Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Soil
2.2. Exposure Assay
2.3. Data Analysis
3. Results and Discussion
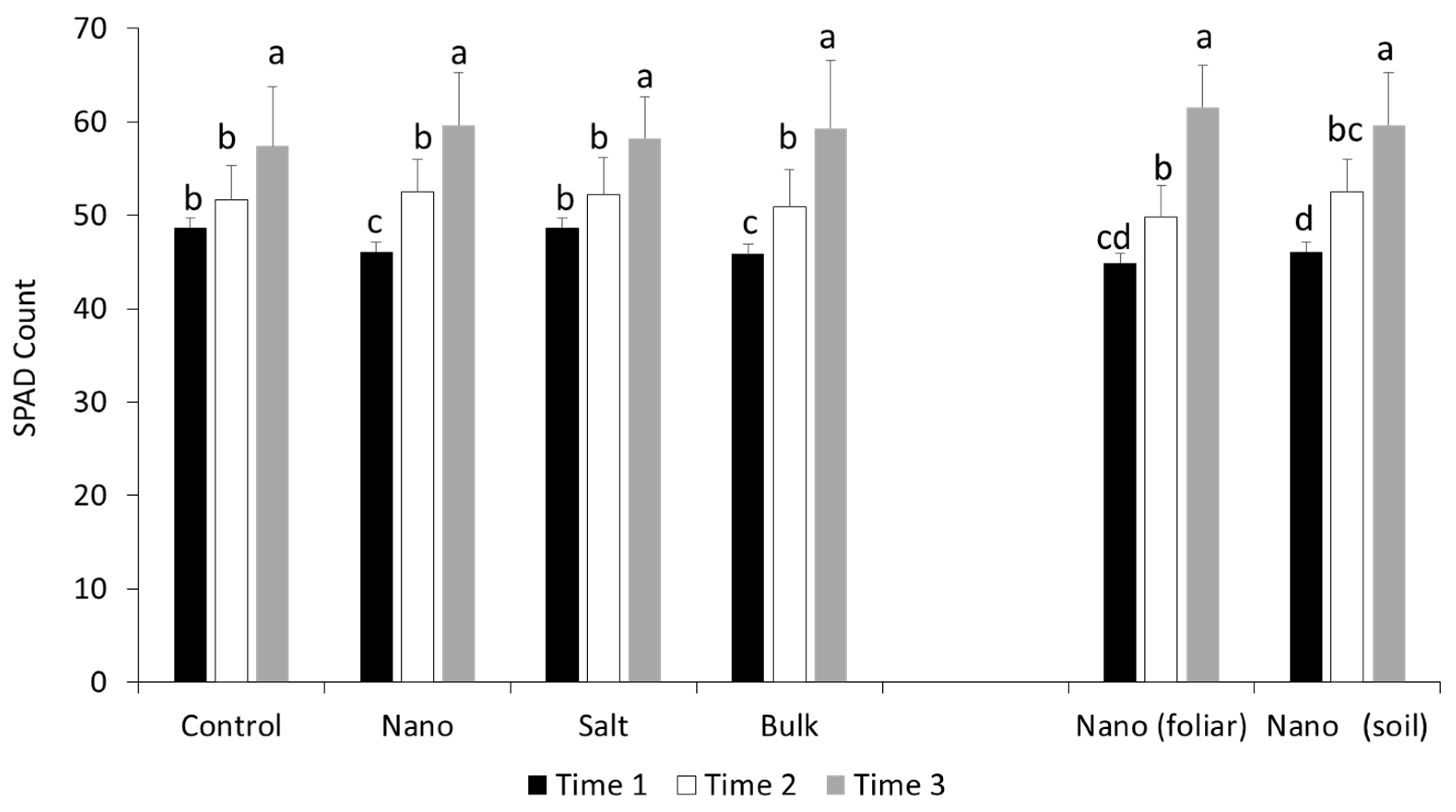
3.1. Early Chlorophyll Formation in Wheat Is Altered by Nano Mn Application
3.2. Effect of Mn Type on the Vegetative and Reproductive Growth of Wheat
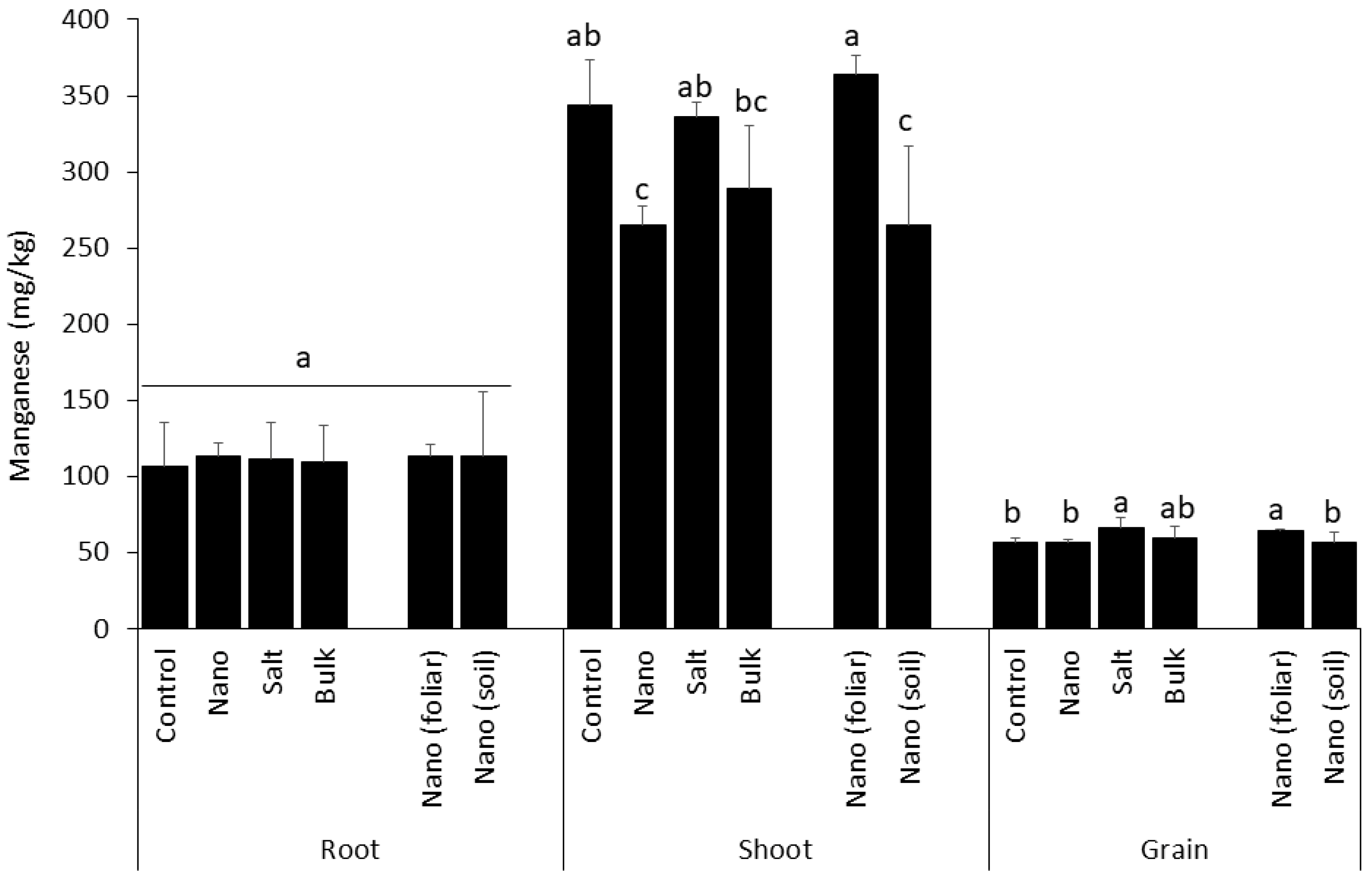
3.3. Shoot Uptake of Manganese Is Inhibited by Soil-Applied Nano Mn
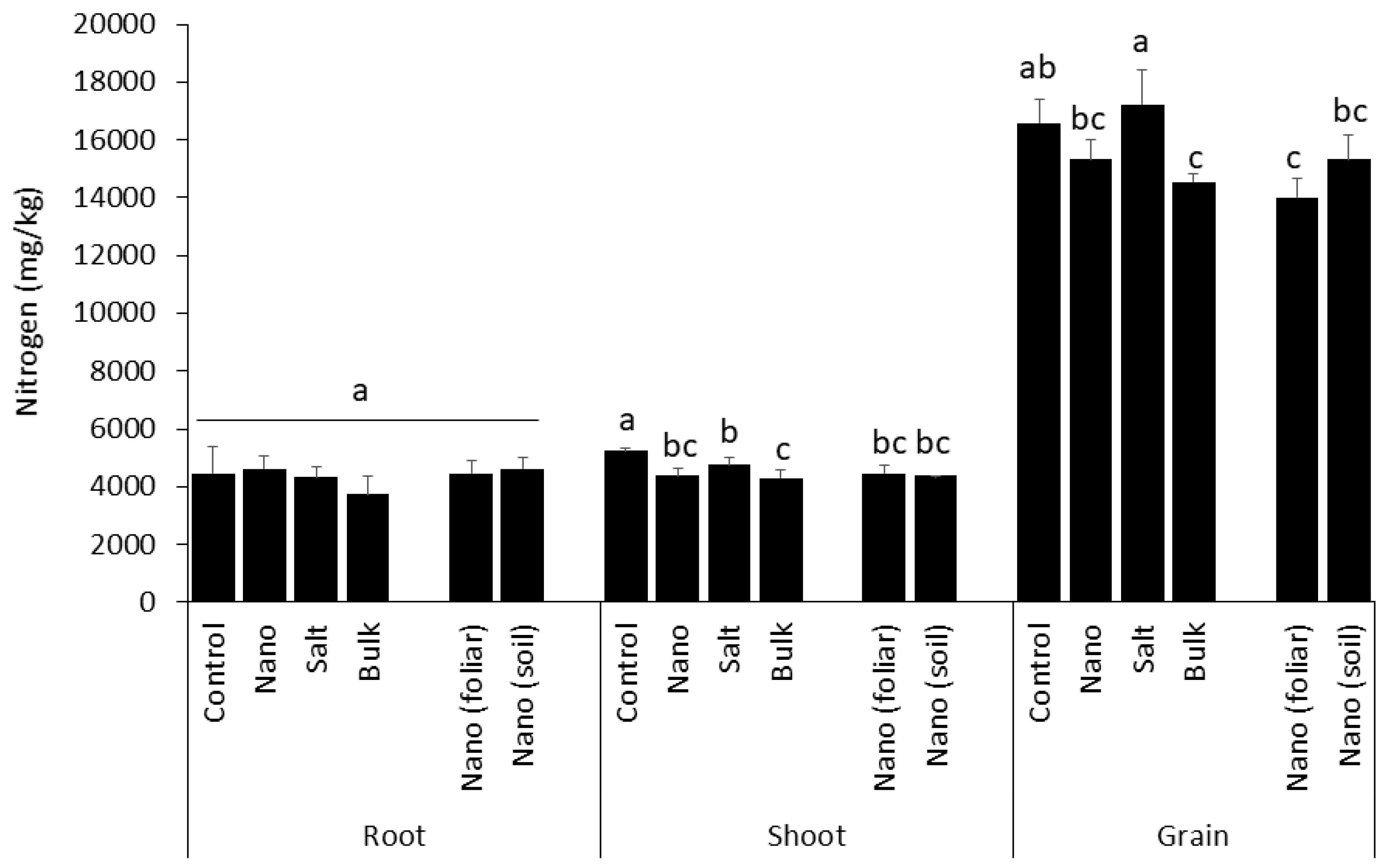
3.4. Accumulation of Nitrogen in Shoot and Grain Is Differently Affected by Mn Type
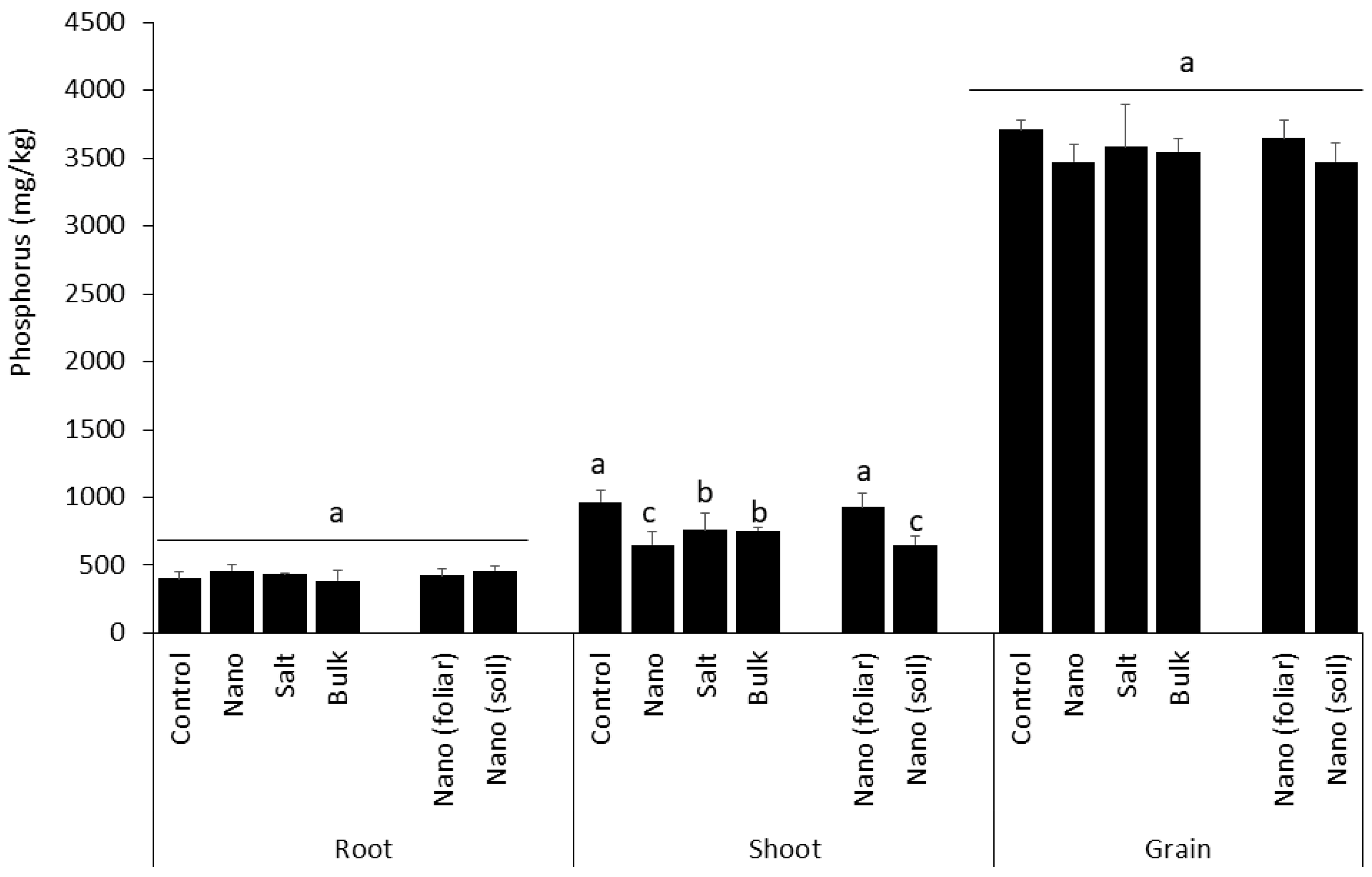
3.5. Shoot Uptake of Phosphorus Is Inhibited by Soil Application of Mn
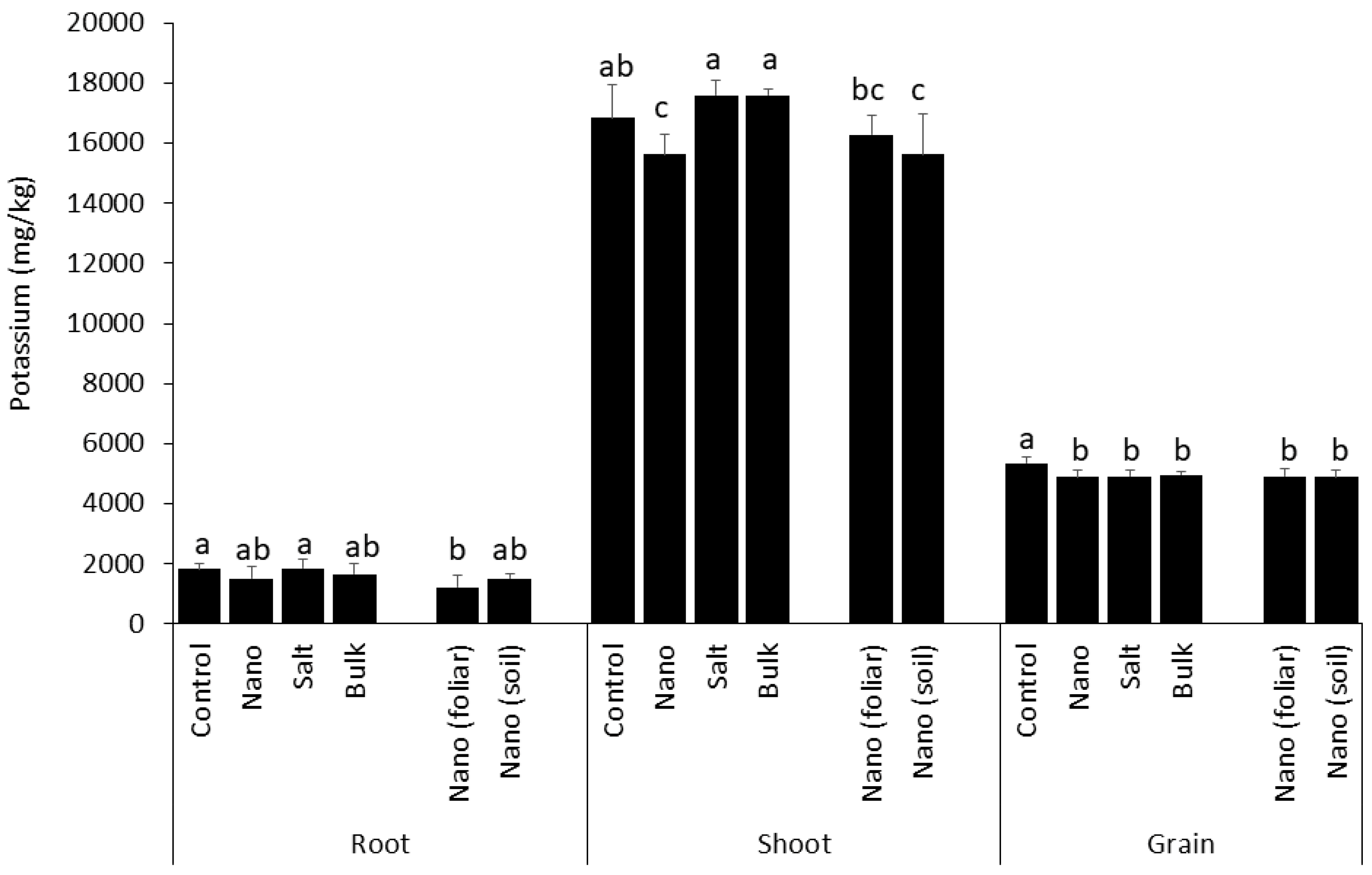
3.6. Above-Ground Accumulation of Potassium Is Inhibited by Soil Applied Nano Mn
3.7. Soil Residual Levels of N, P, and Mn Are Differently Affected by Mn Treatment
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Datnoff, L.E.; Elmer, W.H.; Huber, D.M. Mineral Nutrition and Plant Disease; American Phytopathological Society: Paul, MI, USA, 2007. [Google Scholar]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Pradhan, S.; Patra, P.; Das, S.; Chandra, S.; Mitra, S.; Dey, K.K.; Akbar, S.; Palit, P.; Goswami, A. Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: A detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 2013, 47, 13122–13131. