Seasonal Variation in Starch Accumulation and Starch Granule Size in Cassava Genotypes in a Tropical Savanna Climate
Abstract
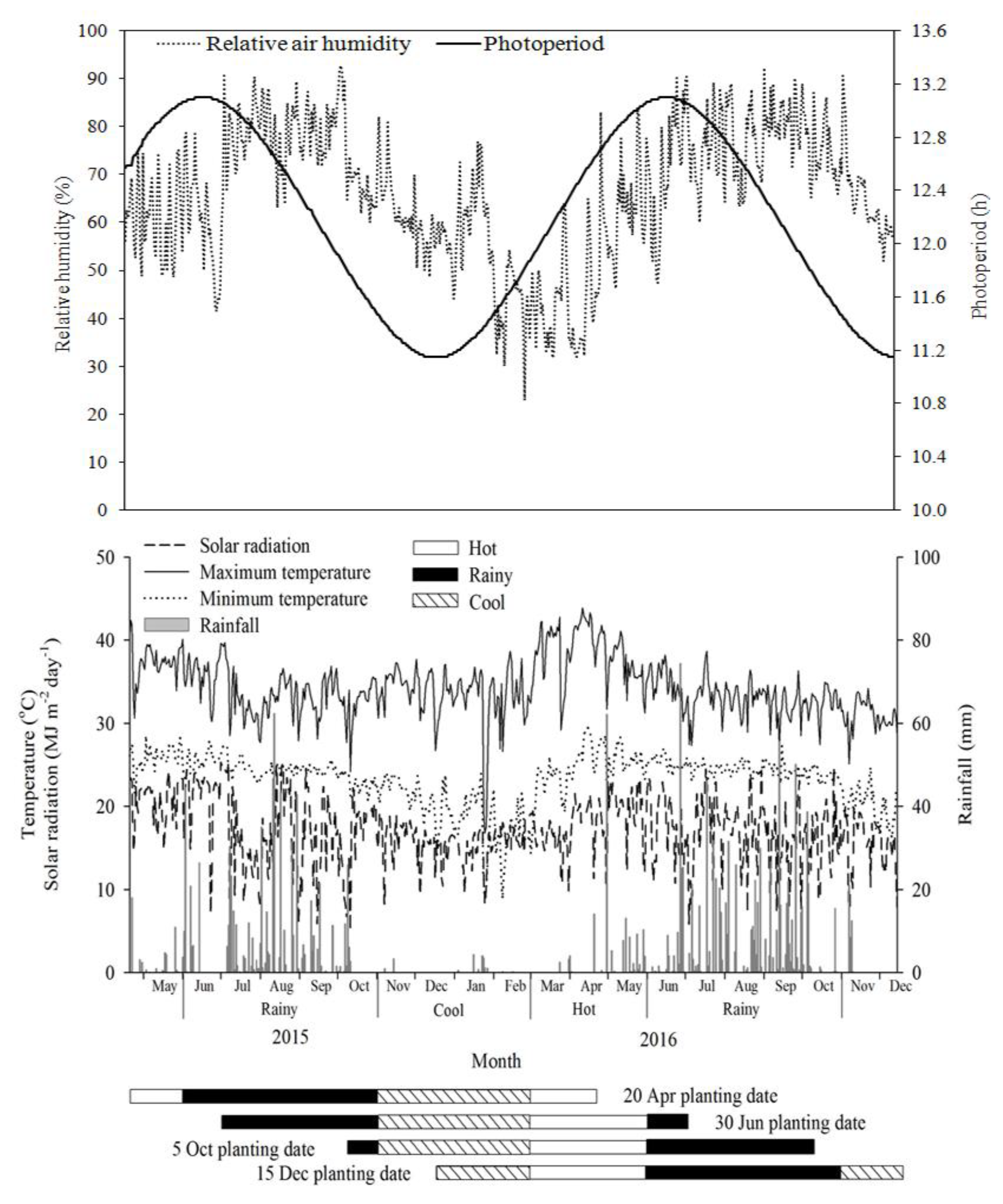
:1. Introduction
2. Materials and Methods
2.1. Experimental Description and Field Trials
2.2. Soil and Plant Determination and Sample Preparation for Laboratory Analyses
2.3. Determination of Starch Content by Polarimetric Method
2.3.1. The Determination of Total Rotary Power (P)
2.3.2. Determination of the Rotary Power (P’) of Substances Soluble
2.4. Determination of Starch Granule Size Distribution by Laser Diffraction
2.5. Determination of Amylose and Amylopectin
2.6. Statistical Analysis
3. Results
3.1. Soil Properties and Growth Conditions
3.2. Combined Analysis of Variance
3.3. Variation in Starch Content and Starch Yield
3.4. The Variation in Amylose Content, Ratio of Amylose and Amylopectin, and Starch Granule Size Distribution
3.5. Stepwise Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Chen, X.; Lu, C.; Ye, J.; Zou, M.; Lu, K.; Feng, S.; Pei, J.; Liu, C.; Zhou, X.; et al. Genome-wide association studies of 11 agronomic traits in cassava (Manihot esculenta Crantz). Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAO). Available online: http://fao.org/faostat/en/#home (accessed on 4 October 2017).
