Irrigation Water Quality and Soil Structural Stability: A Perspective with Some New Insights
Abstract
:1. Introduction
2. Soil Structural Stability in Water
2.1. Aggregation of Soil Particles and Water Stability of Aggregates
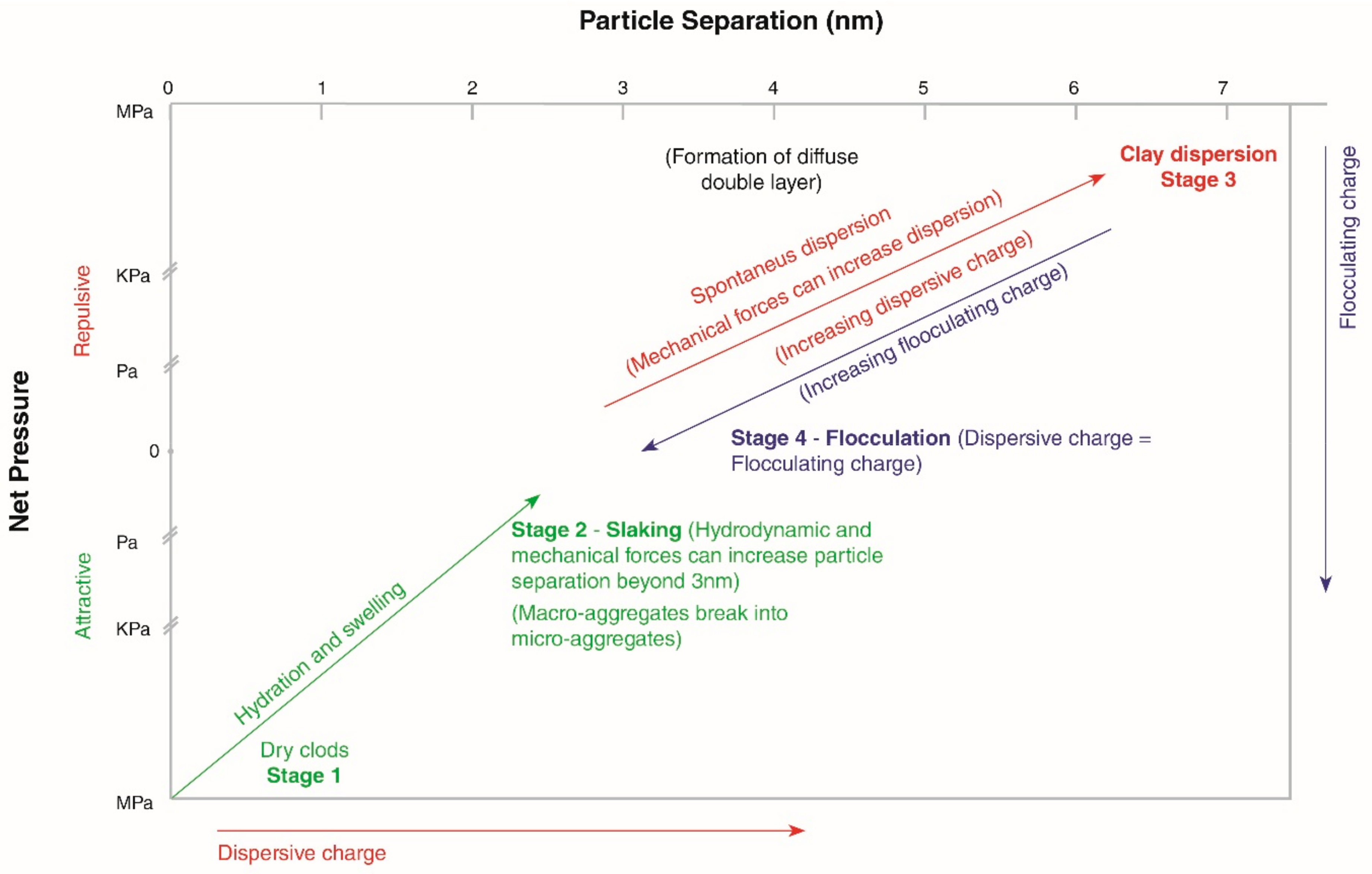
2.2. Processes Leading to Structural Changes on Wetting of Dry Aggregates
2.3. Repulsive Forces in Relation to Cations and Anions
2.4. Net Dispersive Charge in Relation to Clay Dispersion
3. Deficits in the Models Based on Quirk-Schofield Concept on ‘Threshold Electrolyte Concentration’
4. Modification of TEC Concept Based on New Insights
4.1. Use of Net Dispersive Charge to Explain Soil Structural Stability
4.2. Validity of Models Based on Irrigation Water Quality
5. Conclusions and Future Studies
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Oster, J.D.; Jayawardane, N.S. Agricultural management of sodic soils. In Sodic Soils: Distribution, Management and Environmental Consequences; Sumner, M.E., Naidu, R., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 125–147. [Google Scholar]
- Rengasamy, P.; Olsson, K.A. Irrigation and sodicity. Aust. J. Soil Res. 1993, 31, 821–837. [Google Scholar] [CrossRef]
- Quirk, J.P.; Schofield, R.K. The effect of electrolyte concentration on soil permeability. Eur. J. Soil Sci. 1955, 6, 163–178. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Kirk, G.J.D.; Bourrie, G. Landmark papers: No.2. Commentary on the impact of Quirk & Schofield (1955). Eur. J. Soil Sci. 2013, 64, 3–15. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water quality for agriculture. In FAO Irrigation and Drainage; Paper 29 (Rev.1); FAO: Rome, Italy, 1985. [Google Scholar]
- De Menezes, H.R.; de Almeida, B.; Bennett, J.M.; da Silva, E.; Freire, M. Use of threshold electrolyte concentration analysis to determine salinity and sodicity limit of irrigation water. Rev. Bras. Eng. Agric. Ambient. 2014, 18, S53–S58. [Google Scholar] [CrossRef]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach, 2nd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015; 633p. [Google Scholar]
- Ezlit, Y.; Bennett, J.M.; Raine, S.R.; Smith, R. Modification of the McNeal clay swelling model improves prediction of saturated hydraulic conductivity as a function of applied water quality. Soil Sci. Soc. Am. J. 2013, 77, 2149–2156. [Google Scholar] [CrossRef]
- Jayawardane, N.S. An equivalent salt solutions method for predicting hydraulic conductivities of soils for different salt solutions. Aust. J. Soil Res. 1979, 17, 423–428. [Google Scholar] [CrossRef]
- McNeal, B.L.; Coleman, N.T. Effects of solution composition on soil hydraulic conductivity. Soil Sci. Soc. Am. J. 1966, 30, 308–312. [Google Scholar] [CrossRef]
- Rengasamy, P.; Greene, R.; Ford, G.W.; Mehanni, A.H. Identification of dispersive behaviour and the management of red-brown earths. Aust. J. Soil Res. 1984, 22, 413–431. [Google Scholar] [CrossRef]