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Patra, P.; Mitra, S.; Dey, K.K.; Jain, S.; Sarkar, S.; Roy, S.; Palit, P.; Goswami, A. Manganese nanoparticles: Impact on non-nodulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro. J. Agric. Food Chem. 2014, 62, 8777–8785. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. Adequate and toxic levels of copper and manganese in upland rice, common bean, corn, soybean, and wheat grown on an oxisol. Commun. Soil Sci. Plant Anal. 2001, 32, 1659–1676. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Micronutrients fortification for efficient agronomic production. Agron. Sustain. Dev. 2016, 36, 1–26. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, A.; Genaidy, A.; Abdelraheem, W.; Dionysiou, D.; Andersen, C. The effects of metallic engineered nanoparticles upon plant systems: An analytic examination of scientific evidence. Sci. Total Environ. 2017, 579, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Bindraban, P. Nanofertilizers: New products for the industry? J. Agric. Food Chem. 2018, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De La Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.S.; Dimkpa, C.O. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Landa, P.; Cyrusova, T.; Jerabkova, J.; Drabek, O.; Vanek, T.; Podlipna, R. Effect of mtal oxides on plant germination: Phytotoxicity of nanoparticles, bulk materials and metal ions. Water Soil Air Pollut. 2016, 227, 448. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: Nanotoxicants or nanonutrients? Water Air Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Effect of metalloid and metallic oxide nanoparticles on Fusarium wilt of watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Bindraban, P.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Heinlaan, M.; Muna, M.; Juganson, K.; Oriekhova, O.; Stoll, S.; Kahru, A.; Slaveykova, V.I. Exposure to sublethal concentrations of Co3O4 and Mn2O3 nanoparticles induced elevated metal body burden in Daphnia magna. Aquat. Toxicol. 2017, 189, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.G.; Chien, S.H.; Chardon, W.J. Iron oxide-impregnated filter paper (Pi test): II. A review of its application. Nutr. Cycl. Agroecosyst. 1996, 47, 7–18. [Google Scholar] [CrossRef]
- Jhanji, S.; Sadana, U.S.; Shankar, A.; Shukla, A.K. Manganese influx and its utilization efficiency in wheat. Indian J. Exp. Biol. 2014, 52, 650–657. [Google Scholar] [PubMed]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur mediated alleviation of Mn toxicity in polish wheat relates to regulating Mn allocation and improving antioxidant system. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, V.; Sadana, U.; Takkar, T. Methods and rates of application of Mn and its critical levels for wheat following rice on coarse textured soils. Fert. Res. 1985, 8, 173–178. [Google Scholar] [CrossRef]
