Complete Chloroplast Genome Sequence of Broomcorn Millet (Panicum miliaceum L.) and Comparative Analysis with Other Panicoideae Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Chloroplast Genome Assembly and Sequence Analysis of Broomcorn Millet
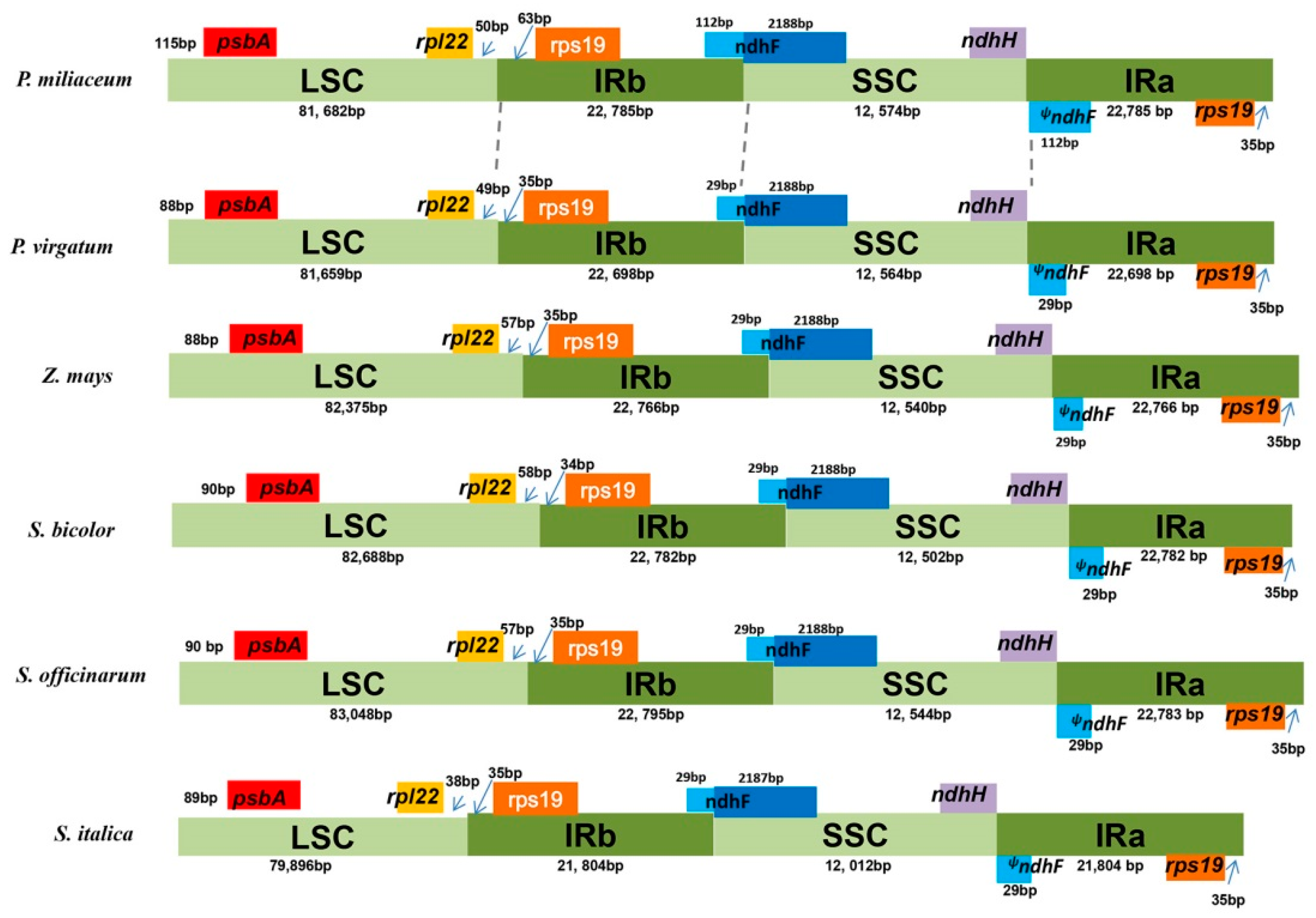
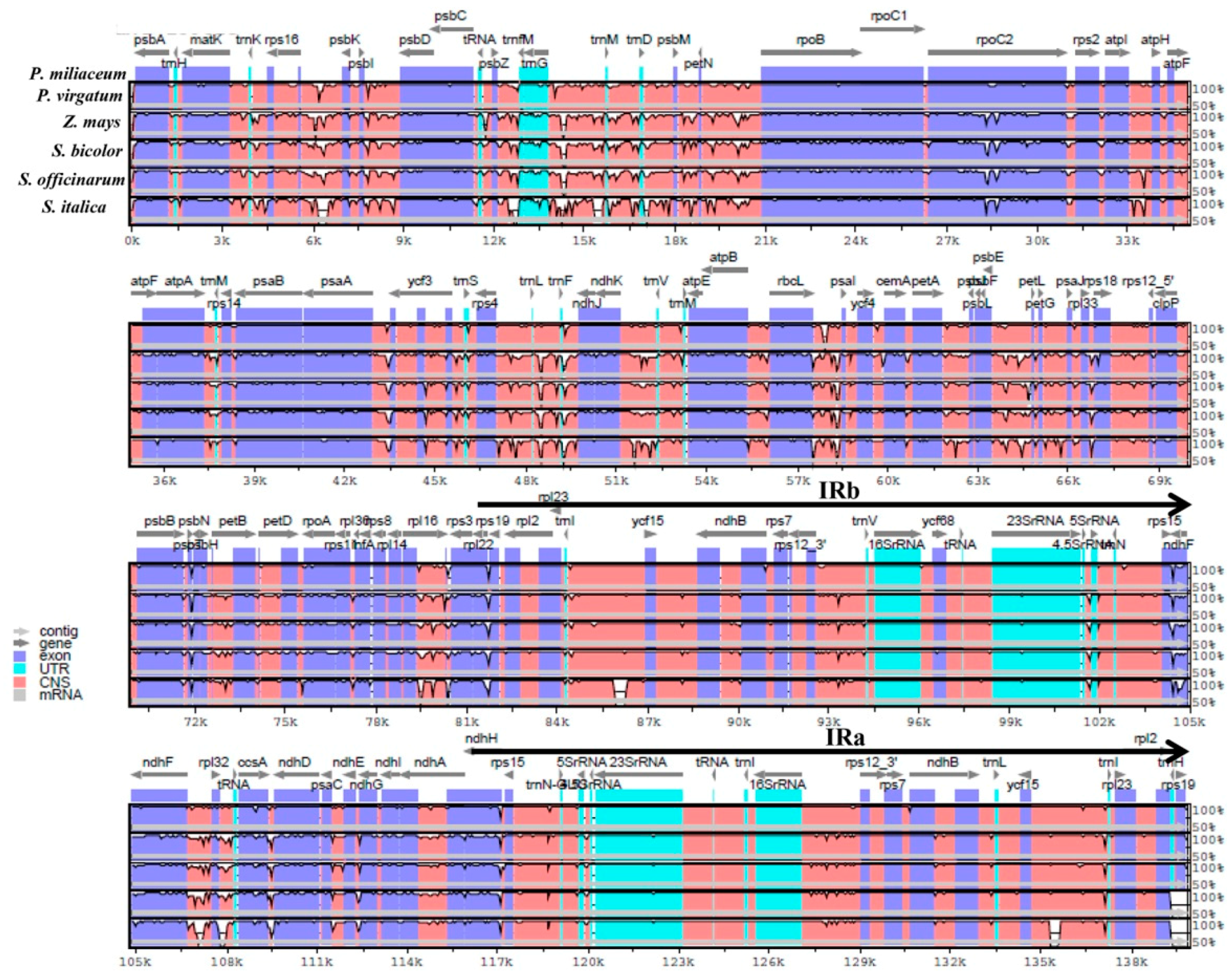
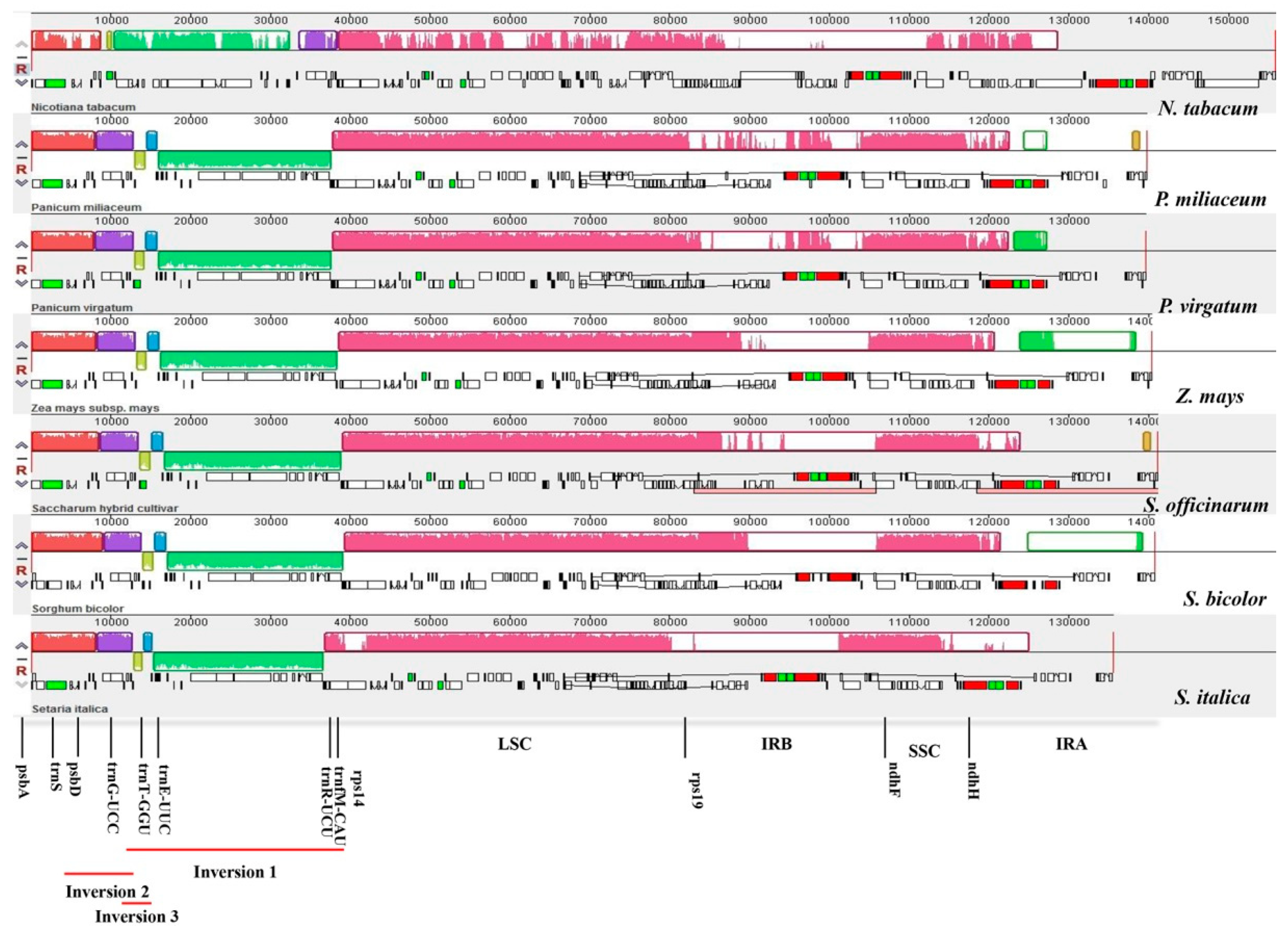
2.2. Comparison of cp Genomes between Broomcorn Millet and Other Five Panicoideae Species
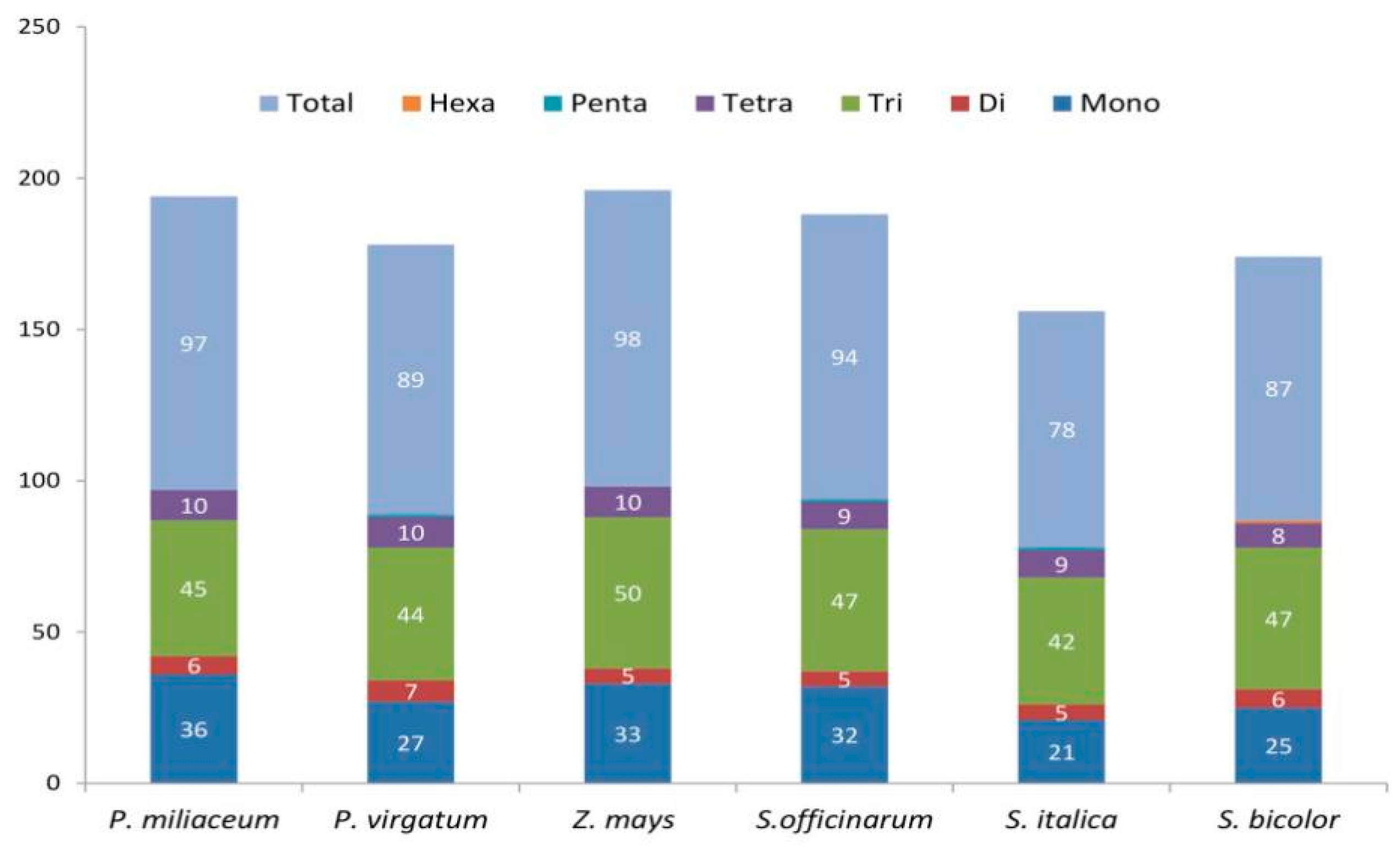
2.3. SSR Loci Identified in Panicoideae cp Genomes
2.4. Prediction of RNA Editing Sites in Panicoideae cp Genomes
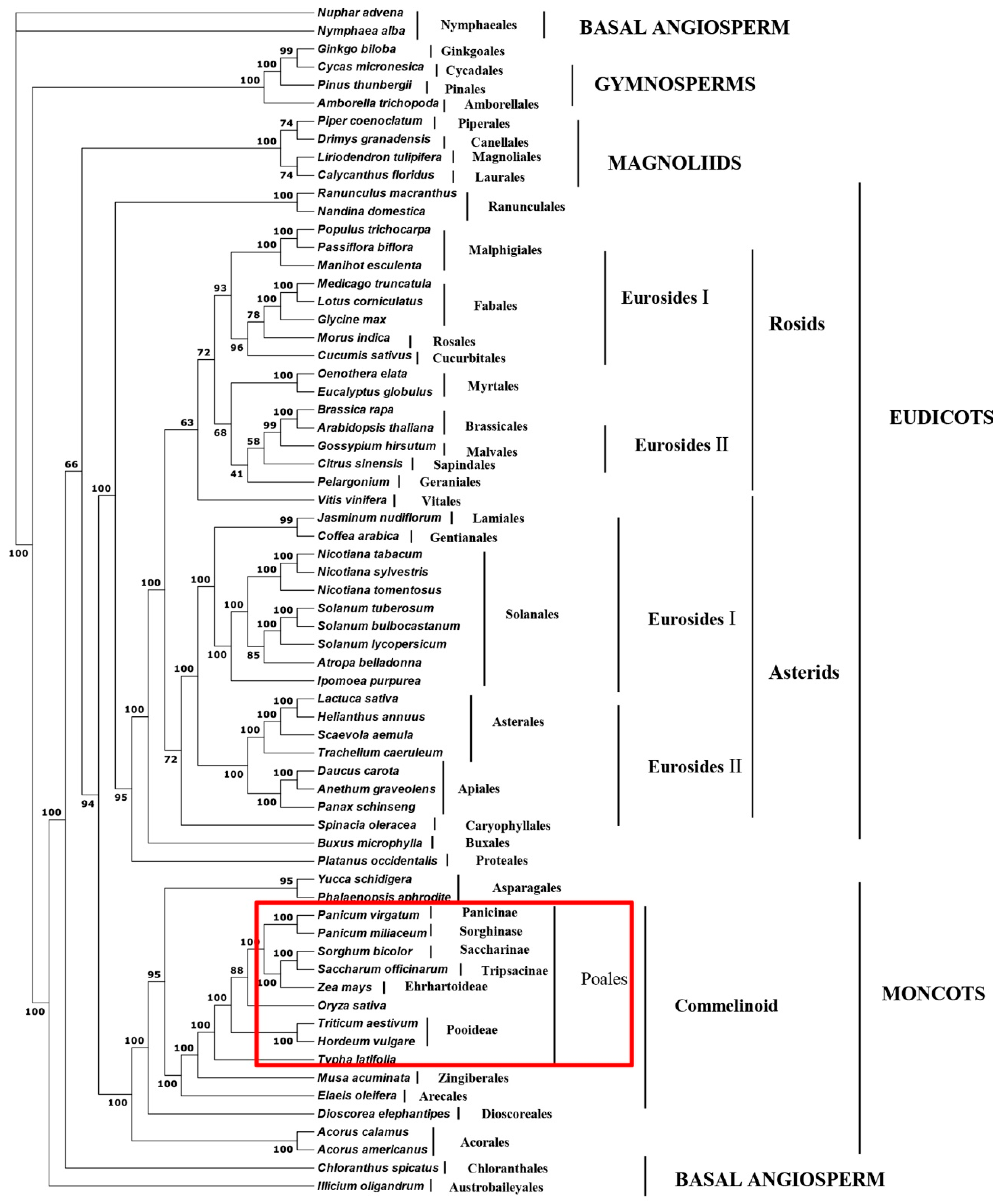
2.5. Phylogenetic Analysis
3. Materials and Methods
3.1. Genome Assembly and Genome Annotation
3.2. Sequence and Structural Comparison in Six Panicoideae cp Genomes
3.3. SSRs Identification and RNA Editing
3.4. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gray, M.W. The evolutionary origin of organelles. Trends Genet. 1989, 5, 294–299. [Google Scholar] [CrossRef]
- Neuhaus, H.E.; Emes, M.J. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.J.; Barbrook, A.C.; Koumandou, V.L.; Nisbet, R.E.R.; Symington, H.A.; Wightman, T.F. Evolution of the chloroplast genome. Phil. Trans. R. Soc. Lond. B 2003, 358, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.C.; Zhang, Y.Z.; Geng, H.M.; Chen, S.L. The complete chloroplast genome sequence of Gentiana lawrencei var. farreri (Gentianaceae) and comparative analysis with its congeneric species. PeerJ 2016, 4, e2540. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Raubeson, L.A.; Boore, J.L.; Chumley, T.W.; Haberle, R.C.; Wyman, S.K.; Alverson, A.J.; Peery, R.; Herman, S.J.; Fourcade, H.M.; et al. Methods for obtaining and analyzing whole chloroplast genome sequences. In In Methods in Enzymology; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; Volume 395, pp. 348–384. [Google Scholar]
- Chumley, T.W.; Palmer, J.D.; Mower, J.P.; Fourcade, H.M.; Calie, P.J.; Boore, J.L.; Jansen, R.K. The complete chloroplast genome sequence of Pelargonium × hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 2006, 23, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Guisinger, M.; Kim, H.G.; Ruck, E.; Blazier, J.C.; McMurtry, V.; Kuehl, J.V.; Boore, J.; Jansen, R.K. Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. J. Mol. Evol. 2008, 67, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- Nie, X.; Lv, S.; Zhang, Y.; Du, X.; Wang, L.; Biradar, S.S.; Tan, X.; Wan, F.; Weining, S. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora). PLoS ONE 2012, 7, e36869. [Google Scholar] [CrossRef] [PubMed]
