Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization
Abstract
1. Introduction
2. Materials and Methods
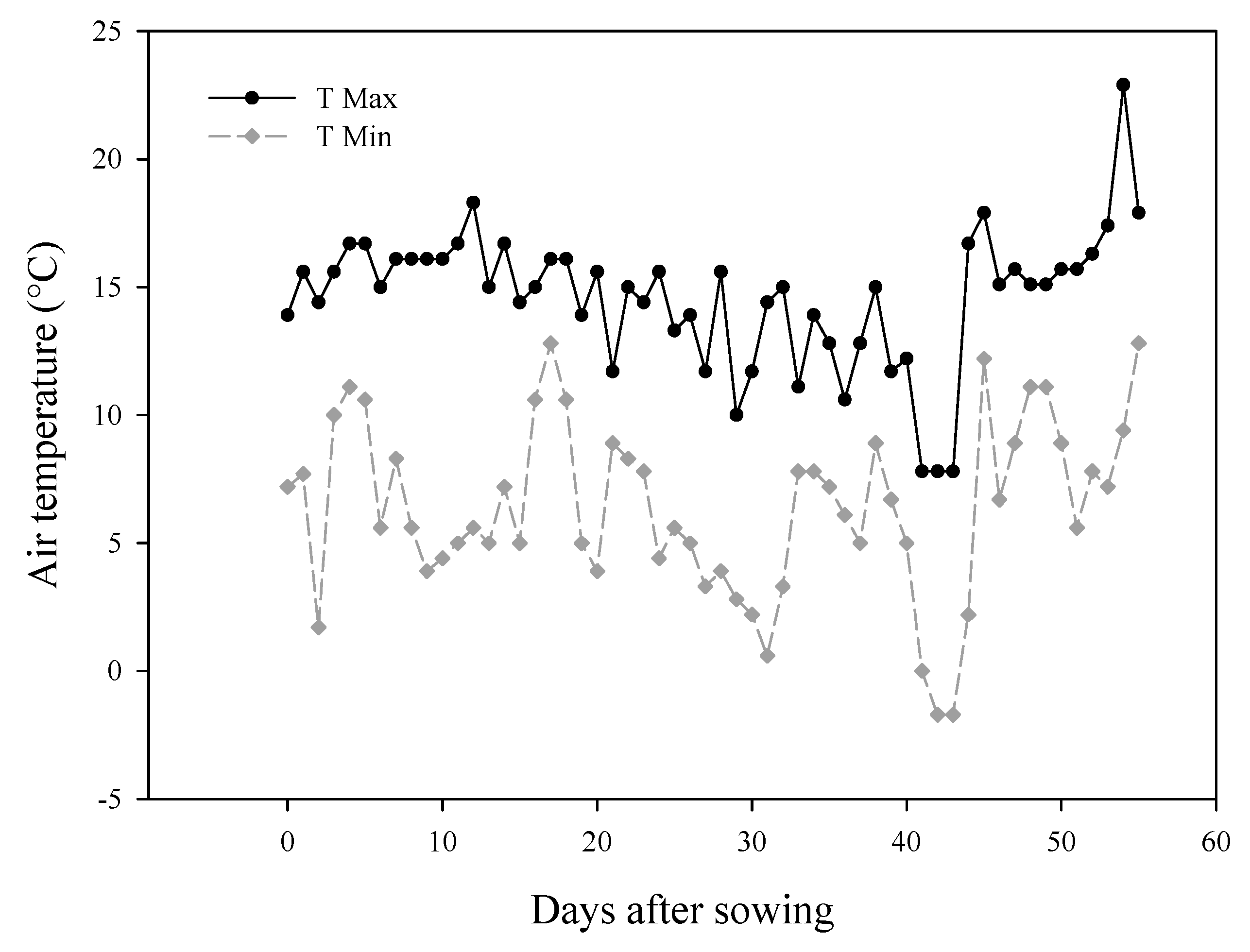
2.1. Experimental Setting, Plant Material and Design
2.2. Nitrogen Fertilization Levels, Cultural Practices and Biostimulants Application
2.3. Plant Growth Parameters, Marketable Yield, Leaf Colorimetry and Sampling
2.4. Antioxidant Capacity Analysis
2.5. Chlorophyllous Pigments and Nitrate Analysis
2.6. Total Ascorbic Acid Analysis
2.7. Statistical Processing
3. Results and Discussion
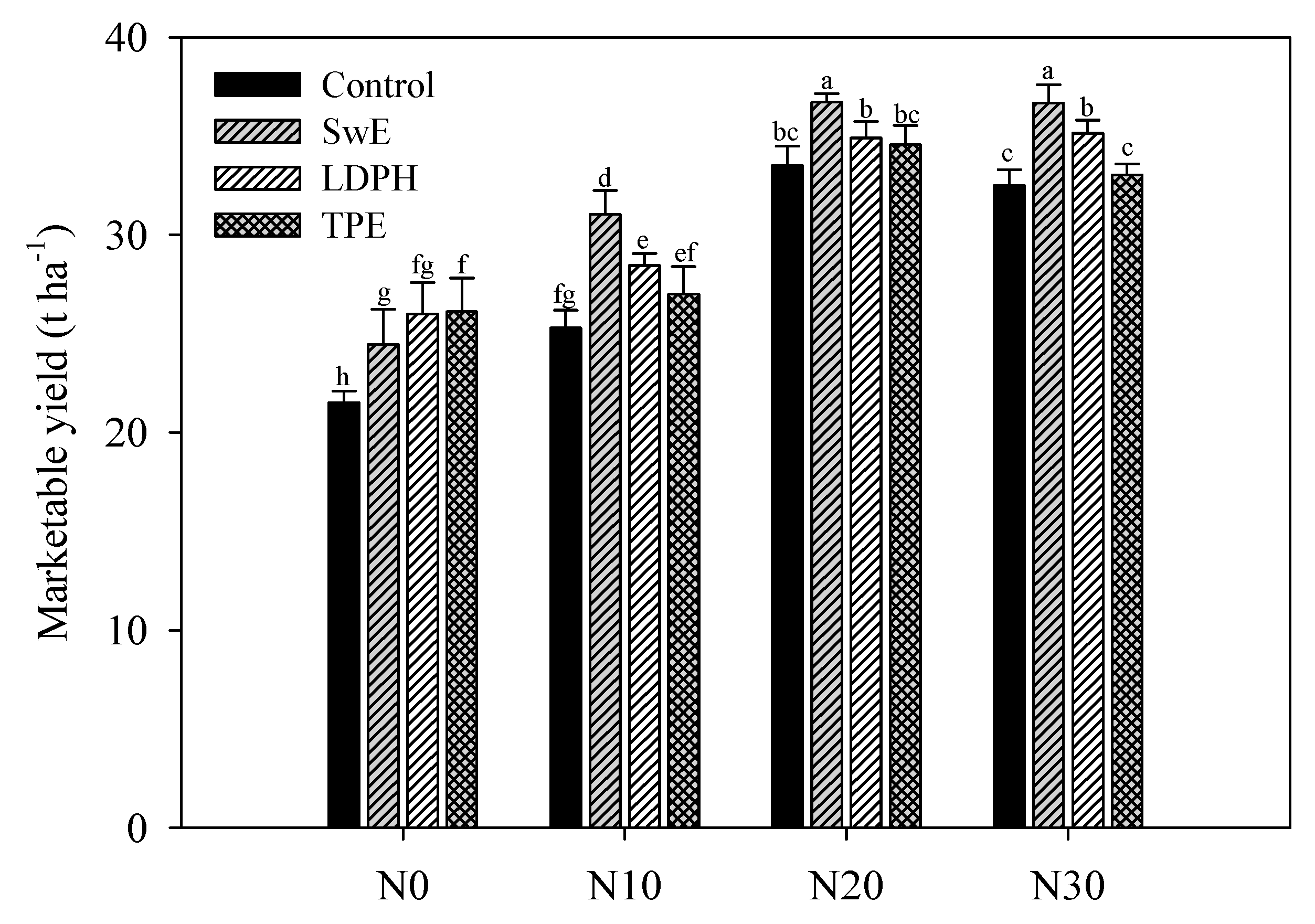
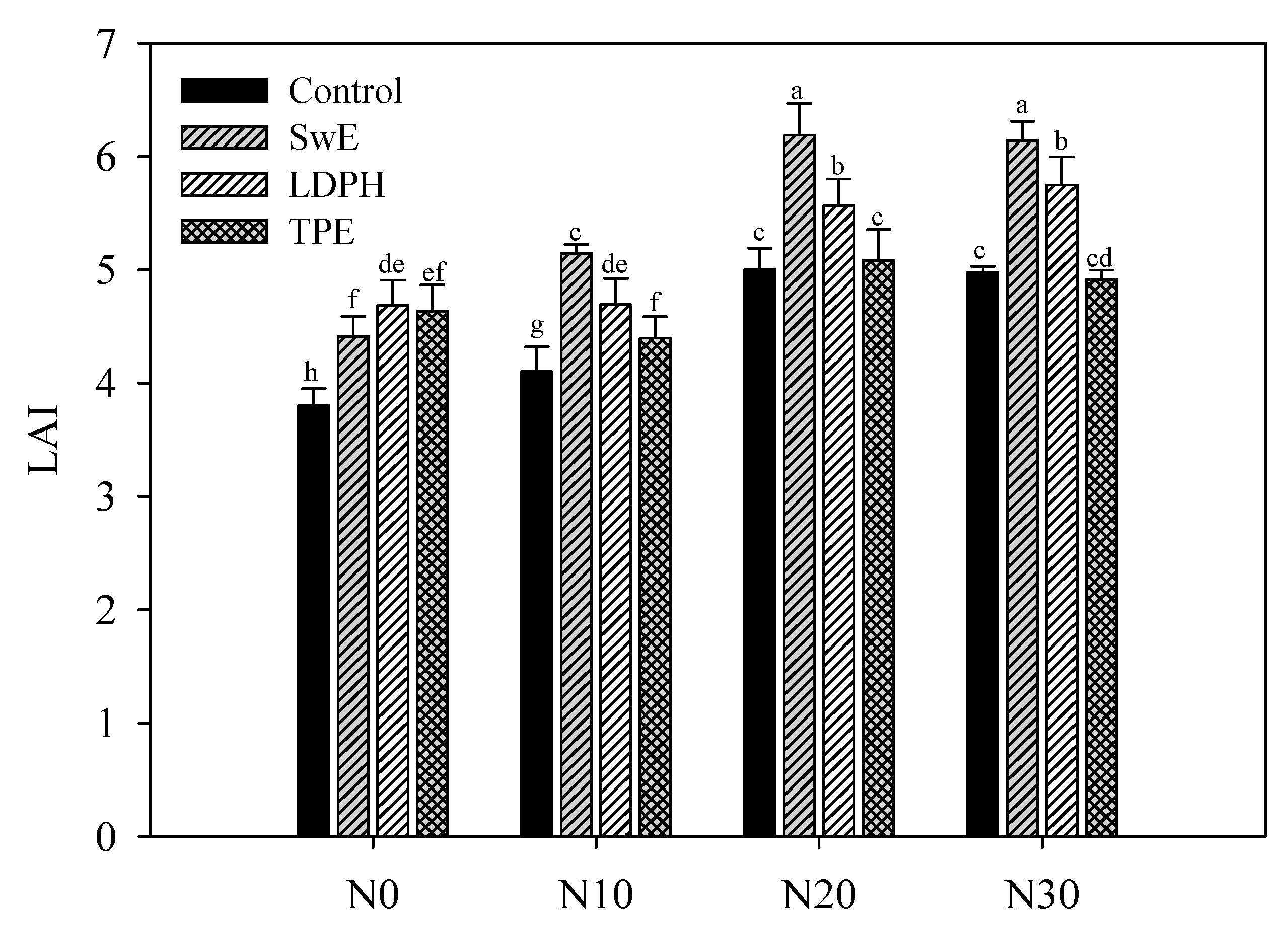
3.1. Effect of N Fertilization Levels and Biostimulant Application on Yield and Growth
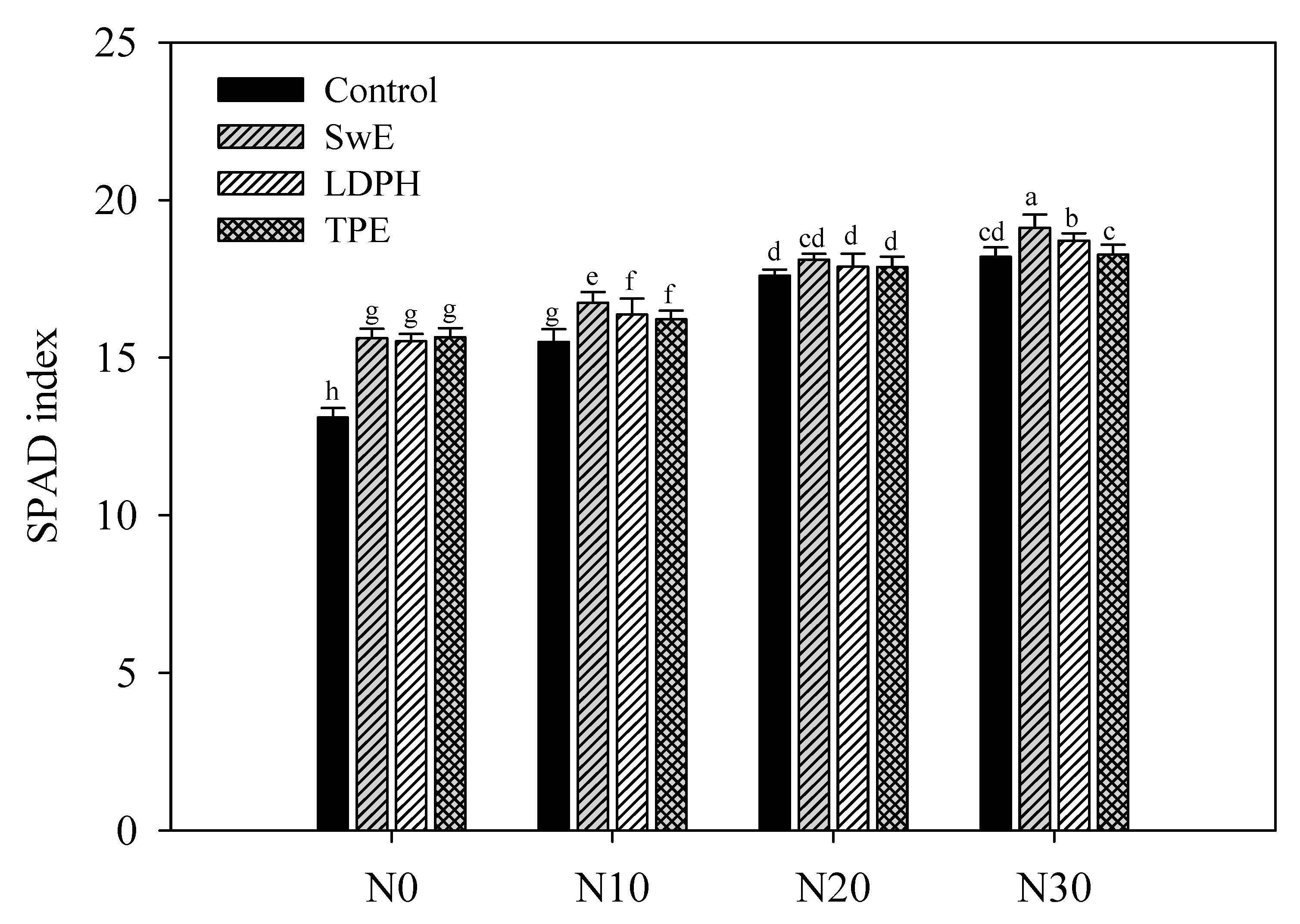
3.2. Effect of N Fertilization Levels and Biostimulant Application on Leaf Colorimetry and SPAD Index
3.3. Effect of N Fertilization Levels and Biostimulant Application on Nitrate Accumulation and Biochemical Parameters
3.4. Effect of N Fertilization Levels and Biostimulant Application on Antioxidant Capacity and Bioactive Content
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, M.; Renjel Encinas, S.; Jensen, O.C.; Andersen, J.H.; Condarco, G.; Jørs, E. Pesticide residues in commercial lettuce, onion, and potato samples from Bolivia—A threat to public health? Environ. Health Insights 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; El-Nakhel, C.; De Pascale, S. The Influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral composition of Brassica rapa L. subsp. sylvestris under nutrient stress conditions. Eur. J. Hortic. Sci. 2017, 82, 286–293. