Continuous Flooding or Alternate Wetting and Drying Differently Affect the Accumulation of Health-Promoting Phytochemicals and Minerals in Rice Brown Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Growth Conditions, and Grain Processing
2.2. Physiological and Yield Performances
2.3. Brown Grain Nutritional Traits
2.4. Brown Grain Ionome
2.5. Statistical Analysis
3. Results
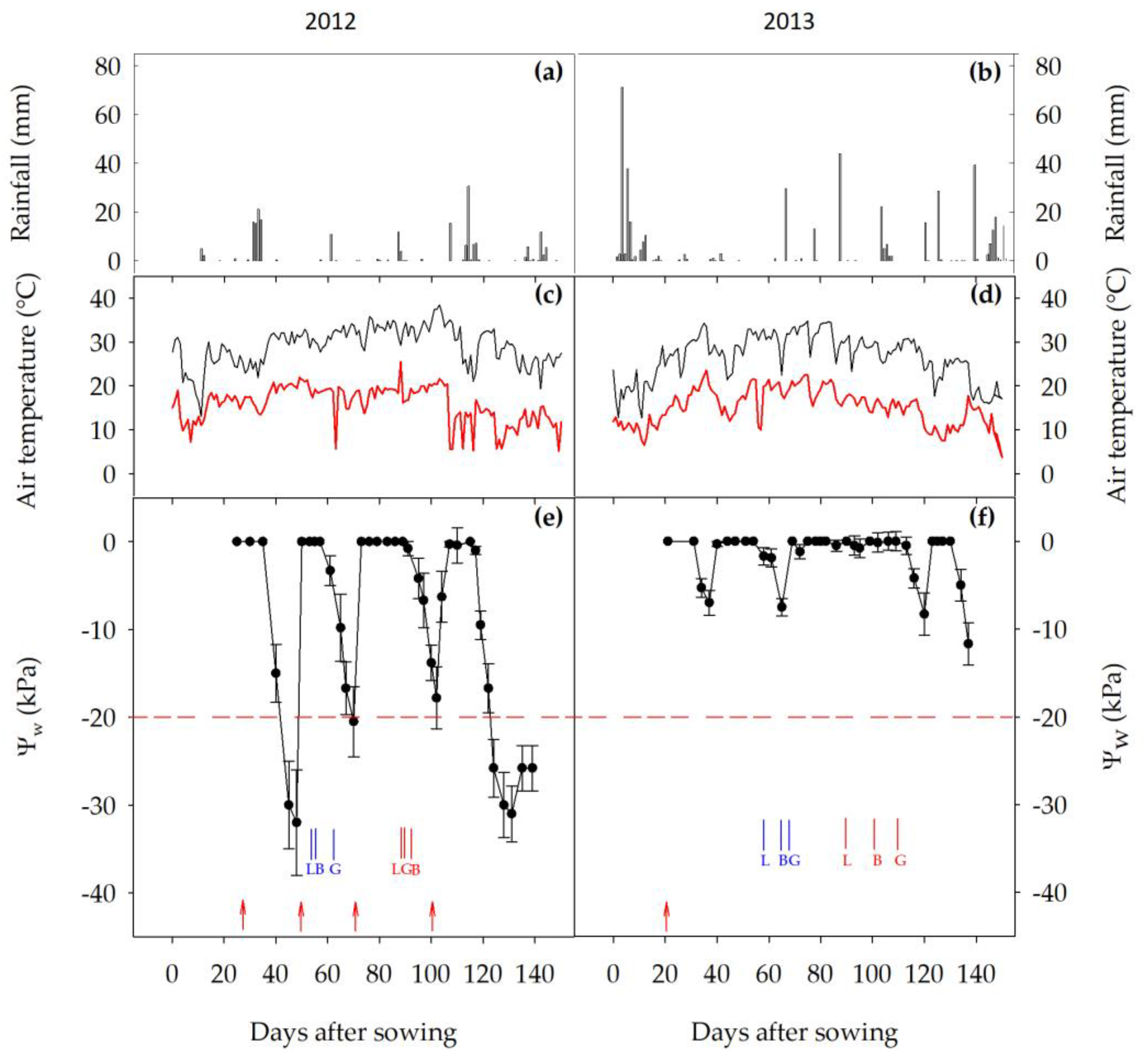
3.1. Weather Conditions and AWD Timing and Severity
3.2. Trait Differences
3.3. Plant Phenology, Yield, Grain Morphology, and Quality
3.4. Concentrations of Health-Promoting Phytochemicals in the Grains
3.5. Effects of AWD on Grain Ionome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maclean, J.L.; Dawe, D.; Hardy, B.; Hettel, G.P. (Eds.) Rice Almanac, 3rd ed.; CABI Publishing: Wallingford, UK, 2002; pp. 30–33. [Google Scholar]
- Tuong, T.P.; Bouman, B.A.M.; Mortimer, M. More rice, less water: Integrated approaches for increasing water productivity in irrigated rice-based system in Asia. Plant Prod. Sci. 2005, 8, 231–241. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Humphreys, E.; Tuong, T.P.; Baker, R. Rice and water. Adv. Agron. 2007, 92, 187–237. [Google Scholar]
- Ringler, C.; Zhu, T. Water resources and food security. Agron. J. 2015, 107, 1533–1538. [Google Scholar] [CrossRef]
- Wassmann, R.; Jagadish, S.V.K.; Heuer, S.; Ismail, A.; Redona, E.; Serraj, R.; Singh, R.K.; Howell, G.; Pathak, H.; Sumfleth, K. Climate change affecting rice production: The physiological and agronomic basis for possible adaptation strategies. Adv. Agron. 2009, 101, 59–122. [Google Scholar] [CrossRef]
- Linquist, B.A.; van Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; van Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, S.; Fu, Z.; Chen, G.; Zou, G.; Song, X. A two-year field measurement of methane and nitrous oxide fluxes from rice paddies under contrasting climate conditions. Sci. Rep. 2016, 6, 28255. [Google Scholar] [CrossRef] [PubMed]
- Price, A.H.; Norton, G.J.; Salt, D.E.; Ebenhoeh, O.; Meharg, A.A.; Meharg, C.; Islam, R.M.; Sarma, R.N.; Dasgupta, T.; Isamil, A.M. Alternate wetting and drying irrigation for rice in Bangladesh: Is it sustainable and has plant breeding something to offer? Food Energy Secur. 2013, 2, 120–129. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Lundy, M.E.; Linquist, B.A. Rice yields and water use under alternate wetting and drying irrigation: A meta-analysis. Field Crops Res. 2017, 203, 173–180. [Google Scholar] [CrossRef]