- Ruhfel, B.R.; Gitzendanner, M.A.; Soltis, P.S.; Soltis, D.E.; Burleigh, J.G. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol. Biol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.V.; Miller, J.T.; Small, I.; Nevill, P.G.; Boykin, L.M. Integration of complete chloroplast genome sequences with small amplicon datasets improves phylogenetic resolution in Acacia. Mol. Phylogenet. Evol. 2016, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Dhingra, A.; Soltis, P.S.; Shaw, R.; Farmerie, W.G.; Folta, K.M.; Soltis, D.E. Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol. 2016, 6, 1–13. [Google Scholar]
- Saarela, J.M.; Wysocki, W.P.; Barrett, C.F.; Soreng, R.J.; Davis, J.I.; Clark, L.G.; Kelchner, S.A.; Pires, J.C.; Edger, P.P.; Mayfield, D.R.; et al. Plastid phylogenomics of the cool-season grass subfamily: Clarification of relationships among early-diverging tribes. Aob Plants 2015, 7, plv046. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, J.; Shimada, H.; Whittier, R.; Ishibashi, T.; Sakamoto, M.; Mori, M.; Kondo, C.; Honji, Y.; Sun, C.R.; Meng, B.Y.; et al. The complete sequence of the rice (Oryza sativa) chloroplast genome: Intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol. Gen. Genet. 1989, 217, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Young, H.A.; Lanzatella, C.L.; Sarath, G.; Tobias, C.M. Chloroplast genome variation in upland and lowland switchgrass. PLoS ONE 2011, 6, e23980. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, R.; Li, X. Comparative Analysis of the Complete Chloroplast Genome of Four Known Ziziphus Species. Genes 2017, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, A.R.; Waqas, M.; Kang, S.M.; Khan, M.A.; Lee, S.M.; Lee, I.J. Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front. Plant Sci. 2016, 7, 843. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Tsudzuki, T.; Takahashi, S.; Shimada, H.; Kadowaki, K.I. Complete nucleotide sequence of the sugarcane (Saccharum officinarum) chloroplast genome: a comparative analysis of four monocot chloroplast genomes. DNA Res. 2004, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.A. Subfamily Panicoideae Link. In Flowering Plants. Monocots: Poaceae; Springer international publishing: Zurich, Switzerland, 2015; Volume 13, pp. 271–345. [Google Scholar]
- Hitchcock, A.S.; Chase, A. Manual of the grasses of the United States; Dover Publications: New York, NY, USA, 1971; Volume 2. [Google Scholar]
- Palmer, N.A.; Saathoff, A.J.; Waters, B.M.; Donze, T.; Heng-Moss, T.M.; Twigg, P.; Tobias, C.M.; Sarath, G. Global changes in mineral transporters in tetraploid switchgrasses (Panicum virgatum L.). Front. Plant Sci. 2014, 4, 549. [Google Scholar] [CrossRef] [PubMed]