- Amezketa, E. Soil aggregate stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Dexter, A.R. Advances in characterization of soil structure. Soil Tillage Res. 1988, 11, 199–238. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Effect of heating on swelling and dispersion of different cationic forms of a smectite. Clays Clay Miner. 1996, 44, 783–790. [Google Scholar] [CrossRef]
- Rengasamy, P.; Sumner, M.E. Processes Involved in Sodic Behaviour. In Sodic Soils: Distribution, Management and Environmental Consequences; Sumner, M.E., Naidu, R., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 35–50. [Google Scholar]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry, 2nd ed.; John Wiley: New York, NY, USA, 1977. [Google Scholar]
- Greene, R.S.B.; Posner, A.M.; Quirk, J.P. Factors affecting the formation of quasi-crystals of montmorillonite. Soil Sci. Soc. Am. J. 1973, 37, 457–460. [Google Scholar] [CrossRef]
- Swartzen-Allen, S.L.; Matijevic, E. Surface and colloid chemistry of clays. Chem. Rev. 1974, 74, 385–400. [Google Scholar] [CrossRef]
- Kjellander, R.; Marcelja, S.; Quirk, J.P. Attractive double-layer interactions between calcium clay particles. J. Colloid Interface Sci. 1988, 126, 194–211. [Google Scholar] [CrossRef]
- Rengasamy, P.; Tavakkoli, E.; McDonald, G.K. Exchangeable cations and clay dispersion: Net dispersive charge, a new concept for dispersive soil. Eur. J. Soil Sci. 2016, 67, 659–665. [Google Scholar] [CrossRef]
- Rengasamy, P.; Olsson, K.A. Sodicity and soil structure. Aust. J. Soil Res. 1991, 29, 935–952. [Google Scholar] [CrossRef]
- Slade, P.G.; Quirk, J.P.; Norrish, K. Crystalline swelling of smectite samples in concentrated NaCl solutions in relation to layer charge. Clays Clay Miner. 1991, 39, 234–238. [Google Scholar] [CrossRef]
- Sposito, G.; Skipper, N.T.; Sutton, R.; Park, S.; Soper, A.K.; Greathouse, J.A. Surface geochemistry of the clay minerals. Proc. Natl. Acad. Sci. USA 1999, 96, 3358–3364. [Google Scholar] [CrossRef] [PubMed]
- Marchuk, A.; Rengasamy, P. Clay behaviour in suspension is related to the ionicity of clay-cation bonds. Appl. Clay Sci. 2011, 53, 754–759. [Google Scholar] [CrossRef]
- Laird, D.A. Influence of layer charge on swelling of smectites. Appl. Clay Sci. 2006, 34, 74–87. [Google Scholar] [CrossRef]
- Norrish, K. The swelling of montmorillonite. Trans. Faraday Soc. 1954, 18, 120–134. [Google Scholar] [CrossRef]
- Hu, F.; Liu, J.; Xu, C.; Wang, Z.; Liu, G.; Li, H.; Zhao, S. Soil internal forces initiate aggregate breakdown and splash erosion. Geoderma 2018, 320, 43–51. [Google Scholar] [CrossRef]
- Emerson, W.W. Inter-particle bonding. In Soils: An Australian Viewpoint; Academic Press: London, UK, 1983; pp. 477–498. [Google Scholar]