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 64, 145–158. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2013, 1009, 29–35. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.; De Pascale, S.; Cozzolino, E.; Pannico, A.; Giordano, M.; Teliban, G.; Cuciniello, A.; Rouphael, Y. Production, leaf quality and antioxidants of perennial wall rocket as affected by crop cycle and mulching type. Agronomy 2019, 9, 194. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of Vesuvian Piennolo tomato PDO as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; El-Shemy, H., Ed.; InTech Europe Publisher: Rijeka, Croatia, 2011; pp. 215–230. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Lucini, L.; Canaguier, R.; Stefanoni, W.; Fiorillo, A.; Cardarelli, M. Protein hydrolysate-based biostimulants: Origin, biological activity and application methods. Acta Hortic. 2016, 1148, 27–34. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Sady, W.; Smoleń, S. The Influence of Pentakeep V and Nitrogen Fertilization on the Yield and Biological Quality of Carrot and Spinach Crop; Final Report for Cosmo Oil Co., Ltd.; Cosmo Oil Co., Ltd.: Tokio, Japan, 2007. [Google Scholar]
- Smoleń, S.; Sady, W. The influence of nitrogen fertilization and Pentakeep V application on contents of nitrates in carrot. Acta Hortic. Regiotec. 2009, 12, 221–223. [Google Scholar]