- Siopongco, J.D.L.C.; Wassmann, R.; Sander, B.O. Alternate Wetting and Drying in Philippine Rice Production: Feasibility Study for a Clean Development Mechanism (No. 2215-2019-1632); International Rice Research Institute: Laguna, Philippines, 2013; p. 14, IRRI Tech. Bulletin No. 17. [Google Scholar]
- Linquist, B.A.; Anders, M.M.; Adviento-Borbe, M.A.A.; Chaney, R.L.; Nalley, L.L.; da Rosa, E.F.F.; van Kessel, C. Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Glob. Chang. Biol. 2015, 21, 407–417. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Crop management techniques to enhance harvest index in rice. J. Exp. Bot. 2010, 61, 3177–3189. [Google Scholar] [CrossRef]
- Yadav, S.; Humphreys, E.; Li, T.; Gurgeet, G.; Kukal, S.S. Evaluation of tradeoffs in land and water productivity of dry seeded rice as affected by irrigation schedule. Field Crops Res. 2012, 128, 180–190. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. An alternate wetting and moderate soil-drying regime improves root and shoot growth in rice. Crop Sci. 2009, 49, 2246–2260. [Google Scholar] [CrossRef]
- Volante, A.; Desiderio, F.; Tondelli, A.; Perrini, R.; Orasen, G.; Biselli, C.; Riccardi, P.; Vattani, A.; Cavalluzzo, D.; Urso, S.; et al. Genome-wide analysis of japonica rice performance under limited water and permanent flooding conditions. Front. Plant Sci. 2017, 8, 1862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, G.; Zhao, G.; Yao, H.; Xu, H. Variation in rice quality of different cultivars and grain positions as affected by water management. Field Crops Res. 2003, 80, 245–252. [Google Scholar] [CrossRef]
- Fofana, M.; Cherif, M.; Kone, B.; Futakuchi, K.; Audebert, A. Effect of water deficit at grain ripening stage on rice grain quality. J. Agric. Biotech. Sustain. Dev. 2010, 2, 100–107. [Google Scholar]
- Xu, Y.; Gu, D.; Li, K.; Zhang, W.; Zhang, H.; Wang, Z.; Yang, J. Response of grain quality to alternate wetting and moderate soil drying irrigation in rice. Crop Sci. 2019, 59, 1261–1272. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Factors influencing antioxidant compounds in rice. Crit. Rev. Food Sci. Nutr. 2017, 57, 893–922. [Google Scholar] [CrossRef] [PubMed]