- Saski, C.; Lee, S.B.; Fjellheim, S.; Guda, C.; Jansen, R.K.; Luo, H.; Tomkins, J.; Rognli, O.A.; Daniell, H.; Clarke, J.L. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor. Appl. Genet. 2007, 115, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.H.; Li, K.T.; Hsu, T.F.; Tsai, Y.C.; Fang, P.H.; Hsing, Y.I.C. Broomcorn and foxtail millet were cultivated in Taiwan about 5000 years ago. Bot. Stud. 2017, 58, 3. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ken, J.G.; Clark, L.G.; Kellogg, E.A.; Kay, E.E. Reinstatement and emendation of subfamily Micrairoideae (Poaceae). Syst. Bot. 2007, 32, 71–80. [Google Scholar] [CrossRef]
- Washburn, J.D.; Schnable, J.C.; Davidse, G.; Pires, J.C. Phylogeny and photosynthesis of the grass tribe Paniceae. Am. J. Bot. 2015, 102, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Giussani, L.M.; Cota-Sánchez, J.H.; Zuloaga, F.O.; Kellogg, E.A. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Am. J. Bot. 2001, 88, 1993–2012. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ken, J.G.; Clark, L.G. Phylogeny and a new tribal classification of the Panicoideae s.l. (Poaceae) based on plastid and nuclear sequence data and structural data. Am. J. Bot. 2010, 97, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Filgueiras, T.S.; Davis, J.I.; Morrone, O. A worldwide phylogenetic classification of the Poaceae(Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Burke, S.V.; Wysocki, W.P.; Zuloaga, F.O.; Craine, J.M.; Pires, J.C.; Edger, P.P.; Mayfield-Jones, D.; Clark, L.G.; Kelchner, S.A.; Duvall, M.R. Evolutionary relationships in Panicoid grasses based on plastome phylogenomics (Panicoideae; Poaceae). BMC Plant Biol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.V.; Denyer, K.; Packman, L.C.; Jones, M.K.; Howe, C.J. Molecular basis of the waxy endosperm starch phenotype in broomcorn millet (Panicum miliaceum L.). Mol. Biol. Evol. 2010, 27, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Xu, Y.; He, J.H.; Zhang, S.; Wang, Y.Y.; Lu, P. Genetic diversity and population structure of broomcorn millet (Panicum miliaceum L.) cultivars and landraces in china based on microsatellite markers. Int. J. Mol. Sci. 2016, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wang, L.; Liu, H.; Yue, W.J.; Du, X.H.; Song, W.N.; Nie, X.J. De novo assembly and characterization of the transcriptome of broomcorn millet (Panicum miliaceum L.) for gene discovery and marker development. Front. Plant Sci. 2016, 7, 1083. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wang, M.; Liu, S.Y.; Du, X.H.; Song, W.N.; Nie, X.J. Transcriptome-wide identification and expression profiles of the WRKY transcription factor family in Broomcorn millet (Panicum miliaceum L.). BMC Genom. 