- Marcar, N.E.; Graham, R.D. Micronutrients: Genotypic variation for manganese efficiency in wheat. J. Plant Nutr. 1987, 10, 2049–2055. [Google Scholar] [CrossRef]
- Narwal, R.P.; Dahiya, R.R.; Malik, R.S.; Kala, R. Influence of genetic variability on zinc, iron and manganese responses in wheat. J. Geochem. Exp. 2012, 121, 45–48. [Google Scholar] [CrossRef]
- Pahlavan-Rad, M.R.; Mohammad Pessarakli, M. Response of wheat plants to zinc, iron, and manganese applications and uptake and concentration of zinc, iron, and manganese in wheat grains. Commun. Soil Sci. Plant Anal. 2009, 40, 1322–1332. [Google Scholar] [CrossRef]
- Stepien, A.; Wojtkowiak, K. Effect of foliar application of Cu, Zn and Mn on yield and quality indicators of winter wheat grain. Chil. J. Agric. Res. 2016, 76. [Google Scholar] [CrossRef]
- Seadh, S.E.; EL-Abady, M.I.; El-Ghamry, A.M.; Farouk, S. Influence of micronutrients foliar application and nitrogen fertilization on wheat yield and quality of grain and seed. J. Biol. Sci. 2009, 9, 851–858. [Google Scholar] [CrossRef]
- Karim, M.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Batsmanova, L.M.; Gonchar, L.M.; Taran, N.Y.; Okanenko, A.A. Using a colloidal solution of metal nanoparticles as micronutrient fertilizer for cereals. Proc. Int. Conf. Nanomater. 2013, 2, 4. [Google Scholar]