- Bergman, C.J.; Xu, Z. Genotype and environment effects on tocopherol, tocotrienol, and γ-oryzanol contents of southern U.S. rice. Cereal Chem. 2003, 80, 446–449. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumierd, G.; Ben Ghozlena, N.; Latouchea, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Biselli, C.; Cavalluzzo, D.; Perrini, R.; Gianinetti, A.; Bagnaresi, P.; Urso, S.; Orasen, G.; Desiderio, F.; Lupotto, E.; Cattivelli, L.; et al. Improvement of marker-based predictability of Apparent Amylose Content in japonica rice through GBSSI allele mining. Rice 2014, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169–1173. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonoids and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their colour properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Bergman, C.J. A rapid procedure for analysing rice bran tocopherol, tocotrienol and γ-oryzanol contents. J. Food Compos. Anal. 2005, 18, 139–151. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, C.; Saghai Maroof, M.A.; Biyashev, R.M.; Grabau, E.A.; Kwanyuen, P.; Burton, J.W.; Buss, G.R. A modified colorimetric method for phytic acid analysis in soybean. Crop Sci. 2007, 47, 1797–1803. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Confalonieri, R.; Mariani, L.; Bocchi, S. Analysis and modelling of water and near water temperature in flooded rice (Oryza sativa, L.). Ecol. Modell. 2005, 183, 269–280. [Google Scholar] [CrossRef]
- Yoshida, S.; Coronel, V. Nitrogen nutrition, leaf resistance, and leaf photosynthetic rate of the rice plant. Soil Sci. Plant Nutr. 1976, 22, 207–211. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B.F. Molecular and environmental factors determining grain quality in rice. Food Energy Secur. 2012, 1, 111–132. [Google Scholar] [CrossRef]
- Britz, S.J.; Prasad, P.V.V.; Moreau, R.A., Jr.; Allen, L.H.; Kremer, D.F.; Boote, K.J. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice. J. Agric. Food Chem. 2007, 55, 7559–7565. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hua, X.; Guo, J.; Qi, D.; Wang, L.; Liu, Z.; Jin, Z.; Chen, S.; Liu, G. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis Thaliana. Biotechnol. Lett. 2008, 30, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Matsuzuka, K.; Kimura, E.; Nakagawa, K.; Murata, K.; Kimura, T.; Miyazawa, T. Investigation of tocotrienol biosynthesis in rice (Oryza sativa L.). Food Chem. 2013, 140, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Matsukawa, T.; Horibata, A. Quantitative trait loci responsible for the difference in γ-oryzanol content in brown rice between japonica-type and indica-type rice cultivars. Plant Prod. Sci. 2017, 20, 459–466. [Google Scholar] [CrossRef]
