Impacts of Trace Element Addition on Lentil (Lens culinaris L.) Agronomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Soil
2.2. Experimental Design and Treatments
2.3. Protein Percentage
2.4. Chemical Analysis of Plant Samples
2.5. Soil Analysis
- DP = Particle density (g cm−3);
- Vs = Volume of soil solid (cm−3);
- Ms = Weight of soil solid (g).
- Db = Bulk density (g cm−3);
- Ms = Mass of soil solid (g);
- Vt = Total volume of soil (cm−3).
- Dp = Particle density (g cm−3);
- Db = Bulk density (g cm−3).
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Micronutrients on Morpho-Physiological Characters of Lentil
3.1.1. Plant Height
3.1.2. Branches Plant−1
3.2. Effect of Micronutrients on Yield Components of Lentil
3.2.1. Number of Pods Plant−1
3.2.2. Number of Seeds Pod−1
3.2.3. Thousand Seed Weight
3.2.4. Seed Yield
3.3. Effects of Micronutrients on the Protein Content of Lentil Seed
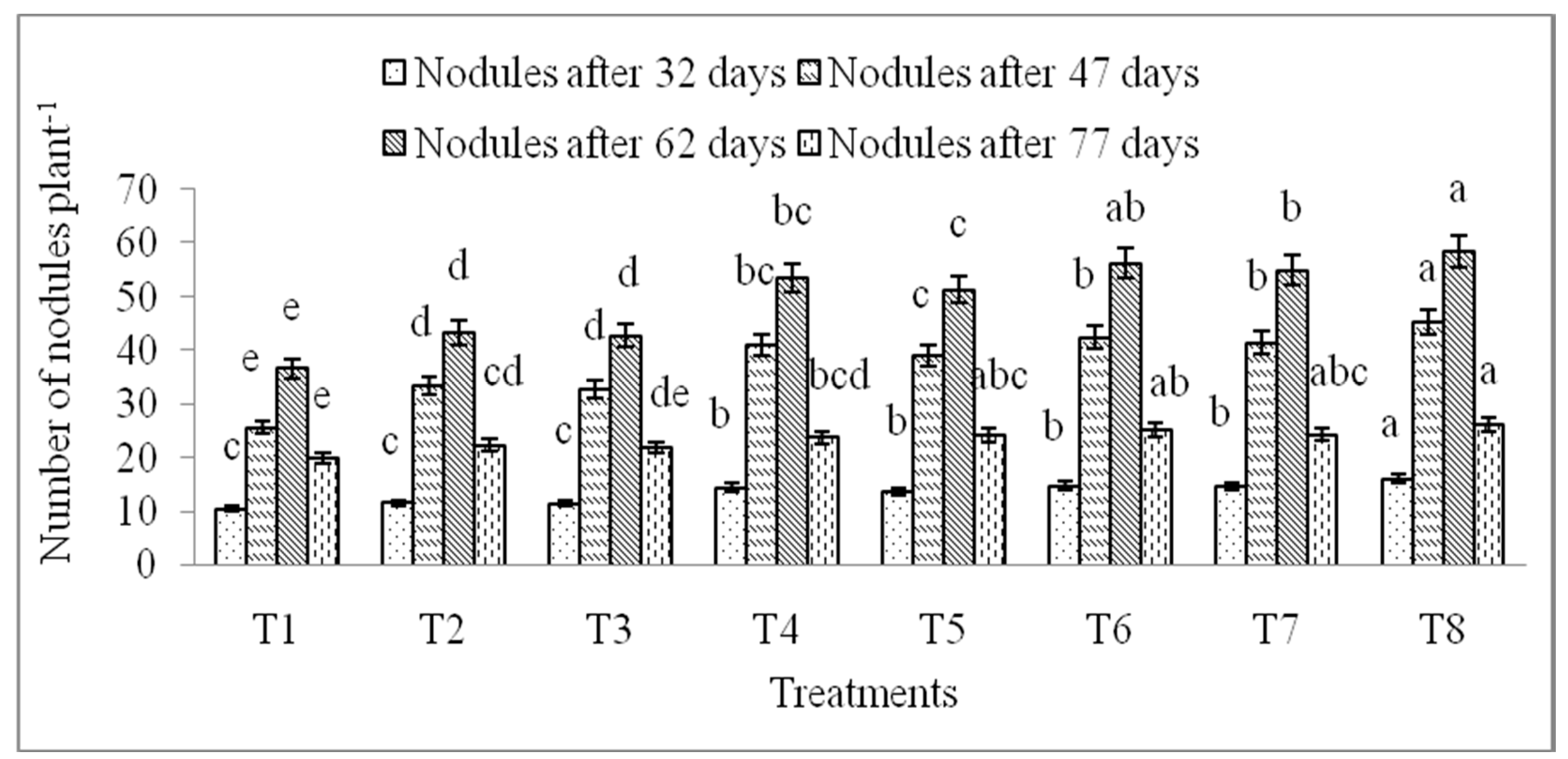
3.4. Effects of Micronutrients on the Nodule Formation of Lentil
3.5. Effects of Micronutrients on the N, P, K, S, Zn, and B Content of Lentil
3.6. Effects of Micronutrients on Postharvest Soil Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faris, M.A.I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterr. J. Nutr. Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Singh, A.K.; Bhatt, B.P.; Upadhya, A.; Singh, B.K.; Kumar, S.; Sundaram, P.K.; Chndra, N.; Bharati, R.C. Improvement of faba bean (Vicia faba L.) yield and quality through biotechnological approach: A review. Afr. J. Biotechnol. 2012, 11, 15264–15271. [Google Scholar]
- Zeidan, M.S.; Hozayn, M.; Abd El-Salam, M.E.E. Yield and Quality of Lentil as Affected by Micronutrient Deficiencies in Sandy Soils. J. Appl. Sci. Res. 2006, 2, 1342–1345. [Google Scholar]
- Deo, K.; Singh, S.B. Effect of Zinc and Boron application on Yield of Lentil and Nutrient Balance in the Soil under Indo-Gangetic Plain Zones. J. AgriSearch 2014, 1, 206–209. [Google Scholar]
- Singh, S.S.; Singh, A.K.; Sundaram, P.K. Agrotechnological options for upscaling agricultural productivity in eastern indo gangetic plains under impending climate change situations: A review. J. AgriSearch 2014, 1, 55–65. [Google Scholar]
- Khan, H.R.; McDonald, G.K.; Rengel, Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arietinum). Plant Soil 2004, 267, 271–284. [Google Scholar] [CrossRef]
- Ahlawat, I.P.S.; Gangaiah, B.; Ashraf Zadid, M. Nutrient management in chickpea. In Chickpea Breeding and Management; Yadav, S.S., Redden, R., Chen, W., Sharma, B., Eds.; CAB International: Wallingford, UK, 2007; pp. 213–232. [Google Scholar]