- Emerson, W.W. Emerson Dispersion Test. In Soil Physical Measurement and Interpretation for Land Evaluation; McKenzie, N., Coughlan, K., Cresswell, K., Eds.; CSIRO Publishing: Collingwood, Australia, 2002; pp. 190–199. [Google Scholar]
- Chorom, M.; Rengasamy, P.; Murray, R.S. Clay dispersion as influenced by pH and net particle charge of sodic soils. Aust. J. Soil Res. 1994, 32, 1243–1252. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Rengasamy, P.; Oades, J.M. Interaction of monomeric and polymeric species of metal ions with clay surfaces. IV. Mixed system of aluminium (III) and iron (III). Aust. J. Soil Res. 1979, 17, 141–153. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Dispersion and zeta potential of pure clays as related to net particle charge under varying pH, electrolyte concentration and cation type. Eur. J. Soil Sci. 1995, 46, 657–665. [Google Scholar] [CrossRef]
- Marchuk, A.; Rengasamy, P.; McNeill, A. Influence of organic matter, clay mineralogy, and pH on the effects of CROSS on soil structure is related to the zeta potential of the dispersed clay. Soil Res. 2013, 51, 34–40. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, L.; Patey, I. The influence of individual clay minerals on formation damage of reservoir sandstones: A critical review with some new insights. Clay Miner. 2014, 49, 147–164. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil chemistry factors confounding crop salinity tolerance—A Review. Agronomy 2016, 6, 53. [Google Scholar] [CrossRef]
- Dang, A.; Bennett, J.M.; Marchuk, A.; Biggs, A.; Raine, S.R. Quantifying the aggregation-dispersion boundary condition in terms of saturated hydraulic conductivity reduction and the threshold electrolyte concentration. Agric. Water Manag. 2018, 203, 172–178. [Google Scholar] [CrossRef]
- Quirk, J.P. The significance of the threshold and turbidity concentrations in relation to sodicity and microstructure. Aust. J. Soil Res. 2001, 39, 1185–1217. [Google Scholar] [CrossRef]
- Sumner, M.E.; Rengasamy, P.; Naidu, R. Sodic soils: A reappraisal. In Sodic Soils: Distribution, Management and Environmental Consequences; Sumner, M.E., Naidu, R., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 3–17. [Google Scholar]
- Blackmore, A.V. Subplasticity in Australian soil. IV. Plasticity and structure related to clay cementation. Aust. J. Soil Res. 1976, 14, 261–272. [Google Scholar] [CrossRef]
- McIntyre, D.S. Exchangeable Na, subplasticity and hydraulic conductivity of some Australian soils. Aust. J. Soil Res. 1979, 17, 115–120. [Google Scholar] [CrossRef]
- So, H.B.; Aylmore, L.A.G. How do sodic soils behave—The effects of sodicity on soil physical behaviour? Aust. J. Soil Res. 1993, 31, 761–777. [Google Scholar] [CrossRef]
- Sumner, M.E. Sodic soils—New perspectives. Aust. J. Soil Res. 1993, 31, 683–750. [Google Scholar] [CrossRef]
- Bennett, J.M.; Raine, S.R. The soil specific nature of threshold electrolyte concentration analysis. In Proceedings of the 5th Joint Australian and New Zealand Soil Conference, Hobart, Australia, 2–7 December 2012; pp. 302–305. [Google Scholar]