- Dimkpa, C.O.; Latta, D.E.; McLean, J.E.; Britt, D.W.; Boyanov, M.I.; Anderson, A.J. Fate of CuO and ZnO nano and micro particles in the plant environment. Environ. Sci. Technol. 2013, 47, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Giehl, R.F.H.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatyev, K.; Andresen, E.; Küpper, H.; Peiter, E.; von Wirén, N. Metal tolerance protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Rengel, Z. Distribution and remobilization of Zn and Mn during grain development in wheat. J. Exp. Bot. 1994, 45, 1829–1835. [Google Scholar] [CrossRef]
- Barman, A.; Pandey, R.N.; Singh, B.; Bappa Das, B. Manganese deficiency in wheat genotypes: Physiological responses and manganese deficiency tolerance index. J. Plant Nutr. 2017, 40, 2691–2708. [Google Scholar] [CrossRef]
- Dimkpa, C.O. Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Curr. Opin. Environ. Sci. Health 2018. [Google Scholar] [CrossRef]
- Neilsen, D.; Neilsen, G.H.; Sinclair, A.H.; Linehan, D.J. Soil phosphorus status, pH and the manganese nutrition of wheat. Plant Soil 1992, 145, 45–50. [Google Scholar] [CrossRef]
- Zeidan, M.S.; Mohamed, M.F.; Hamouda, H.A. Effect of foliar fertilization of Fe, Mn and Zn on wheat yield and quality in low sandy soils fertility. World J. Agric. Sci. 2010, 6, 696–699. [Google Scholar]
- Abbas, G.; Khan, M.Q.; Khan, M.J.; Tahir, M.; Ishaque, M.; Hussain, F. Nutrient uptake, growth and yield of wheat (Triticum aestivum L.) as affected by manganese application. Pakistan J. Bot. 2011, 43, 607–616. [Google Scholar]
- Rawat, M.; Nayan, R.; Negi, B.; Zaidi, M.G.H.; Arora, S. Physio-biochemical basis of iron-sulfide nanoparticle induced growth and seed yield enhancement in B. juncea. Plant Physiol. Biochem. 2017, 118, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ortiz, C.; Adeleye, A.S.; Hu, Q.; Zhou, H.; Huang, Y.; Keller, A.A. Metabolomics to Detect Response of Lettuce (Lactuca sativa) to Cu(OH)2 Nanopesticides: Oxidative stress response and detoxification mechanisms. Environ. Sci. Technol. 2016, 50, 9697–9707. [Google Scholar] [CrossRef] [PubMed]
- Pedas, P.; Husted, S.; Skytte, K.; Schjoerring, J.K. Elevated phosphorus impedes manganese acquisition by barley plants. Front. Plant Sci. 2011, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Fageria, N.K. Influence of micronutrients on dry matter yield and interaction with other nutrients in annual crops. Pesq. Agropec. Bras. 2002, 37, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Lidon, F.J.C. Rice adaptation to excess manganese: Nutrient accumulation and implications of the quality of crops. J. Plant Physiol. 2000, 156, 652–658. [Google Scholar] [CrossRef]