2016, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Bosacchi, M.; Gurdon, C.; Maliga, P. Plastid Genotyping Reveals Uniformity of cms-T Maize Cytoplasms. Plant Physiol. 2015, 169, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, L.Z. Complete chloroplast genome sequence of green foxtail (Setaria viridis), a promising model system for C4 photosynthesis. Mitochondrial DNA Part A 2016, 27, 3707–3708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Nie, X.J.; Jia, X.O.; Zhao, C.Z.; Biradar, S.S.; Wang, L.; Du, X.H.; Weining, S. Analysis of codon usage patterns of the chloroplast genomes in the Poaceae family. Aust. J. Bot. 2012, 60, 461–470. [Google Scholar] [CrossRef]
- Maier, R.M.; Neckermann, K.; Igloi, G.L.; Kössel, H. Complete sequence of the maize chloroplast genome: Gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J. Mol. Biol. 1995, 251, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Guisinger, M.M.; Chumley, T.W.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Implications of the Plastid Genome Sequence of Typha (Typhaceae, Poales) for Understanding Genome Evolution in Poaceae. J. Mol. Evol. 2010, 70, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Blaner, A.; Schneider, J. Phylogenetic relationships in the grass family (Poaceae) based on the nuclear single copy locus topoisomerase 6 compared with chloroplast DNA. Syst. Biodivers. 2014, 12, 111–124. [Google Scholar] [CrossRef]
- Howe, C.J.; Barker, R.F.; Bowman, C.M.; Dyer, T.A. Common features of three inversions in wheat chloroplast DNA. Curr. Genet. 1988, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Davis, J.I.; Soreng, R.J.; Garvin, D.; Anderson, M.J. Chloroplast DNA inversions and the origin of the grass family (Poaceae). Proc. Natl. Acad. Sci. USA 1992, 89, 7722–7726. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.A.; Aliscioni, S.; Morrone, O.; Pensiero, J.; Zuloaga, F. A Phylogeny of Setaria (Poaceae, Panicoideae, Paniceae) and Related Genera Based on the Chloroplast Gene ndhF. Int. J. Plant Sci. 2009, 170, 117–131. [Google Scholar] [CrossRef]
- Nybom, H.; Weising, K.; Rotter, B. DNA fingerprinting in botany: Past, present, future. Investig. Genet. 2014, 5, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.; Polsakiewicz, M.; Knoop, V. Frequent chloroplast RNA editing in early-branching flowering plants: Pilot studies on angiosperm-wide coexistence of editing sites and their nuclear specificity factors. BMC Evol. Biol. 2016, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Liu, H.; Ge, L.Q.; Xing, G.W.; Wang, M.; Song, W.N.; Nie, X.J. Identification and analysis of RNA editing sites in the chloroplast transcripts of Aegilops tauschii L. Genes 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Kusumegi, T.; Tsudzuki, T.; Sugiura, M. RNA editing sites in tobacco chloroplast transcripts: Editing as a possible regulator of chloroplast RNA polymerase activity. Mol. Gen. Genet. 