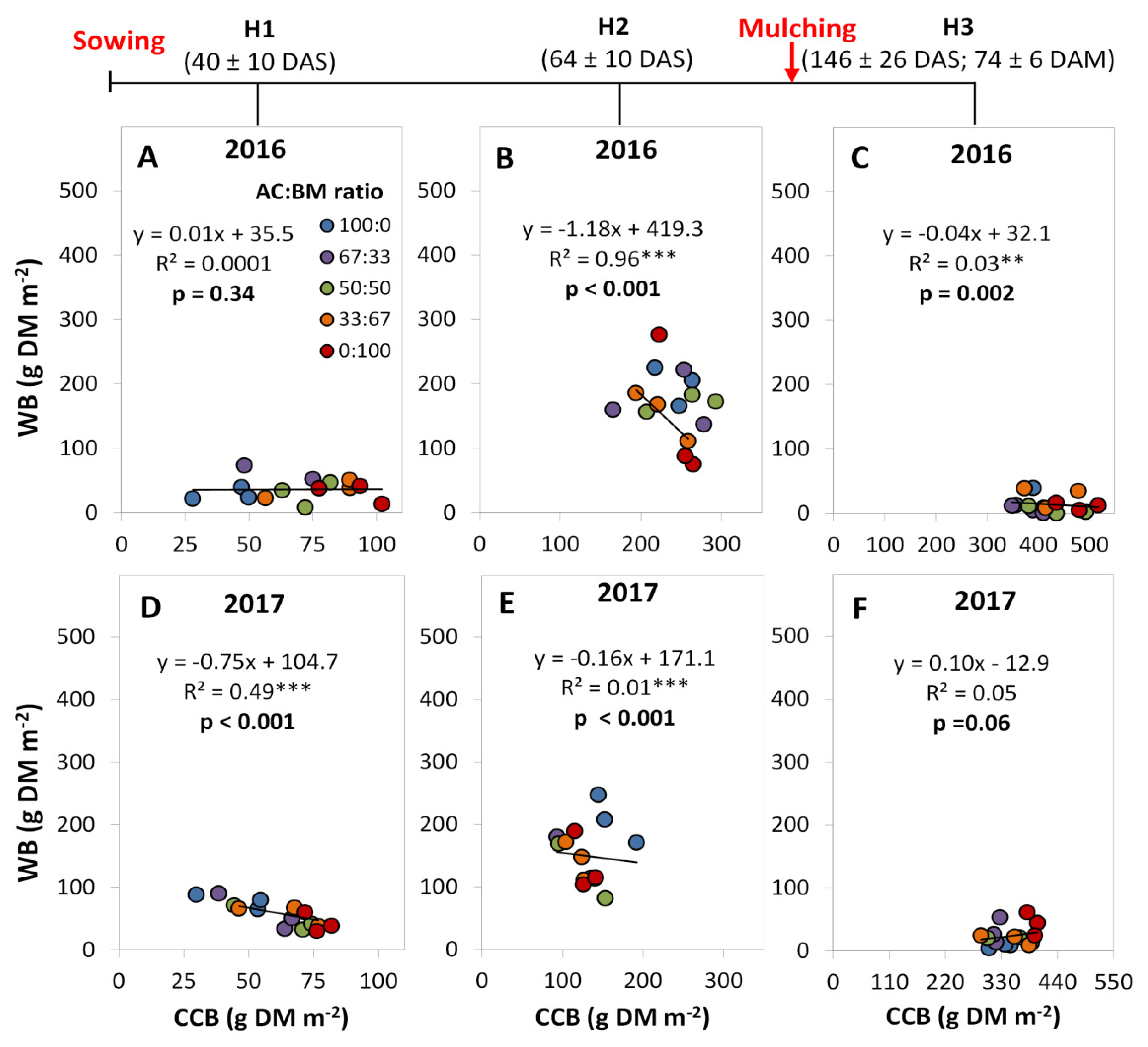
4.5. Relationship between Crop Biomass and Weed Biomass
4.6. Mulching Effects
4.7. Site and Year Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chauhan, B.S.; Gill, G.S. Ecologically based weed management strategies. In Recent Advances in Weed Management; Chauhan, B.S., Mahajan, G., Eds.; Springer: New York, NY, USA, 2014; pp. 1–11. [Google Scholar]
- Abouziena, H.F.; Haggag, W.M. Weed Control in Clean Agriculture: A Review. Planta Daninha 2016, 34, 377–392. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: An alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Lemessa, F.; Wakjira, M. Cover crops as a means of ecological weed management in agroecosystems. J. Crop Sci. Biotechnol. 2015, 18, 133–145. [Google Scholar] [CrossRef]
- Finney, D.M.; Kaye, J.P. Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J. Appl. Ecol. 2017, 54, 509–517. [Google Scholar] [CrossRef]
- Lorin, M.; Jeuffroy, M.H.; Butier, A.; Valantin-Morison, M. Undersowing winter oilseed rape with Frost-Sensitive legume living mulches to improve weed control. Eur. J. Agron. 2015, 71, 96–105. [Google Scholar] [CrossRef]
- Hiltbrunner, J.; Jeanneret, P.; Liedgens, M.; Stamp, P.; Streit, B. Response of weed communities to legume living mulches in winter wheat. J. Agron. Crop Sci. 2007, 193, 93–102. [Google Scholar] [CrossRef]
- Fradgley, N.S.; Creissen, H.E.; Pearce, H.; Howlett, S.A.; Pearce, B.D.; Döring, T.F.; Girling, R.D. Weed Suppression and Tolerance in Winter Oats. Weed Technol. 2017, 31, 740–751. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; von Gillhaussen, P.; Delory, B.M.; Blossfeld, S.; Poorter, H.; Temperton, V.M. The Importance of Being First: Exploring Priority and Diversity Effects in a Grassland Field Experiment. Front. Plant Sci. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mangum, R.W.; Radhakrishnan, J.; Cavigelli, M.A. Weed seedbank dynamics in three organic farming crop rotations. Agron. J. 2004, 96, 1429–1435. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.; Lima, M.; Davis, A.S.; Gonzalez-Andujar, J.L. Weed Diversity Affects Soybean and Maize Yield in a Long Term Experiment in Michigan, USA. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruisi, P.; Frangipane, B.; Amato, G.; Frenda, A.S.; Plaia, A.; Giambalvo, D.; Saia, S. Nitrogen uptake and nitrogen fertilizer recovery in old and modern wheat genotypes grown in the presence or absence of interspecific competition. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, L.S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef]
- Mahmood, A.; Ihsan, M.Z.; Khaliq, A.; Hussain, S.; Cheema, Z.A.; Naeem, M.; Daur, I.; Hussain, H.A.; Alghabari, F. Crop Residues Mulch as Organic Weed Management Strategy in Maize. Clean-Soil Air Water 2016, 44, 317–324. [Google Scholar] [CrossRef]