- Hamilton, M.A.; Westermann, D.T.; James, D.W. Factors affecting zinc uptake in cropping systems. Soil Sci. Soc. Am. J. 1993, 57, 1310–1315. [Google Scholar] [CrossRef]
- Singh, M.; Ram, N. Effect of soil enrichment with zinc on crop yields and its replenishment in Mollisols of northern India. Agrochimica 1996, 40, 19–24. [Google Scholar]
- Singh, A.K.; Khan, M.A.; Srivastava, A. Effect of boron and molybdenum application on seed yield of mungbean. Asian J. Biol. Sci. 2014, 9, 169–172. [Google Scholar] [CrossRef]
- Subasinghe, S.; Dayatilake, G.A.; Senaratne, R. Effect of B, Co and Mo on nodulation, growth and yield of cowpea (Vigna unguiculata). Trop. Agric. Res. Ext. 2003, 6, 108–112. [Google Scholar]
- Hansch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A. Growth and yield of lentil (Lens culinaris L.) as affected by Boron and Molybdenum application in lateritic soil. J. Crop Weed 2009, 5, 88–91. [Google Scholar]
- Chatterjee, R.; Bandyopadhyay, S. Effect of boron, molybdenum and biofertilizers on growth and yield of cowpea (Vigna unguiculata L. Walp.) in acid soil of eastern Himalayan region. J. Saudi Soc. Agric. Sci. 2017, 16, 332–336. [Google Scholar] [CrossRef]
- Valenciano, J.B.; Marcelo, V.; Boto, J.A. Response of chickpea (Cicer arietinum) yield to micronutrient application under pot conditions in Spain. Span. J. Agric. Res. 2010, 8, 797–807. [Google Scholar] [CrossRef]
- Quddus, M.A.; Mian, M.J.A.; Naser, H.M.; Hossain, M.A.; Sultana, S. Maximizing Yields, Nutrient Uptake and Balance for Mustard-Mungbean-T. Aman Rice Cropping Systems through Nutrient Management Practices in Calcareous Soils. J. Agric. Sci. 2017, 9, 210–229. [Google Scholar] [CrossRef]
- Birru, A. Agricultural Field Experiment Management Manual Part III; IAR (Institute of Agricultural Research): Addis Ababa, Ethiopia, 1979; pp. 35–42. [Google Scholar]
- Food and Agriculture Origination Publication (FAO). Manuals of Food Quality Control, Food Analysis: General Techniques Additives, Contaminants, and Composition; FAO Food and Nutrition Paper 14/7; Centre for Food Safety, UN: Rome, Italy, 1986.
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Bombay, India, 1966; pp. 368–392. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Page, A.L.; Miller, R.H.; Kuny, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of American Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Black, C.A. Methods of Soil Analysis Part-II; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; p. 770. [Google Scholar]
- Piper, G.S. Soil and Plant Analysis; Adelaide University, Hassell Press: Adelaide, Australia, 1950. [Google Scholar]