- Perera, I.; Seneweera, S.; Hirotsu, N. Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice 2018, 11, 4–17. [Google Scholar] [CrossRef]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef]
- Fageria, N.K.; Slaton, N.A.; Baligar, V.C. Nutrient Management for Improving Lowland Rice Productivity and Sustainability. Adv. Agron. 2003, 80, 63–152. [Google Scholar]
- Weber, F.-A.; Voegelin, A.; Kaegi, R.; Kretzschmar, R. Contaminant mobilization by metallic copper and metal sulphide colloids in flooded soil. Nat. Geosci. 2009, 2, 267–271. [Google Scholar] [CrossRef]
- Hu, P.; Li, Z.; Yuan, C.; Ouyang, Y.; Zhou, L.; Huang, J.; Huang, Y.; Luo, Y.; Christie, P.; Wu, L. Effect of water management on cadmium and arsenic accumulation by rice (Oryza sativa L.) with different metal accumulation capacities. J. Soils Sediments 2013, 13, 916–924. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M. Redox chemistry of nickel in soils and sediments: A review. Chemosphere 2017, 179, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.M.H.; Turner, F.T. Effect of redox potential on manganese transformation in waterlogged soil. Nature 1968, 220, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Silveira, S.F.D.S.; Daloso, D.M.; Mendes, G.C.; Merchant, A.; Kuki, K.N.; Oliva, M.A.; Loureiro, M.E.; Almeida, A.M. Ecophysiological responses to excess iron in lowland and upland rice cultivars. Chemosphere 2017, 189, 123–133. [Google Scholar] [CrossRef]
- Norton, G.J.; Travis, A.J.; Danku, J.M.; Salt, D.E.; Hossain, M.; Islam, M.R.; Price, A.H. Biomass and elemental concentrations of 22 rice cultivars grown under alternate wetting and drying conditions at three field sites in Bangladesh. Food Energy Secur. 2017, 6, 98–112. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Akbar, N.; Reis, A.F.; Li, C.; Gaudin, A.C.; Parikh, S.J.; Green, P.G.; Linquist, B.A. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics. Field Crops Res. 2018, 222, 101–110. [Google Scholar] [CrossRef]
- Chaney, R.L. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr. Pollution Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Briat, J.F. Arsenic tolerance in plants: “Pas de deux” between phytochelatin synthesis and ABCC vacuolar transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 20853–20854. [Google Scholar] [CrossRef]
| Trait | Year | cv. ‘Baldo’ | cv. ‘Gladio’ | cv. ‘Loto’ | |||
|---|---|---|---|---|---|---|---|
| CF | AWD | CF | AWD | CF | AWD | ||
| Flowering time, d | 2012 | 91bcd | 91bcd | 83e | 88cde | 87de | 87de |
| 2013 | 96b | 105a | 94bc | 110a | 85e | 97b | |