- Smoleń, S.; Sady, W.; Wierzbińska, J. The effect of plant biostimulation with ‘Pentakeep V’ and nitrogen fertilization on yield, nitrogen metabolism and quality of spinach. Acta Sci. Pol. Hortorum Cultus 2010, 9, 25–36. [Google Scholar]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carote-Noids–Measurement and Characterisation by UV-VIS.–Cur-rent Protocols in Food Analytical Chemistry (CPFA), (Sup-plement 1), F4.3.1–F4.3.8; John Wiley: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Sah, R.N. Nitrate-nitrogen determination: A critical review. Commun. Soil Sci. Plant Anal. 1994, 25, 2841–2869. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S.A. Novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Szczepanek, M.; Siwik-Ziomek, A. P and K accumulation by rapeseed as affected by biostimulant under different NPK and S fertilization doses. Agronomy 2019, 9, 477. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.M.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum Lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Tsouvaltzis, P.; Koukounaras, A.; Siomos, S.A. Application of amino acids improves lettuce crop uniformity and inhibits nitrate accumulation induced by the supplemental inorganic nitrogen fertilization. Int. J. Agric. Biol. 2014, 16, 951–955. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 231–238. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Z.H.; Hayat, S.; Ahmad, H.; Ghani, M.I.; LIU, T. Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 2019, 18, 1001–1013. [Google Scholar] [CrossRef]