- Barker, D.E.; Surh, N.H. Atomic Absorption and Flame Emission Spectroscopy. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; SSSA Book Series No. 9; SSSA: Madison, WI, USA; ASA: Madison, WI, USA, 1982; pp. 13–26. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedure for Agricultural Research; John Willey and Sons: New York, NY, USA, 1984. [Google Scholar]
- Singh, A.; Singh, B.B.; Patel, C.S. Response of vegetable pea (Pisum sativum) to zinc, boron and molybdenum in an acidi Alsifol of Meghalaya. Indian J. Agron. 1992, 37, 615–616. [Google Scholar]
- Basavrajeswari, C.P.; Hosamani, R.M.; Ajjappalavara, P.S.; Naik, B.H.; Smitha, R.P.; Ukkund, K.C. Effect of foliar application of micronutrients on growth, yield components of Tomato (Lycopersicon esculentum Mill.). Karnataka J. Agric. Sci. 2008, 21, 428–430. [Google Scholar]
- Sharma, S.K. Effect of boron and calcium on seed production of Bell pepper (Capsicum annuum L.). Veg. Sci. 1999, 26, 87–88. [Google Scholar]
- Kiran, J.V.; Yakaranchal, B.S.; Raikar, S.D.; Ravikumar, G.H.; Deshpande, V.K. Seed yield and quality of brinjal as influenced by crop nutrition. Indian J. Agric. Res. 2010, 44, 1–7. [Google Scholar]
- Mohanty, S.K.; Sahoo, L.P.; Dash, S. Effect of bio-fertilizer and micronutrients on vegetative and reproductive growth behaviour and seed yield in tomato. In Proceedings of the XII National Seed Seminar, UAS Bengaluru, Bengaluru, India, 8–10 June 2013; Abstracts. p. 41. [Google Scholar]
- Hatwar, G.P.; Gondane, S.M.; Urkade, S.M.; Ahukar, O.V. Effect of micronutrients on growth and yield of chilli. Soils Crops 2003, 13, 123–125. [Google Scholar]
- Bhatia, V.S.; Singh, B.N.; Lal, S. Variability and interrelationship of yield and its attributes in chickpea. Indian J. Pulses Res. 1993, 6, 1–5. [Google Scholar]
- Taliee, A.; Sayadian, K. Effect of supplemental irrigation and plant nutrient in chickpea (dry farming). J. Agron. Crop Sci. 2000, 2, 12–19. [Google Scholar]
- Nadergoli, M.S.; Yarnia, M.; Khoei, F.R. Effect of zinc and manganese and their application method on yield and yield components of common bean. Middle-East J. Sci. Res. 2011, 8, 859–865. [Google Scholar]
- Quddus, M.A.; Rashhid, M.H.; Hossain, M.A.; Naser, H.M. Effect of zinc and boron on yield and yield contributing characters of mungbean in low ganges river floodplain soil at madaripur, Bangladesh. Bangladesh J. Agric. Res. 2011, 36, 75–85. [Google Scholar] [CrossRef]
- Valenciano, J.B.; Boto, J.A.; Marcelo, V. Chickpea (Cicer arietinum L.) response to zinc, boron and molybdenum application under field conditions. N. Z. J. Crop Hortic. Sci. 2011, 39, 217–229. [Google Scholar] [CrossRef]