1999, 262, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2014, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Sugiura, M. Regulation of gene expression in chloroplasts of higher plants. Plant Mol. Biol. 1996, 32, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW(OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]

| Genome Characteristics | P. miliaceum | P. virgatum [15] | Z. mays [34] | S. bicolor [22] | S. officinarum [18] | S. italic [35] |
|---|---|---|---|---|---|---|
| Genome Size (bp) | 139,826 | 139,619 | 140,447 | 140,754 | 141,182 | 135,516 |
| LSC length (bp) | 81,682 | 81,659 | 82,375 | 82,688 | 83,048 | 79,896 |
| SSC length (bp) | 12,574 | 12,564 | 12,540 | 12,502 | 12,544 | 12,012 |
| IR length (bp) | 22,785 | 22,698 | 22,766 | 22,782 | 22,795 | 21,804 |
| Genome length with one copy of IR (bp) | 117,068 | 116,921 | 117,681 | 117,972 | 118,387 | 113,712 |
| No. of different genes | 108 | 111 | 110 | 110 | 110 | 110 |
| No. of different protein-coding genes | 76 | 77 | 76 | 78 | 76 | 76 |
| No. of different tRNA genes | 28 | 30 | 30 | 30 | 30 | 30 |
| No. of rRNA genes | 4 | 4 | 4 | 4 | 4 | 4 |
| No. of genes duplicated by IR | 15 | 16 | 18 | 18 | 20 | 18 |
| No. of different genes with introns | 15 | 16 | 15 | 16 | 16 | 15 |
| coding sequence length (bp) | 60,903 | 60,315 | 60,348 | 60,966 | 61,272 | 60,168 |
| No. of codons | 20,301 | 20,105 | 20,116 | 20,322 | 20,424 | 20,056 |
| % GC content in LSC | 36.47 | 33.1 | 36.26 | 36.38 | 36.28 | 36.82 |
| % GC content in SSC | 33.09 | 36.43 | 32.83 | 32.63 | 32.86 | 33.27 |
| % GC content in IR | 43.95 | 44.01 | 43.93 | 43.99 | 43.91 | 43.15 |
| % GC content of genome | 38.61 | 38.59 | 38.44 | 38.26 | 38.44 | 38.97 |
| Coding % GC content | 39.07 | 39.00 | 39.19 | 39.10 | 39.15 | 39.14 |
| Category | Group of Genes | Name of Genes |
|---|---|---|
| Transcription and translation related genes (27) | Large subunit ribosomal proteins | rpl2b, c, 14, 16 b, 20, 22, 23c, 32, 33, 36 |
| Small subunit ribosomal proteins | rps2, 3, 4, 7c, 8, 11, 12b, c, d, 14, 15c, 16b, 18, 19 c | |
| translation initiation factor | infA | |
| DNA dependent RNA olymerase | rpoA, B, C1, C2 | |
| RNA genes (32) | Ribosomal RNAs | rrn23c, 16c, 5c, 4.5c |
| Transfer RNAs | trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAUc, trnG-UCC, trnH-GUGc, trnI-CAUc, trnI-GAUc, trnK-UUU, trnL-CAAc, trnL-UAA, trnL-UAG, trnM-CAUc, trnN-GUUc, trnP-UGG, trnQ-UUG, trnR-ACGc, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GACc, trnV-UAC, trnW-CCA, trnY-GUA | |