- Tadros, M.J.; Omari, H.J.; Turk, M.A. The morphological, physiological and biochemical responses of sweet corn to foliar application of amino acids biostimulants sprayed at three growth stages. Aust. J. Crop Sci. 2019, 13, 412–417. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Fun. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale food production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Li, S.X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Vasantharaja, R.; Stanley Abraham, L.; Inbakandan, D.; Thirugnanasambandam, R.; Senthilvelan, T.; Ayesha Jabeen, S.K.; Prakash, P. Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal. Agric. Biotechnol. 2019, 17, 589–594. [Google Scholar] [CrossRef]
| Soil Properties | Units | Mean Values |
|---|---|---|
| Texture | ||
| Coarse sand | % | 69.1 |
| Fine sand | % | 21.9 |
| Silt | % | 4.5 |
| Clay | % | 4.5 |
| Chemical properties | ||
| pH | - | 6.54 |
| Electrical conductivity | dS·m−1 | 0.64 |
| Organic matter | g·kg−1 | 32.4 |
| Total N (Kjeldahl method) | g·kg−1 | 1.2 |
| P2O5 (Olsen method) | mg·kg−1 | 312.8 |
| K2O (Tetraphenylborate method) | mg·kg−1 | 620.7 |
| NO3-N | mg·kg−1 | 10.0 |
| NH4-N | mg·kg−1 | 9.0 |
| Treatments | Succulence | SLW |
|---|---|---|
| (mg H2O·cm−2) | (mg dm·cm−2) | |
| Fertilization (F) | ||
| N0 | 59.9 b | 2.99 a |
| N10 | 71.5 a | 3.26 a |
| N20 | 69.9 a | 2.66 b |
| N30 | 67.7 a | 2.60 b |
| Biostimulant (B) | ||
| Control | 60.5 c | 2.94 a |
| SwE | 65.2 b | 2.56 b |
| LDPH | 70.4 a | 2.92 a |