- Begum, R. Effect of Some Micronutrients Application on Seed Yield and Quality in Green Gram. Master’s Thesis, Orissa University of Agriculture and Technology, Bhubaneswar, Odisha, India, 2014. [Google Scholar]
- Nasir, M.; Khalatbari, M.; Farahani, H.M. Zn-foliar application influence on quality and quality features in Phaseolus vulgaris under different levels of N and K fertilizers. Adv. Environ. Biol. 2011, 5, 839–846. [Google Scholar]
- Dordas, C.; Apostolides, G.E.; Goundra, O. Boron application affects seed yield and seed quality of sugar beets. J. Agric. Sci. 2007, 145, 377–384. [Google Scholar] [CrossRef]
- Ramu, Y.R.; Reddy, D.S. Effect of micronutrient management on growth, yield, quality and economics of hybrid maize. Crop Res. 2007, 33, 46–49. [Google Scholar]
- Punia, R.C.; Dahiya, O.S.; Jakhar, S.S. Effect of micronutrients on seed yield and quality in wheat. In Proceedings of the XII National Seed Seminar, UAS Bengaluru, Bengaluru, India, 8–10 June 2013; Abstracts. p. 75. [Google Scholar]
- Yang, M.; Shi, L.; Xu, F.S.; Lu, J.W.; Wang, Y.H. Effects of B, Mo, Zn and their interactions on seed yield of rapeseed. Pedosphere 2009, 19, 53–59. [Google Scholar] [CrossRef]
- Jain, G.L. Secondary and micronutrients in relation to ‘mothbean’ (Phaseolus aconitifolius Jacq.). Effect of soil application on uptake and fixation of N. Indian J. Agron. 1973, 18, 517–519. [Google Scholar]
- Barik, T.; Rout, D. Effect of foliar spray of commercial micronutrient mixtures on growth, yield and quality of urdbean. Legum. Res. 1990, 13, 50–62. [Google Scholar]
- Rosolem, C.A.; Caires, E.F. Yield and nitrogen uptake of peanuts as affected by lime, cobalt and molybdenum. J. Plant Nutr. 1998, 21, 827–835. [Google Scholar] [CrossRef]
- O'Hara, G.W. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Bolanos, L.; Brewin, N.J.; Bonilla, I. Effects of boron on Rhizobium-legume cell-surface interactions and nodule development. Plant Physiol. 1996, 110, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.M.; Roll, J.T.; Rangaraj, P.; Shah, V.K.; Roberts, G.P.; Ludden, P.W. Incorporation of molybdenum into the iron-molybdenum cofactor of nitrogenase. J. Biol. Chem. 1999, 274, 15869–15874. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.A.; Munns, D.N. Response of Phaseolus vulgaris L. to molybdenum under acid conditions. Soil Sci. Soc. Am. J. 1981, 45, 1144–1148. [Google Scholar] [CrossRef]