- Smiles, D.; Smith, C. A survey of the cation content of piggery effluents and some consequences of their use to irrigate soil. Aust. J. Soil Res. 2004, 42, 231–246. [Google Scholar] [CrossRef]
- Marchuk, A.; Rengasamy, P. Threshold electrolyte concentration and dispersive potential in relation to CROSS in dispersive soils. Soil Res. 2012, 50, 473–481. [Google Scholar] [CrossRef]
- Rengasamy, P. Clay Dispersion. In Soil Physical Measurement and Interpretation for Land Evaluation; McKenzie, N.J., Coughlan, K., Cresswell, H., Eds.; CSIRO Publishing: Collingwood, Australia, 2002; Chapter 14; pp. 200–210. [Google Scholar]
- Schofield, R.K. A ratio law governing the equilibrium of cations in soil solutions. In Proceedings of the 11th International Congress of Pure and Applied Chemistry, London, UK; 1947; Volume 3, pp. 257–261. [Google Scholar]
- Arienzo, M.; Christen, E.W.; Jayawardane, N.S.; Quayle, W.C. The relative effects of Na and K on soil hydraulic conductivity and implications for winery wastewater management. Geoderma 2012, 173–174, 303–310. [Google Scholar] [CrossRef]
- Buelow, M.C.; Steenwerth, K.; Parikh, S.J. The effect of mineral-ion interactions on soil hydraulic conductivity. Agric. Water Manag. 2015, 152, 777–785. [Google Scholar] [CrossRef]
- Oster, J.D.; Sposito, G.; Smith, C.J. Accounting for potassium and magnesium in irrigation water quality assessment. Calif. Agric. 2016, 70, 71–76. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Keller, T. Impact of monovalent cations on soil structure. Part II. Results of two Swiss soils. Int. Agrophysics 2018, 32, 69–80. [Google Scholar] [CrossRef]
- Jayawardane, N.S.; Christen, E.W.; Arienzo, M.; Quayle, W.C. Evaluation of the effects of cation combinations on soil hydraulic conductivity. Soil Res. 2011, 49, 56–64. [Google Scholar] [CrossRef]
- Jenkins, B.; Morand, D. An overview of acid-sodic soils in two regions of New South Wales, Australia. In Proceedings of the 3rd Australian New Zealand Soils Conference (SuperSoil 2004), Sydney, Australia, 5–9 December 2004. [Google Scholar]
- Apesteguia, M.; Virto, I.; Orcary, L.; Bescansa, P.; Enrique, A.; Imaz, M.J.; Karlen, D.L. Tillage effects on soil quality after three years of irrigation in northern Spain. Sustainability 2017, 9, 1476. [Google Scholar] [CrossRef]
- Bower, C.A.; Ogata, G.; Tucker, J.M. Root zone soil profiles and alfalfa growth as influenced by irrigation water salinity and leaching fraction. Agron. J. 1969, 61, 783–785. [Google Scholar] [CrossRef]
- Mmolawa, K.; Or, D. Root zone solute dynamics under drip irrigation: A review. Plant Soil 2000, 222, 163–190. [Google Scholar] [CrossRef]
- Prendergast, J.B. A model of crop yield response to irrigation water salinity: Theory, testing and application. Irrig. Sci. 1993, 13, 157–164. [Google Scholar] [CrossRef]