| TPE | 72.9 a | 3.10 a |
| Significance | ||
| F | ** | ** |
| B | * | * |
| F × B | NS | NS |
| Table | L* | a* | b* |
|---|---|---|---|
| Fertilization (F) | |||
| N0 | 55.9 d | −20.8 a | 39.8 b |
| N10 | 56.7 c | −21.2 a | 40.1 b |
| N20 | 58.7 b | −22.7 b | 41.8 a |
| N30 | 59.5 a | −22.7 b | 42.4 a |
| Biostimulants (B) | |||
| Control | 56.5 c | −21.5 | 39.8 b |
| SwE | 58.5 a | −22.2 | 41.6 a |
| LDPH | 57.5 b | −21.8 | 41.0 a |
| TPE | 58.3 a | −21.9 | 41.6 a |
| Significance | |||
| F | ** | * | * |
| B | * | NS | * |
| F × B | NS | NS | NS |
| Treatments | Nitrate | Chlorophyll a | Chlorophyll b | Total chlorophyll | Carotenoids |
|---|---|---|---|---|---|
| (mg·kg−1 fw) | (mg·g−1 fw) | (mg·g−1 fw) | (mg·g−1 fw) | (µg·g−1 fw) | |
| Fertilization (F) | |||||
| N0 | 703.3 d | 0.298 b | 0.211 | 0.508 b | 156 b |
| N10 | 1476.0 c | 0.330 a | 0.210 | 0.540 a | 178 a |
| N20 | 6206.7 b | 0.334 a | 0.201 | 0.535 a | 170 a |
| N30 | 7288.1 a | 0.338 a | 0.209 | 0.546 a | 170 a |
| Biostimulants (B) | |||||
| Control | 3366.5 b | 0.302 b | 0.191 c | 0.493 b | 155 c |
| SwE | 4467.6 a | 0.319 b | 0.192 c | 0.511 b | 181 a |
| LDPH | 3504.4 b | 0.342 a | 0.214 b | 0.556 a | 173 ab |
| TPE | 4426.5 a | 0.337 a | 0.232 a | 0.569 a | 164 bc |
| Significance | |||||
| F | ** | * | NS | * | * |
| B | * | * | ** | * | * |
| F × B | NS | NS | NS | NS | NS |
| Treatments | LAA | HAA | TAA | |
|---|---|---|---|---|
| mmol Trolox eq. 100 g−1 dw | mmol ascorbic acid eq. 100 g−1 dw | mg g−1 fw | ||