- Vieira, R.F.; Cardoso, E.J.B.N.; Vieira, C.; Cassini, S.T.A. Foliar application of molybdenum in common beans. I. Nitrogenase and reductase activities in a soil of high fertility. J. Plant Nutr. 1998, 21, 169–180. [Google Scholar] [CrossRef]
- Yanni, Y.G. Performance of chickpea, lentil and lupin nodulated with indigenous or inoculated rhizobia micropartners under nitrogen, boron, cobalt and molybdenum fertilization schedules. World J. Microbiol. Biotechnol. 1992, 8, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Kumar, T.N.; Ahmed, S. Effect of Zinc, Boron and Molybdenum application on the yield and nutrient uptake by BRRI Dhan 30. Online J. Biol. Sci. 2001, 1, 698–700. [Google Scholar]
- Sarker, S.K.; Chowdhury, M.A.H.; Zakir, H.M. Sulphur and boron fertilization on yield quality and nutrient uptake by Bangladesh Soybean-4. Online J. Biol. Sci. 2002, 2, 729–733. [Google Scholar]
- Okaz, A.M.A.; El-Gareib, E.A.; Kadry, W.; Negm, A.Y.; Zahran, F.A.F. Micronutrient application to lentil plants grown on newly reclaimed sandy soils. Proceedings of 6th Conference of Agronomy, Al-Azhar University, Cairo, Egypt, 2–4 September 1994; Volume II, pp. 737–752. [Google Scholar]
- Singh, A.K.; Meena, M.K.; Bharati, R.C.; Gade, R.M. Effect of sulphur and zinc management on yield, nutrient uptake, changes in soil fertility and economics in rice (Oryza sativa)–lentil (Lens culinaris) cropping system. Indian J. Agric. Sci. 2013, 83, 344–348. [Google Scholar]
- Iqtidar, A.; Rahman, S.F. Effect of boron on the protein and amino acid composition of wheat grain. J. Agric. Sci. 1984, 103, 75–80. [Google Scholar] [CrossRef]
- Ogbodo, E.N. Effect of crop residue on soil chemical properties and rice yield on an Ultisol at Abakaliki, Southeastern Nigeria. World J. Agric. Sci. 2011, 7, 13–18. [Google Scholar]
- Hinsinger, P. How do plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Adv. Agron. 1998, 64, 225–265. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Hinsinger, P. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–50. [Google Scholar] [CrossRef]
- Ahlawat, I.P.S.; Srivastava, T.K. Fertility management in pulse based cropping systems. In Recent Advances in Pulses Research; Asthana, A.N., Masood, A., Eds.; Indian Society of Pulses Research and Development: Kanpur, India, 1997; pp. 509–523. [Google Scholar]
- Rao, J.V.; Bhardwaj, R.B.L. Influence of residual fertility on green gram in intensive cropping system. Indian J. Agron. 1980, 25, 97–101. [Google Scholar]
- Singh, S.K.; Varma, S.C.; Singh, R.P. Economics and nutrient uptake in Rice-Lentil cropping sequence. Agric. Sci. Dig. 2003, 23, 122–124. [Google Scholar]
| Soil Properties | Value |
|---|---|
| Sand % | 26.28 |