- Ranaivoson, L.; Naudin, K.; Ripoche, A.; Affholder, F.; Rabeharisoa, L.; Corbeels, M. Agro-Ecological functions of crop residues under conservation agriculture. A review. Agron. Sustain. Dev. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Caamal-Maldonado, J.A.; Jiménez-Osornio, J.J.; Torres-Barragán, A.; Anaya, A.L. The use of allelopathic legume cover and mulch species for weed control in cropping systems. Agron. J. 2001, 93, 27–36. [Google Scholar] [CrossRef]
- Reddy, P.P. Crop Residue Management and Organic Amendments. In Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Singapore, 2017; pp. 29–41. [Google Scholar]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by Cereal-Grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Dibet, A.; Hauggaard-Nielsen, H.; Crozat, Y.; Gooding, M.; Ambus, P.; Dahlmann, C.; von Fragstein, P.; Pristeri, A.; Monti, M.; et al. The competitive ability of Pea-Barley intercrops against weeds and the interactions with crop productivity and soil N availability. Field Crop. Res. 2011, 122, 264–272. [Google Scholar] [CrossRef]
- Bilalis, D.; Papastylianou, P.; Konstantas, A.; Patsiali, S.; Karkanis, A.; Efthimiadou, A. Weed-Suppressive effects of Maize-Legume intercropping in organic farming. Int. J. Pest Manag. 2010, 56, 173–181. [Google Scholar] [CrossRef]
- Stoltz, E.; Nadeau, E. Effects of intercropping on yield, weed incidence, forage quality and soil residual N in organically grown forage maize (Zea mays L.) and faba bean (Vicia faba L.). Field Crop. Res. 2014, 169, 21–29. [Google Scholar] [CrossRef]
- Connolly, J.; Sebastià, M.T.; Kirwan, L.; Finn, J.A.; Llurba, R.; Suter, M.; Collins, R.P.; Porqueddu, C.; Helgadóttir, Á.; Baadshaug, O.H.; et al. Weed suppression greatly increased by plant diversity in intensively managed grasslands: A Continental-Scale Experiment. J. Appl. Ecol. 2018, 55, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Atwood, L.W.; Warren, N.D. Increased productivity of a cover crop mixture is not associated with enhanced agroecosystem services. PLoS ONE 2014, 9, e97351. [Google Scholar] [CrossRef]
- Cong, W.F.; Suter, M.; Lüscher, A.; Eriksen, J. Species interactions between forbs and Grass-Clover contribute to yield gains and weed suppression in forage grassland mixtures. Agric. Ecosyst. Environ. 2018, 268, 154–161. [Google Scholar] [CrossRef]
- Yu, Y.; Makowski, D.; Stomph, T.J.; van der Wopke, W. Robust increases of land equivalent ratio with temporal niche differentiation: A Meta-Quantile regression. Agron. J. 2016, 108, 2269–2279. [Google Scholar] [CrossRef]
- Döring, T.F.; Baddeley, J.A.; Brown, R.; Collins, R.; Crowley, O.; Cuttle, S.; Howlett, S.A.; Jones, H.E.; Mccalman, H.; Measures, M.; et al. Using Legume-Based mixtures to enhance the nitrogen use efficiency and economic viability of cropping systems: Project Report (RD-2007-3447). HGCA/Agric. Hort. Dev. Board 2013, 190. [Google Scholar]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-Year Continental-Scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Storkey, J.; Döring, T.; Baddeley, J.; Marshall, A.; Roderick, S.; Jones, H. Modelling the ability of legumes to suppress weeds. Asp. Appl. Biol. 2011, 109, 53–58. [Google Scholar]