| photosynthesis related genes (46) | Photosystem I | psaA, B, C, I, J, ycf3a, ycf4 |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z | |
| NADH oxidoreductase | ndhAb, Bb, c, C, D, E, F, G, H, I, J, K | |
| Cytochrome b6/f | petA, Bb, Db, G, L, N | |
| ATP synthase | atpA, B, E, Fb, H, I | |
| Rubisco | rbcL | |
| Other genes (4) | fatty acid synthesis | ccsA |
| carbon metabolism | cemA | |
| Proteolysis | clpP | |
| RNA processing | matK | |
| Proteins of unknown function (3) | conserved open reading frames | ycf15c, ycf68c,orf42 |
| Gene | Location | ExonI | IntronI | ExonII | IntronII | ExonIII |
|---|---|---|---|---|---|---|
| rps16 | LSC | 218 | 840 | 40 | ||
| atpF | LSC | 159 | 816 | 408 | ||
| ycf3 | LSC | 159 | 732 | 228 | 739 | 132 |
| petB | LSC | 6 | 764 | 645 | ||
| rps12 * | LSC | 114 | -- | 29 | 541 | 232 |
| petD | LSC | 8 | 740 | 475 | ||
| rpl2 | IR | 390 | 661 | 429 | ||
| ndhB | IR | 753 | 711 | 777 | ||
| ndhA | SSC | 534 | 1022 | 537 | ||
| trnK-UUU | LSC | 33 | 2472 | 38 | ||
| trnA-UGC | IR | 38 | 811 | 35 | ||
| trnL-UAA | LSC | 35 | 541 | 50 | ||
| trnV-UAC | LSC | 37 | 601 | 39 | ||
| rpl16 | LSC | 402 | 1001 | 9 | ||
| trnI-GAU | IR | 42 | 947 | 35 |
| Amino Acid | Codon | No. * | tRNA | Amino Acid | Codon | No. | tRNA | Amino Acid | Codon | No. | tRNA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phe | UUU | 719 | trnF-GAA | Ser | UCU | 383 | trnS-GGA | Tyr | UAU | 578 | trnY-GUA |
| Phe | UUC | 405 | Ser | UCC | 291 | Tyr | UAC | 149 | |||
| Leu | UUA | 734 | trnL-UAA | Ser | UCA | 235 | stop | UAA | 43 | ||
| Leu | UUG | 396 | trnL-CAA | Ser | UCG | 129 | stop | UAG | 23 | ||
| Leu | CUU | 463 | trnL-UAG | Pro | CCU | 344 | trnP-UGG | His | CAU | 341 | trnH-GUG |
| Leu | CUC | 159 | Pro | CCC | 208 | His | CAC | 120 | |||
| Leu | CUA | 324 | Pro | CCA | 239 | Gln | CAA | 525 | trnQ-UUG | ||
| Leu | CUG | 123 | Pro | CCG | 94 | Gln | CAG | 151 | |||
| Ile | AUU | 823 | trnI-GAU | Thr | ACU | 444 | trnT-GGU | Asn | AAU | 598 | trnN-GUU |
| Ile | AUC | 324 | Thr | ACC | 209 | Asn | AAC | 206 | |||
| Ile | AUA | 524 | trnI-CAU | Thr | ACA | 302 | trnT-UGU | Lys | AAA | 754 | trnK-UUU |
| Met | AUG | 464 | trnM-CAU | Thr | ACG | 132 | Lys | AAG | 291 | ||
| trnfM-CAU | |||||||||||
| Val | GUU | 444 | trnV-GAC | Ala | GCU | 555 | trnA-UGC | Asp | GAU | 570 | trnD-GUC |
| Val | GUC | 145 | Ala | GCC | 191 | Asp | GAC | 161 | |||
| Val | GUA | 447 | trnV-UAC | Ala | GCA | 376 | Glu | GAA | 801 | trnE-UUC | |
| Val | GUG | 159 | Ala | GCG | 144 | Glu | GAG | 264 | |||
| Cys | UGU | 170 | trnC-GCA | Arg | CGU | 294 | trnR-ACG | Ser | AGU | 293 | trnS-GCU |