- Haynes, R.J. Ion exchange properties of roots and ionic interactions within the root apoplasm: Their role in ion accumulation by plants. Bot. Rev. 1980, 46, 75–99. [Google Scholar] [CrossRef]
- Tong, Y.; Rengel, Z.; Graham, R.D. Interactions between nitrogen and manganese nutrition of barley genotypes differing in manganese efficiency. Ann. Bot. 1997, 79, 53–58. [Google Scholar] [CrossRef]
- Sims, T.J. Soil effects on the distribution and plant availability of manganese, copper and zinc. Soil Sci. Soc. Ame. J. 1986, 50, 367–373. [Google Scholar] [CrossRef]
- Xin, X.P.; Wright, A.L.; He, Z.L.; Jiang, X.J. Manganese oxide affects nitrification and N2O emissions in a subtropical paddy soil with variable water regimes. Eur. J. Soil Sci. 2017, 68, 749–757. [Google Scholar] [CrossRef]
| Treatment | Tiller Number (Anthesis) | Plant Height | Root Dry Weight (g/Plant) | Shoot Dry Weight (g/Plant) | Grain Yield (g/Plant) |
|---|---|---|---|---|---|
| Control | 36 ± 5 | 98 ± 5 | 11 ± 6 | 84 ± 7 | 32 ± 7 |
| Nano | 41 ± 6 | 95 ± 4 | 11 ± 4 | 81 ± 4 | 37 ± 8 |
| Salt | 43 ± 8 | 96 ± 6 | 10 ± 3 | 82 ± 7 | 35 ±13 |
| Bulk | 38 ± 5 | 98 ± 6 | 10 ± 3 | 83 ± 4 | 36 ± 9 |
| Nano (foliar) | 39 ± 5 | 97 ± 7 | 8 ± 4 | 84 ± 5 | 39 ± 7 |
| ANOVA | NS | NS | NS | NS | NS |