- Döring, T.F.; Storkey, J.; Baddeley, J.A.; Collins, R.P.; Crowley, O.; Howlett, S.A.; Jones, H.E.; McCalman, H.; Measures, M.; Pearce, H.; et al. Weeds in Organic Fertility-Building Leys: Aspects of Species Richness and Weed Management. Org. Farming 2017, 3. [Google Scholar] [CrossRef]
- Dhamala, N.R.; Eriksen, J.; Carlsson, G.; Søegaard, K.; Rasmussen, J. Highly productive forage legume stands show no positive biodiversity effect on yield and N2-Fixation. Plant Soil 2017, 417, 169–182. [Google Scholar] [CrossRef]
- Emery, S.M.; Gross, K.L. Dominant species identity, not community evenness, regulates invasion in experimental grassland plant communities. Ecology 2007, 88, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Hauggaard-Nielsen, H.; Andersen, M.K.; Jørnsgaard, B.; Jensen, E.S. Density and relative frequency effects on competitive interactions and resource use in pea-barley intercrops. Field Crop. Res. 2006, 95, 256–267. [Google Scholar] [CrossRef]
- Kirwan, L.; Lüscher, A.; Sebastià, M.T.; Finn, J.A.; Collins, R.P.; Porqueddu, C.; Helgadottir, A.; Baadshaug, O.H.; Brophy, C.; Coran, C.; et al. Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. J. Ecol. 2007, 95, 530–539. [Google Scholar] [CrossRef]
- Tracy, B.F.; Renne, I.J.; Gerrish, J.; Sanderson, M.A. Effects of plant diversity on invasion of weed species in experimental pasture communities. Basic Appl. Ecol. 2004, 5, 543–550. [Google Scholar] [CrossRef]
- Tracy, B.F.; Sanderson, M.A. Forage productivity, species evenness and weed invasion in pasture communities. Agric. Ecosyst. Environ. 2004, 102, 175–183. [Google Scholar] [CrossRef]
- Polley, H.W.; Wilsey, B.J. Reductions in grassland species evenness increase dicot seedling invasion and spittle bug infestation. Ecol. Lett. 2002, 5, 676–684. [Google Scholar]
- Sanderson, M.A.; Brink, G.; Stout, R.; Ruth, L. Grass-Legume proportions in forage seed mixtures and effects on herbage yield and weed abundance. Agron. J. 2013, 105, 1289–1297. [Google Scholar] [CrossRef]
- Bulson, H.A.J.; Snaydon, R.W.; Stopes, C.E. Effects of plant density on intercropped wheat and field beans in an organic farming system. J. Agric. Sci. 1997, 128, 59–71. [Google Scholar] [CrossRef]
- Weiner, J.; Andersen, S.B.; Wille, W.K.M.; Griepentrog, H.W.; Olsen, J.M. Evolutionary Agroecology: The potential for cooperative, high density, Weed-Suppressing cereals. Evol. Appl. 2010, 3, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef]
- Bybee-Finley, K.; Mirsky, S.B.; Ryan, M.R. Crop Biomass Not Species Richness Drives Weed Suppression in Warm-Season Annual Grass–Legume Intercrops in the Northeast. Weed Sci. 2017, 65, 669–680. [Google Scholar] [CrossRef]
- Clark, A. Managing Cover Crops Profitably. In Sustainable Agriculture Research and Education, 3rd ed.; Sustainable Agriculture Network: Beltsville, MD, USA, 2007; pp. 1–244. [Google Scholar]
- FAO. Ecocrop, Data Sheet, Black medic (Medicago Lupulina). Available online: http://ecocrop.fao.org/ecocrop/srv/en/dataSheet?id=7654 (accessed on 15 September 2019).