| N0 | Control | 26.2 b | 6.7 c | 25.0 b |
| SwE | 27.6 b | 6.6 c | 33.4 a | |
| LDPH | 32.3 a | 8.2 a | 18.0 c | |
| TPE | 25.2 b | 7.2 bc | 18.6 c | |
| N10 | Control | 21.0 c | 7.6 b | 17.3 c |
| SwE | 20.4 c | 7.5 b | 16.8 c | |
| LDPH | 20.9 c | 7.7 b | 18.1 c | |
| TPE | 30.9 a | 7.6 b | 16.9 c | |
| N20 | Control | 22.0 c | 4.9 d | 14.0 de |
| SwE | 22.2 c | 4.8 d | 14.3 d | |
| LDPH | 19.9 c | 4.4 d | 12.6 de | |
| TPE | 22.1 c | 3.0 e | 14.5 d | |
| N30 | Control | 21.8 c | 3.3 e | 13.2 de |
| SwE | 20.1 c | 3.2 e | 11.7 e | |
| LDPH | 21.9 c | 4.5 d | 12.6 de | |
| TPE | 21.5 c | 3.4 e | 6.8 f | |
| Significance | ||||
| Fertilization (F) | ** | ** | ** | |
| Biostimulant (B) | * | * | ** | |
| F × B | ** | ** | ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. https://doi.org/10.3390/agronomy9100571
Di Mola I, Cozzolino E, Ottaiano L, Giordano M, Rouphael Y, Colla G, Mori M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy. 2019; 9(10):571. https://doi.org/10.3390/agronomy9100571
Chicago/Turabian StyleDi Mola, Ida, Eugenio Cozzolino, Lucia Ottaiano, Maria Giordano, Youssef Rouphael, Giuseppe Colla, and Mauro Mori. 2019. "Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization" Agronomy 9, no. 10: 571. https://doi.org/10.3390/agronomy9100571
APA StyleDi Mola, I., Cozzolino, E., Ottaiano, L., Giordano, M., Rouphael, Y., Colla, G., & Mori, M. (2019). Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy, 9(10), 571. https://doi.org/10.3390/agronomy9100571