| Solvent | Dielectric Constant at 25 °C | Slaking % < 2 mm ESP 1 | Slaking % < 2 mm ESP 20 | Dispersed Clay as % of Total Clay ESP 1 | Dispersed Clay as % of Total Clay ESP 20 |
|---|---|---|---|---|---|
| Water | 78.5 | 67 | 80 | 0 | 26 |
| Ethanol | 24.3 | 24 | 12 | 0 | 6 |
| Benzene | 2.3 | 0 | 0 | 0 | 0 |
| n-Hexane | 1.9 | 0 | 0 | 0 | 0 |
| Soil Factors | Mechanism |
|---|---|
| 1. Clay mineralogy and clay content | Charge originates in clay structures because of isomorphous substitution and broken bonds. Location of charge in the tetrahedral structure is not available for hydration reactions. Thus, the total charge depends on the mineralogy and the amount of clay in soils. |
| 2. Soil pH | Alters the charge on broken bonds by adsorption of H+ or OH- ions. With increasing concentration of carbonate anions, pH increases, and also negative charge on soil particles increases. When pH decreases, as observed in acidic soils, negative charge decreases. |
| 3. Organic matter | Organic molecules bonded to clays by covalent bonding reduce the hydration charge of clay particles. Unbound, charged organic molecules can increase the hydration charge. Soil particles covered by hydrophobic organic matter are not affected by water interaction. |
| 4. Inner sphere complexes | Cations such as Fe, Al, and K fixed by clay minerals by inner sphere complexation (covalent bonding) reduce the hydration charge. |
| 5. Cementation | Cementation of soil particles by Fe and Al oxides or calcium carbonate can block the charge available for water interaction. |
| 6. Exchangeable cations | Exchangeable cations are attached to charged soil particles by a mixture of ionic and covalent bonding. The resultant ionicity of these bindings determines the net hydration charge. Dispersive charge depends on the dispersive power of the exchangeable cations. |
| 7. Electrolytes | Free (unbound) electrolytes in soil water contribute to the cationic flocculating charge, which is a function of the flocculating power and the concentration of individual cations. |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengasamy, P. Irrigation Water Quality and Soil Structural Stability: A Perspective with Some New Insights. Agronomy 2018, 8, 72. https://doi.org/10.3390/agronomy8050072
Rengasamy P. Irrigation Water Quality and Soil Structural Stability: A Perspective with Some New Insights. Agronomy. 2018; 8(5):72. https://doi.org/10.3390/agronomy8050072
Chicago/Turabian StyleRengasamy, Pichu. 2018. "Irrigation Water Quality and Soil Structural Stability: A Perspective with Some New Insights" Agronomy 8, no. 5: 72. https://doi.org/10.3390/agronomy8050072
APA StyleRengasamy, P. (2018). Irrigation Water Quality and Soil Structural Stability: A Perspective with Some New Insights. Agronomy, 8(5), 72. https://doi.org/10.3390/agronomy8050072