| Flowering-maturing time, d | 2012 | 56a | 52abc | 45bc | 42c | 50abc | 49abc |
| 2013 | 59ab | 58ab | 57abc | 48abc | 62a | 63a | |
| Linear yield, kg m−1 | 2012 | 319ab | 254cd | 201ef | 165f | 222de | 191ef |
| 2013 | 327a | 276bc | 247cd | 169f | 261cd | 264cd | |
| Fertile tillers m−1, n | 2012 | 66c | 53c | 81abc | 74abc | 92abc | 67bc |
| 2013 | 65c | 84abc | 93abc | 119a | 86abc | 112ab | |
| Seeds panicle−1, n | 2012 | 114ab | 100bcde | 107abc | 84fg | 92def | 85fg |
| 2013 | 115a | 95cdef | 103abcd | 66e | 86efg | 76ge | |
| Weight of 100 grains, g | 2012 | 3.70a | 3.20b | 2.12f | 1.90fg | 2.65de | 2.46e |
| 2013 | 3.65a | 3.05bc | 2.11f | 1.71g | 2.82cd | 2.51e | |
| Grain length, mm | 2012 | 7.26ab | 7.09abc | 7.46a | 6.77bcd | 6.53de | 6.40de |
| 2013 | 7.55a | 6.89bcd | 7.45a | 6.82bcd | 6.59cde | 6.26e | |
| Grain width, mm | 2012 | 3.28a | 3.20a | 2.19d | 2.46cd | 3.00ab | 2.76bc |
| 2013 | 3.23a | 2.99ab | 2.11d | 2.21d | 2.99ab | 2.88abc | |
| Trait | Year | cv. ‘Baldo’ | cv. ‘Gladio’ | cv. ‘Loto’ | |||
|---|---|---|---|---|---|---|---|
| CF | AWD | CF | AWD | CF | AWD | ||
| CHL | 2012 | 36.52ab | 23.10b | 29.08ab | 27.56ab | 30.27ab | 33.03ab |
| 2013 | 30.71ab | 30.50ab | 41.12a | 31.43ab | 38.04a | 33.20ab | |
| NBI | 2012 | 28.93a | 16.47b | 20.93ab | 20.27ab | 23.05ab | 23.48ab |
| 2013 | 17.73b | 20.31ab | 25.84ab | 19.69ab | 25.91ab | 22.15ab | |
| Apparent amylose content (%) | 2012 | 18.57cd | 16.91def | 24.36a | 21.30bc | 14.22f | 16.28def |
| 2013 | 17.52de | 19.06cd | 22.91ab | 25.86a | 16.94def | 14.76ef | |
| N-protein content (%) | 2012 | 7.14abc | 6.81bc | 6.67bc | 7.11abc | 7.90ab | 7.41abc |
| 2013 | 6.14c | 8.33a | 7.60ab | 7.31abc | 7.53ab | 8.22a | |
| Trait | Year | cv. ‘Baldo’ | cv. ‘Gladio’ | cv. ‘Loto’ | |||
|---|---|---|---|---|---|---|---|
| CF | AWD | CF | AWD | CF | AWD | ||
| Total Flavonoids, g RE kg−1 | 2012 | 1.34f | 2.08b | 2.01bc | 2.52a | 1.67de | 2.09b |
| 2013 | 1.44ef | 1.93bcd | 1.86bcd | 2.39a | 1.75cd | 2.41a | |
| Total Tocols, mg kg−1 | 2012 | 14.26d | 33.85ab | 15.54d | 14.28d | 11.14d | 27.40bc |
| 2013 | 17.03d | 28.62b | 18.61cd | 19.42cd | 17.34d | 38.58a | |
| γ-oryzanol, mg kg−1 | 2012 | 54.10cd | 57.00cd | 44.21d | 57.57cd | 49.95cd | 91.87ab |
| 2013 | 60.44bcd | 78.47abc | 42.09d | 33.87d | 60.43bcd | 92.97a | |
| Phytic acid, g kg−1 | 2012 | 10.03a | 10.49a | 10.82a | 11.30a | 10.71a | 10.23a |
| 2013 | 10.44a | 10.87a | 8.63a | 9.12a | 9.71a | 10.29a | |