| Cys | UGC | 58 | Arg | CGC | 111 | Ser | AGC | 110 | |||
| stop | UGA | 21 | Arg | CGA | 275 | Arg | AGA | 370 | trnR-UCU | ||
| Trp | UGG | 350 | trnW-CCA | Arg | CGG | 94 | Arg | AGG | 128 | ||
| Gly | GGU | 487 | trnG-UCC | Gly | GGC | 160 | |||||
| Gly | GGA | 602 | Gly | GGG | 274 |
| Gene. | Site | Nucleotide Position | Amino Acid Position | Edited Codon | Amino Acid Change | Panicum miliaceum L. | Panicum virgatum | Sorghum | Seteria | Saccharum | Zea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| matK | 1 a | 331 | 111 | Ctt-Ttt | L-F | + | + | + | + | + | + |
| 2 | 1015 | 339 | Cca-Tca | P-S | + | + | (−) | (−) | (−) | (−) | |
| 3 | 1258 | 420 | Cat-Tat | H-Y | + | + | + | + | + | (−) | |
| 4 b | 424 | 142 | Ctc-Ttc | L-F | (−) | (–) | (−) | (−) | (−) | + | |
| 5 b | 1351 | 451 | Cat-Tat | H-Y | (−) | (−) | (−) | (−) | (−) | + | |
| ropB | 1 a | 467 | 156 | tCg-tTg | S-L | + | + c | + | + | + | + c |
| 2 a | 545 | 182 | tCa-tTa | S-L | + | + c | + | + | + | + c | |
| 3 | 560 | 187 | tCg-tTg | S-L | (−) | (−) | + | + | + | + c | |
| 4 a | 617 | 206 | cCg-cTg | P-L | + | + | + | + | + | + c | |
| rpoC2 | 1 | 2209 | 737 | Cat-Tat | H-Y | + | (−) | (−) | (−) | (−) | (−) |
| 2 | 2312 | 771 | tCa-tTa | S-L | + | + | (−) | (−) | (−) | (−) | |
| 3 | 2804 | 935 | tCg-tTg | S-L | + | + | (−) | (−) | (−) | (−) | |
| 4 | 2753 | 918 | tCg-tTg | S-L | (−) | (−) | + | + | (−) | (−) | |
| 5 | 2795 | 932 | tCg-tTg | S-L | (−) | (−) | (−) | (−) | + | (−) | |
| 6 | 1732 | 578 | Ctc-Ttc | L-F | (−) | (−) | (−) | (−) | (−) | + | |
| 7 | 2774 | 925 | tCg-tTg | S-L | (−) | (−) | (−) | (−) | (−) | + c | |
| atpA | 1 a | 1148 | 383 | tCa-tTa | S-L | + | + c | + c | + | + | + |
| rps14 | 1 a | 80 | 27 | tCa-tTa | S-L | + | + c | + | + | + | + |
| ycf3 | 1 a | 44 | 15 | tCc-tTc | S-F | + | + c | + | + | + | + c |
| 2 | 191 | 64 | aCg-aTg | S-F | + | + c | + | + | (−) | (−) | |
| 3 | 185 | 62 | aCg-aTg | T-M | (−) | (−) | (−) | (−) | + | + c | |
| rpl20 | 1 a | 308 | 103 | tCa-tTa | S-L | + | + | + | + | + | + c |
| petB | 1 | 617 | 206 | cCa-cTa | P-L | + | + c | (−) | (−) | (−) | (−) |
| 2 | 668 | 223 | cCa-cTa | P-L | (−) | (−) | + | + | (−) | + | |
| 3 | 611 | 204 | cCa-cTa | P-L | (−) | (−) | (−) | (−) | + | (−) | |
| rps8 | 1 a | 182 | 61 | tCa-tTa | S-L | + | + c | + | + | + | + |
| rpl2 | 1 | 2 | 1 | aCg-aTg | T-M | (−) | (−) | (−) | (−) | + | + |
| ndhBa | 1 | 467 | 156 | cCa−cTa | P-L | + | + | + | + | + | + |
| 2 | 586 | 196 | Cat-Tat | H-Y | + | + | + | + | + | + | |
| 3 | 611 | 204 | tCa-tTa | S-L | + | + | + | + | + | + | |
| 4 | 737 | 246 | cCa-cTa | P-L | + | + | + | + | + | + | |
| 5 | 830 | 277 | tCa-tTa | S-L | + | + | + | + | + | + c | |
| 6 | 1481 | 494 | cCa-cTa | P-L | + | + | + | + | + | + | |