| Treatment | pH | Mn | Ammonium-N | Nitrate-N | P | K |
|---|---|---|---|---|---|---|
| Pre-growth | 6.87 | 12.4 | ||||
| Post-harvest | ||||||
| Control | 6.11 a | 4.65 b | 4.99 a | 1.33 ab | 8.40 d | 177 a |
| Nano | 6.12 a | 5.62 b | 5.45 a | 1.73 a | 10.40 c | 184 a |
| Salt | 6.13 a | 6.16 a | 5.03 a | 1.01 b | 12.20 b | 174 a |
| Bulk | 6.19 a | 4.61 b | 4.31 a | 1.06 b | 16.70 a | 173 a |
| Nano (foliar) | 6.26 a | 4.63 b | 4.70 a | 1.03 b | 12.20 b | 166 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. https://doi.org/10.3390/agronomy8090158
Dimkpa CO, Singh U, Adisa IO, Bindraban PS, Elmer WH, Gardea-Torresdey JL, White JC. Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.). Agronomy. 2018; 8(9):158. https://doi.org/10.3390/agronomy8090158
Chicago/Turabian StyleDimkpa, Christian O., Upendra Singh, Ishaq O. Adisa, Prem S. Bindraban, Wade H. Elmer, Jorge L. Gardea-Torresdey, and Jason C. White. 2018. "Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.)" Agronomy 8, no. 9: 158. https://doi.org/10.3390/agronomy8090158
APA StyleDimkpa, C. O., Singh, U., Adisa, I. O., Bindraban, P. S., Elmer, W. H., Gardea-Torresdey, J. L., & White, J. C. (2018). Effects of Manganese Nanoparticle Exposure on Nutrient Acquisition in Wheat (Triticum aestivum L.). Agronomy, 8(9), 158. https://doi.org/10.3390/agronomy8090158