| Antioxidant Activity, mmol TEAC kg−1 | 2012 | 5.22de | 5.39bcde | 6.24ab | 6.15abc | 3.92fg | 4.80ef |
| 2013 | 4.91e | 5.33cde | 6.31a | 5.92abcd | 3.40g | 4.71ef | |
| Tocochromanol | Year | CF | AWD | CF | AWD | CF | AWD |
|---|---|---|---|---|---|---|---|
| αT3, mg kg−1 | 2012 | 0.67cd | 1.39cd | 0.35d | 0.56cd | 3.95b | 2.25bc |
| 2013 | 0.50d | 5.95a | 0.56cd | 0.68cd | 0.55cd | 3.30b | |
| γT3, mg kg−1 | 2012 | 11.08bc | 23.64a | 10.27bc | 10.17bc | 6.84c | 25.60a |
| 2013 | 10.55bc | 16.06b | 13.39b | 14.55b | 12.80b | 27.80a | |
| δT3, mg kg−1 | 2012 | 0.53de | 1.69c | 0.27e | 1.13cde | 0.89cde | 2.71b |
| 2013 | 1.55c | 1.44c | 1.38cd | 0.98cde | 1.29cd | 3.88a | |
| αT, mg kg−1 | 2012 | 0.09c | 0.04c | 0.67bc | 0.39bc | 0.21c | 0.84bc |
| 2013 | 0.77bc | 1.46ab | 0.98bc | 0.74bc | 0.87bc | 2.25a | |
| γT, mg kg−1 | 2012 | 0.09e | 2.45b | 0.96cd | 0.88cd | 0.93cd | 3.49a |
| 2013 | 2.47b | 1.19c | 1.00cd | 1.04c | 0.46de | 0.83cd | |
| δT, mg kg−1 | 2012 | 0.92c | 0.27d | 0.11d | 0.09d | 0.31d | 1.39b |
| 2013 | 0.26d | 2.23a | 0.20d | nd | nd | nd |
| Component | Year | cv. ‘Baldo’ | cv. ‘Gladio’ | cv. ‘Loto’ | |||
|---|---|---|---|---|---|---|---|
| CF | AWD | CF | AWD | CF | AWD | ||
| CAF, mg kg−1 | 2012 | 5.59ab | 5.65ab | 6.34ab | 4.18ab | 4.00ab | 4.89ab |
| 2013 | 3.83ab | 6.11ab | 4.94ab | 1.40b | 7.14a | 9.05a | |
| 24Me-CAF, mg kg−1 | 2012 | 31.05bcd | 34.26bcd | 23.24cd | 26.94cd | 25.05cd | 59.85a |
| 2013 | 32.89bcd | 40.20abc | 21.62cd | 15.03d | 29.16cd | 49.97ab | |
| CSF, mg kg−1 | 2012 | 17.42c | 15.40c | 16.37c | 15.44c | 17.82c | 27.97b |
| 2013 | 23.27bc | 33.23ab | 15.24c | 15.50c | 24.43bc | 40.09a | |
| Element | Year | cv. ‘Baldo’ | cv. ‘Gladio’ | cv. ‘Loto’ | |||
|---|---|---|---|---|---|---|---|
| CF | AWD | CF | AWD | CF | AWD | ||
| K, g kg−1 | 2012 | 2.72def | 3.04abc | 2.86bcdef | 2.74def | 3.13a | 2.91abcde |
| 2013 | 2.81cdef | 2.67f | 3.09ab | 2.70ef | 2.94abcd | 2.71def | |
| P, g kg−1 | 2012 | 3.50efg | 3.62defg | 4.05bc | 4.01bc | 4.22ab | 3.97bcd |
| 2013 | 3.33g | 3.44fg | 4.47a | 3.80cdef | 3.86bcde | 3.77cdef | |
| Ca, mg kg−1 | 2012 | 80.21abc | 66.18e | 68.32de | 73.75cde | 82.72abc | 79.60abcd |
| 2013 | 75.11bcde | 65.95e | 85.94ab | 80.61abc | 87.53a | 78.80abcd | |
| Cu, mg kg−1 | 2012 | 2.80de | 4.90a | 3.31cde | 5.10a | 3.32cde | 4.82a |
| 2013 | 2.39e | 4.30abc | 3.70bcd | 4.81a | 2.73de | 4.42ab | |
| Fe, mg kg−1 | 2012 | 5.65d | 6.22cd | 10.12ab | 9.83ab | 8.73abc | 7.68bcd |
| 2013 | 7.61bcd | 8.24abcd | 10.63a | 9.20ab | 9.01ab | 10.60a | |