| ndhF | 1 a | 62 | 21 | tCa-tTa | S-L | + | + | + | + | + | + |
| 2 | 1834 | 612 | Ctt-Ttt | L-F | (−) | (−) | (−) | + | (−) | (−) | |
| 3 | 1840 | 614 | Ctt-Ttt | L-F | + | + | (−) | (−) | (−) | (−) | |
| 4 b | 1915 | 639 | Ctt-Ttt | L-F | + | + | + | (−) | + | + | |
| 5 | 1909 | 637 | Ctt-Ttt | L-F | (−) | (−) | (−) | + | (−) | (−) | |
| ccsA | 1 | 647 | 216 | aCt-aTt | T-I | + | (−) | (−) | (−) | (−) | (−) |
| 2 | 644 | 215 | aCc-aTc | T-I | (−) | + | + | (−) | + | + | |
| 3 | 641 | 214 | aCt-aTt | T-I | (−) | (−) | (−) | + | (−) | (−) | |
| ndhD | 1 a | 878 | 293 | tCa-tTa | S-L | + | + c | + | + c | + | + |
| ndhA | 1 | 35 | 12 | tCg-tTg | S-L | + | + | (−) | (−) | (−) | (−) |
| 2 | 458 | 153 | tCa-tTa | S-L | + | + c | (−) | (−) | (−) | (−) | |
| 3 | 548 | 183 | tCa-tTa | S-L | + | + c | (−) | (−) | (−) | (−) | |
| 4 | 1055 | 352 | tCc-tTc | S-F | + | (−) | (−) | (−) | (−) | (−) | |
| 5 | 50 | 17 | tCg-tTg | S-L | (−) | (−) | + | (−) | + | + | |
| 6 | 473 | 158 | tCa-tTa | S-L | (−) | (−) | + | (−) | + | + | |
| 7 | 563 | 188 | tCa-tTa | S-L | (−) | (−) | + | (−) | + | + | |
| 8 | 1070 | 357 | tCc-tTc | S-F | (−) | (−) | + c | (−) | + | + | |
| 9 b | 47 | 16 | tCg-tTg | S-L | (−) | (−) | (−) | + | (−) | (−) | |
| 10 b | 470 | 157 | tCa-tTa | S-L | (−) | (−) | (−) | + | (−) | (−) | |
| 11 b | 560 | 187 | tCa-tTa | S-L | (−) | (−) | (−) | + | (−) | (−) | |
| Number of genes | 14 | 14 | 14 | 14 | 15 | 15 | |||||
| Number of edited sites | 31 | 29 | 28 | 28 | 29 | 30 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Zhao, X.; Wang, S.; Zhang, T.; Li, C.; Liu, H.; Tong, W.; Guo, Y. Complete Chloroplast Genome Sequence of Broomcorn Millet (Panicum miliaceum L.) and Comparative Analysis with Other Panicoideae Species. Agronomy 2018, 8, 159. https://doi.org/10.3390/agronomy8090159
Nie X, Zhao X, Wang S, Zhang T, Li C, Liu H, Tong W, Guo Y. Complete Chloroplast Genome Sequence of Broomcorn Millet (Panicum miliaceum L.) and Comparative Analysis with Other Panicoideae Species. Agronomy. 2018; 8(9):159. https://doi.org/10.3390/agronomy8090159
Chicago/Turabian StyleNie, Xiaojun, Xian Zhao, Sue Wang, Ting Zhang, Chong Li, Hui Liu, Wei Tong, and Yuan Guo. 2018. "Complete Chloroplast Genome Sequence of Broomcorn Millet (Panicum miliaceum L.) and Comparative Analysis with Other Panicoideae Species" Agronomy 8, no. 9: 159. https://doi.org/10.3390/agronomy8090159
APA StyleNie, X., Zhao, X., Wang, S., Zhang, T., Li, C., Liu, H., Tong, W., & Guo, Y. (2018). Complete Chloroplast Genome Sequence of Broomcorn Millet (Panicum miliaceum L.) and Comparative Analysis with Other Panicoideae Species. Agronomy, 8(9), 159. https://doi.org/10.3390/agronomy8090159