| Silt % | 38.20 |
| Clay % | 35.52 |
| Textural class (0–15 cm) | Clay loam |
| Particle density (g cm−3) | 2.51 |
| Bulk density (g cm−3) | 1.35 |
| Porosity (%) | 46.22 |
| pH | 6.6 |
| Exchangeable K (meq. 100 g−1) | 0.11 |
| Exchangeable Ca (meq. 100 g−1) | 6.01 |
| Exchangeable Mg (meq. 100 g−1) | 2.02 |
| Organic matter (%) | 1.28 |
| Total N (%) | 0.057 |
| Available P (µg g−1) | 23.5 |
| Available S (µg g−1) | 26.0 |
| Available Zn (µg g−1) | 1.31 |
| Available B (µg g−1) | 0.16 |
| Available Mo (µg g−1) | 0.072 |
| Treatments | Plant Height (cm) | Branches Plant−1 | Pods Plant−1 | Seeds Pod−1 | 1000 Seeds wt. (gm) | Seed Yield (Kg/ha) | Seed Protein (%) |
|---|---|---|---|---|---|---|---|
| T1 = Control | 31.8 e | 2.54 c | 36.9 e | 1.62 d | 17.3 c | 822 d | 20.8 b |
| T2 = Zn 2.0 kg ha−1 | 34.7 bc | 2.74 b | 51.1 bcd | 1.77 bc | 18.7 b | 1081 bc | 24.8 a |
| T3 = B 1.5 kg ha−1 | 34.1 cd | 2.76 b | 48.2 cd | 1.80 b | 18.6 b | 1066 c | 24.6 a |
| T4 = Mo 1.0 kg ha−1 | 33.5 d | 2.69 bc | 48.0 d | 1.76 bc | 18.3 b | 1015 c | 25.3 a |
| T5 = Zn2.0B1.5 | 35.1 ab | 3.04 a | 62.0 a | 1.88 a | 19.6 a | 1203 ab | 25.2 a |
| T6 = Zn2.0Mo1.0 | 34.9 bc | 2.85 b | 52.9 b | 1.79 bc | 18.4 b | 1105 bc | 25.6 a |
| T7 = B1.5Mo1.0 | 34.8 bc | 2.84 b | 52.4 bc | 1.78 bc | 18.2 b | 1096 bc | 25.8 a |
| T8 = Zn2.0B1.5Mo1.0 | 35.9 a | 3.06 a | 65.0 a | 1.90 a | 19.7 a | 1256 a | 26.7 a |
| CV (%) | 1.70 | 3.33 | 4.72 | 1.46 | 1.55 | 6.61 | 6.71 |
| LSD (0.05) | 1.04 | 0.164 | 4.29 | 0.046 | 0.504 | 1.27 | 2.92 |
| Treatments | N (%) | P (%) | K (%) | S (%) | Zn (μg g−1) | B (μg g−1) |
|---|---|---|---|---|---|---|
| T1 = Control | 3.92 b | 0.22 e | 0.61 c | 0.13 d | 59.0 d | 31.2 d |
| T2 = Zn 2.0 kg ha−1 | 4.68 a | 0.29 cd | 0.62 c | 0.15 cd | 70.8 ab | 32.3 d |
| T3 = B 1.5 kg ha−1 | 4.65 a | 0.30 bcd | 0.69 bc | 0.14 d | 65.6 c | 37.5 c |
| T4 = Mo 1.0 kg ha−1 | 4.78 a | 0.28 d | 0.76 ab | 0.17 cd | 67.5 bc | 32.7 d |
| T5 = Zn2.0B1.5 | 4.76 a | 0.33 abc | 0.79 ab | 0.20 bc | 70.0 abc | 40.1 ab |
| T6 = Zn2.0Mo1.0 | 4.83 a | 0.31 bcd | 0.77 ab | 0.23 b | 69.3 abc | 38.7 bc |
| T7 = B1.5Mo1.0 | 4.88 a | 0.34 ab | 0.81 a | 0.25 b | 68.1 abc | 40.6 ab |
| T8 = Zn2.0B1.5Mo1.0 | 5.04 a | 0.36 a | 0.86 a | 0.34 a | 72.4 a | 41.5 a |
| CV (%) | 6.79 | 9.14 | 7.84 | 10.2 | 3.83 | 3.25 |
| LSD (0.05) | 0.56 | 0.049 | 0.102 | 0.076 | 4.56 | 2.10 |
| Treatments | N (%) | P (%) | K (%) | S (%) | Zn (μg g−1) | B (μg g−1) |
|---|---|---|---|---|---|---|
| T1 = Control | 1.18 e | 0.12 e | 0.59 e | 0.45 e | 42.1 d | 25.6 d |
| T2 = Zn 2.0 kg ha−1 | 1.42 d | 0.17 a | 0.63 d | 0.49 d | 48.2 ab | 26.1 d |