| Mg, g kg−1 | 2012 | 1.16f | 1.27def | 1.33bcd | 1.31cde | 1.49a | 1.44ab |
| 2013 | 1.20ef | 1.18f | 1.47a | 1.32cd | 1.38abcd | 1.41abc | |
| Mn, mg kg−1 | 2012 | 15.05c | 7.94e | 17.00bc | 14.69c | 19.54ab | 11.31d |
| 2013 | 15.62c | 8.23de | 17.91abc | 10.25de | 21.12a | 16.51bc | |
| Ni, mg kg−1 | 2012 | 0.40ef | 1.40cd | 0.71e | 2.16ab | 0.19f | 1.26d |
| 2013 | 0.21ef | 1.58cd | 0.49ef | 2.57a | 0.25ef | 1.79bc | |
| Zn, mg kg−1 | 2012 | 22.64ab | 24.38a | 22.16abc | 23.63a | 25.13a | 23.96a |
| 2013 | 24.28a | 19.24c | 24.00a | 20.18bc | 22.62ab | 19.14c | |
| As, µg kg−1 | 2012 | 148.00ab | 79.60de | 170.21a | 63.42e | 146.19abc | 86.09de |
| 2013 | 107.38cd | 18.68f | 109.71bcd | 10.75f | 119.43bcd | 20.84f | |
| Cd, µg kg−1 | 2012 | 10.52g | 99.92a | 26.28de | 53.26b | 12.83fg | 43.48bc |
| 2013 | 19.52efg | 39.14cd | 12.83fg | 25.17ef | 11.63g | 29.93de | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orasen, G.; De Nisi, P.; Lucchini, G.; Abruzzese, A.; Pesenti, M.; Maghrebi, M.; Kumar, A.; Nocito, F.F.; Baldoni, E.; Morgutti, S.; et al. Continuous Flooding or Alternate Wetting and Drying Differently Affect the Accumulation of Health-Promoting Phytochemicals and Minerals in Rice Brown Grain. Agronomy 2019, 9, 628. https://doi.org/10.3390/agronomy9100628
Orasen G, De Nisi P, Lucchini G, Abruzzese A, Pesenti M, Maghrebi M, Kumar A, Nocito FF, Baldoni E, Morgutti S, et al. Continuous Flooding or Alternate Wetting and Drying Differently Affect the Accumulation of Health-Promoting Phytochemicals and Minerals in Rice Brown Grain. Agronomy. 2019; 9(10):628. https://doi.org/10.3390/agronomy9100628
Chicago/Turabian StyleOrasen, Gabriele, Patrizia De Nisi, Giorgio Lucchini, Alessandro Abruzzese, Michele Pesenti, Moez Maghrebi, Ajay Kumar, Fabio Francesco Nocito, Elena Baldoni, Silvia Morgutti, and et al. 2019. "Continuous Flooding or Alternate Wetting and Drying Differently Affect the Accumulation of Health-Promoting Phytochemicals and Minerals in Rice Brown Grain" Agronomy 9, no. 10: 628. https://doi.org/10.3390/agronomy9100628
APA StyleOrasen, G., De Nisi, P., Lucchini, G., Abruzzese, A., Pesenti, M., Maghrebi, M., Kumar, A., Nocito, F. F., Baldoni, E., Morgutti, S., Negrini, N., Valè, G., & Sacchi, G. A. (2019). Continuous Flooding or Alternate Wetting and Drying Differently Affect the Accumulation of Health-Promoting Phytochemicals and Minerals in Rice Brown Grain. Agronomy, 9(10), 628. https://doi.org/10.3390/agronomy9100628