- Floulds, W. Response To Soil Moisture Supply in Three Leguminous Species: I.; Growth, Reproduction and Mortality. New Phytol. 1978, 80, 535–545. [Google Scholar] [CrossRef]
- Dzyubenko, N.I.; Dzyubenko, E.A. Crops. Medicago lupulina L.-Black medic. In Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds; 2009; Available online: http://www.agroatlas.ru/en/content/cultural/Medicago_lupulina_K/index.html (accessed on 15 September 2019).
- Watschke, T.L.; Dernoeden, P.H.; Shetlar, D.J. Managing Turfgrass Pests, 2nd ed.; CRC Press: London, UK, 2013; p. 519. [Google Scholar]
- Heuzé, V.; Thiollet, H.; Tran, G.; Hassoun, P.; Lebas, F. Feedipedia, Black medic (Medicago lupulina), a program by INRA, CIRAD, AFZ, and FAO. Available online: https://www.feedipedia.org/node/277 (accessed on 15 September 2019).
- Chapman, H.M.; Dodds, K.G.; Keoghan, J.M. Evaluation of the agronomic potential of pasture legume introductions on droughty outwash soils. N. Z. J. Agric. Res. 1990, 33, 21–27. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Ehlke, N.J.; Albrecht, K.A.; Peterson, P.R. Forage Legumes: Clovers, Birdsfoot Trefoil, Cicer Milkvetch, Crownvetch and Alfalfa, 2nd ed.; Agric. Exp. Stn.: Saint Paul, Minnesota, 2003; pp. 608–2003. [Google Scholar]
- Fairey, D.T. Alsike clover. Commun. Branch Agric. Can. 1989, 30, 410–415. [Google Scholar]
- Agricultural Climatology of the Humboldt-University of Berlin. Available online: https://www.agrar.hu-berlin.de/de/institut/departments/dntw/agrarmet/service/wo (accessed on 15 September 2018).
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.H.; Justes, E. Determination of germination response to temperature and water potential for a wide range of cover crop species and related functional groups. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package version 1.3-0. 2019. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 15 September 2019).
- R Core Team language and environment for statistical computing. R Foundation for Statistical Computing; R Core Team language and environment for statistical computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/. (accessed on 1 August 2019).