| T3 = B 1.5 kg ha−1 | 1.41 d | 0.16 ab | 0.64 d | 0.50 cd | 43.3 cd | 28.1 c |
| T4 = Mo 1.0 kg ha−1 | 1.52 cd | 0.15 bc | 0.65 cd | 0.48 d | 44.5 cd | 27.6 c |
| T5 = Zn2.0B1.5 | 1.59 bc | 0.14 cd | 0.69 b | 0.52 b | 47.8 ab | 30.0 b |
| T6 = Zn2.0Mo1.0 | 1.64 abc | 0.13 de | 0.68 b | 0.51 bc | 45.8 bc | 28.0 c |
| T7 = B1.5Mo1.0 | 1.67 ab | 0.16 ab | 0.67 bc | 0.50 cd | 48.5 ab | 29.2 b |
| T8 = Zn2.0B1.5Mo1.0 | 1.74 a | 0.18 a | 0.72 a | 0.54 a | 49.1a | 31.7 a |
| CV (%) | 4.62 | 6.17 | 2.56 | 1.90 | 3.48 | 1.93 |
| LSD (0.05) | 0.123 | 0.016 | 0.030 | 0.017 | 2.82 | 0.960 |
| Treatment | pH | OM (%) | Total N (%) | Ca | Mg | K | P | S | Zn | B |
|---|---|---|---|---|---|---|---|---|---|---|
| meq. 100 g−1 | µg g−1 | |||||||||
| Initial | 6.61 | 1.28 | 0.057 | 6.01 | 2.02 | 0.11 | 23.5 | 26.0 | 1.31 | 0.16 |
| Critical level | 0.12 | 2.0 | 0.80 | 0.20 | 10.0 | 10.0 | 0.60 | 0.2 | ||
| T1 = Zn0B0Mo0 | 6.71 | 1.35 | 0.062 | 5.90 | 2.00 | 0.10 | 24.0 | 25.2 | 1.28 | 0.14 |
| T2 = Zn2.0 | 6.78 | 1.45 | 0.064 | 6.01 | 2.00 | 0.11 | 24.5 | 25.3 | 1.41 | 0.15 |
| T3 = B1.5 | 6.77 | 1.38 | 0.065 | 6.01 | 2.01 | 0.11 | 24.6 | 25.5 | 1.31 | 0.20 |
| T4 = Mo1.0 | 6.82 | 1.39 | 0.067 | 6.00 | 2.02 | 0.10 | 24.8 | 25.8 | 1.32 | 0.16 |
| T5 = Zn2.0B1.5 | 6.93 | 1.47 | 0.069 | 5.89 | 2.00 | 0.11 | 24.6 | 25.7 | 1.43 | 0.23 |
| T6 = Zn2.0Mo1.0 | 6.93 | 1.47 | 0.068 | 5.91 | 2.01 | 0.10 | 24.7 | 25.6 | 1.44 | 0.17 |
| T7 = B1.5Mo1.0 | 6.96 | 1.49 | 0.070 | 5.92 | 2.02 | 0.10 | 24.6 | 25.7 | 1.36 | 0.26 |
| T8 = Zn2.0B1.5Mo1.0 | 7.01 | 1.50 | 0.072 | 5.88 | 2.00 | 0.11 | 24.7 | 25.9 | 1.45 | 0.27 |
| NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Karim, M.R.; Oliver, M.M.H.; Urmi, T.A.; Hossain, M.A.; Haque, M.M. Impacts of Trace Element Addition on Lentil (Lens culinaris L.) Agronomy. Agronomy 2018, 8, 100. https://doi.org/10.3390/agronomy8070100
Islam MM, Karim MR, Oliver MMH, Urmi TA, Hossain MA, Haque MM. Impacts of Trace Element Addition on Lentil (Lens culinaris L.) Agronomy. Agronomy. 2018; 8(7):100. https://doi.org/10.3390/agronomy8070100
Chicago/Turabian StyleIslam, Md. Moshiul, Md. Razaul Karim, Md. Moinul Hosain Oliver, Tahmina Akter Urmi, Md. Ashraf Hossain, and M. Moynul Haque. 2018. "Impacts of Trace Element Addition on Lentil (Lens culinaris L.) Agronomy" Agronomy 8, no. 7: 100. https://doi.org/10.3390/agronomy8070100
APA StyleIslam, M. M., Karim, M. R., Oliver, M. M. H., Urmi, T. A., Hossain, M. A., & Haque, M. M. (2018). Impacts of Trace Element Addition on Lentil (Lens culinaris L.) Agronomy. Agronomy, 8(7), 100. https://doi.org/10.3390/agronomy8070100