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current Status. Allelopath. J. 2006, 18, 57–80. [Google Scholar]
- Liu, Q.; Xu, R.; Yan, Z.; Jin, H.; Cui, H.; Lu, L.; Zhang, D.; Qin, B. Phytotoxic allelochemicals from roots and root exudates of Trifolium pratense. J. Agric. Food Chem. 2013, 61, 6321–6327. [Google Scholar] [CrossRef]
- Elsalahy, H.; Bellingrath-Kimura, S.; Döring, T. Allelopathic effects in species mixtures of legumes. In Proceedings of the Improving Sown Grasslands through Breeding and Management, Proceedings of the Joint 20th Symposium of the European Grassland Federation and the 33rd Meeting of the EUCARPIA, Zurich, Switzerland, 24–27 June 2019; Huguenin-Elie, O., Studer, B., Kölliker, R., Reheul, D., Probo, M., Barre, P., Feuerstein, U., Roldán-Ruiz, I., Mariotte, P., Hopkins, A., Eds.; Organising Committee: Zurich, Switzerland, 2019; p. 128. [Google Scholar]
- Abu-Dieyeh, M.; Watson, A. Impact of mowing and weed control on broadleaf weed population dynamics in turf. J. Plant Interact. 2005, 1, 239–252. [Google Scholar] [CrossRef]

| Site (Year) | Soil Type | pH | Organic Matter | N | P | K | Mg | Ca | Fe | Soil Texture | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Clay | Silt | ||||||||||
| % | mg kg−1 | % | ||||||||||
| FU9 (2016) | Sandy clay loam | 6.3 | 1.24 | 0.13 | 251 | 90 | 52 | 1471 | 7354 | 69 | 22 | 9 |
| S5 (2017) | Sandy loam | 5.6 | 0.72 | 0.09 | 121 | 83 | 37 | 1242 | 5044 | 76 | 14 | 10 |
| Density (%) | Contrast: Mixture vs. Average of Respective Monocultures | 2016 | 2017 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H1 | H2 | H3 | ||||||||
| Effect Size | p | Effect Size | p | Effect Size | p | Effect Size | p | Effect Size | p | Effect Size | P | ||
| 50 | MixB-Avg_mono | −7.0 | 0.49 | −65.1 | 0.49 | 27.4 | 0.13 | −7.9 | 0.48 | −46.5 | 0.05 * | −2.3 | 0.81 |
| MixC-Avg_mono | 4.7 | 0.81 | −94.0 | 0.30 | 4.3 | 0.27 | −2.6 | 0.87 | −49.3 | 0.05 * | −6.7 | 0.50 | |
| MixA-Avg_mono | 43.6 | 0.19 | −91.1 | 0.34 | −2.4 | 0.48 | 15.9 | 0.31 | −37.9 | 0.34 | 26.6 | 0.14 | |
| 100 | MixB-Avg_mono | −2.4 | 0.89 | 27.8 | 0.79 | 12.8 | 0.58 | 15.7 | 0.02 * | 10.5 | 0.73 | −10.5 | 0.14 |
| MixC-Avg_mono | −32.2 | 0.01 ** | 43.0 | 0.59 | −25.9 | 0.07 | −19.6 | 0.03 * | −55.9 | 0.05 * | −19.9 | 0.07 | |
| MixA-Avg_mono | 11.8 | 0.53 | 81.6 | 0.44 | −25.6 | 0.07 | −18.3 | 0.03 * | −23.5 | 0.37 | −12.5 | 0.24 | |
| 150 | MixB-Avg_mono | 32.1 | 0.09 | −15.8 | 0.79 | −6.3 | 0.23 | −17.2 | 0.17 | −50.3 | 0.05 * | −7.9 | 0.15 |
| MixC-Avg_mono | 28.2 | 0.02 * | 45.8 | 0.36 | −12.5 | < 0.01 ** | −13.4 | 0.09 | −48.1 | 0.06 | 4.7 | 0.48 | |
| MixA-Avg_mono | 19.2 | 0.17 | 10.2 | 0.90 | −3.0 | 0.63 | −4.4 | 0.57 | −46.4 | 0.09 | 9.3 | 0.46 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsalahy, H.; Döring, T.; Bellingrath-Kimura, S.; Arends, D. Weed Suppression in Only-Legume Cover Crop Mixtures. Agronomy 2019, 9, 648. https://doi.org/10.3390/agronomy9100648
Elsalahy H, Döring T, Bellingrath-Kimura S, Arends D. Weed Suppression in Only-Legume Cover Crop Mixtures. Agronomy. 2019; 9(10):648. https://doi.org/10.3390/agronomy9100648
Chicago/Turabian StyleElsalahy, Heba, Thomas Döring, Sonoko Bellingrath-Kimura, and Danny Arends. 2019. "Weed Suppression in Only-Legume Cover Crop Mixtures" Agronomy 9, no. 10: 648. https://doi.org/10.3390/agronomy9100648
APA StyleElsalahy, H., Döring, T., Bellingrath-Kimura, S., & Arends, D. (2019). Weed Suppression in Only-Legume Cover Crop Mixtures. Agronomy, 9(10), 648. https://doi.org/10.3390/agronomy9100648