- Howeler, R.H. Cassava production practices in Asia-can they maintain soil productivity? In Proceedings of the International symposium held in Nanning, Guangxi, China, 11–15 November 1996; CIAT: Bangkok, Thailand, 2000.
- Maung Aye, T.; Howeler, R.H. Integrated crop management for cassava cultivation in asia. In Achieving Sustainable Cultivation of Cassava Volume 1: Cultivation Techniques; Clair, H., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2017; pp. 1–29. [Google Scholar]
- Westby, A. Cassava utilization, storage and small-scale processing. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 281–300. [Google Scholar]
- Osiru, M.O.; Olanya, O.M.; Adipala, E.; Kaping, R.; Lemaga, B. Yield stability analysis of Ipomoea batatus L. cultivars in diverse environments. Aust. J. Crop. Sci. 2009, 3, 213–220. [Google Scholar]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Patanothai, A. Responses of growth, physiological traits and tuber yield in Helianthus tuberosus to seasonal variations under tropical area. Sci. Hortic. 2015, 195, 108–115. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Holbrook, C.C.; Patanothai, A. Responses of inulin content and inulin yield of Jerusalem artichoke to seasonal environments. Int. J. Plant Prod. 2015, 9, 599–608. [Google Scholar] [CrossRef]
- Office of Agricultural Economics. Available online: http://www.oae.go.th (accessed on 13 April 2018).
- Asaoka, M.; Okuno, K.; Sugimoto, Y.; Kawakami, J.; Fuwa, H. Effect of environmental temperature during development of rice plants on some properties of endosperm starch. Starch-Stärke 1984, 36, 189–193. [Google Scholar] [CrossRef]
- Defloor, I.; Dehing, I.; Delcour, J.A. Physico-chemical properties of cassava starch. Starch-Stärke 1998, 50, 58–64. [Google Scholar] [CrossRef]
- Sriroth, K.; Piyachomkwan, K.; Santisopasri, V.; Oates, C.G. Environmental conditions during root development: Drought constraint on cassava starch quality. Euphytica 2001, 120, 95–101. [Google Scholar] [CrossRef]
- Santisopasri, V.; Kurotjanawong, K.; Chotineeranat, S.; Piyachomkwan, K.; Sriroth, K. Impact of water stress on yield and quality of cassava starch. Ind. Crops Prod. 2001, 13, 115–129. [Google Scholar] [CrossRef]
- Teerawanichpan, P.; Lertpanyasampatha, M.; Netrphan, S.; Varavinit, S.; Boonseng, O.; Narangajavana, J. Influence of cassava storage root development and environmental conditions on starch granule size distribution. Starch-Stärke 2008, 60, 696–705. [Google Scholar] [CrossRef]
- Molenda, M.; Stasiak, M.; Horabik, J.; Fornal, J.; Blaszczak, W.; Ornowski, A. Microstructure and mechanical parameters of five types of starch. Pol. J. Food Nutr. Sci. 2006, 15, 161–168. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Gu, B.; Yao, Q.Q.; Li, K.M.; Chen, S.B. Change in physicochemical traits of cassava roots and starches associated with genotypes and environmental factors. Starch-Stärke 2013, 65, 253–263. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Good Agricultural Practices for Cassava. Available online: http://www.acfs.go.th/standard/download/eng/GAP_cassava.pdf (accessed on 21 October 2017).
- Howeler, R.H. Cassava mineral nutrition and fertilization. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 281–300. [Google Scholar]
- Janket, A.; Vorasoot, N.; Kesmala, T.; Jogloy, S. Influence of zinc, copper and manganese on dry matter yield and physiological traits of three cassava genotypes grown on soil micronutrient deficiencies. Pak. J. Bot. 2018, 50, 1719–1725. [Google Scholar]
- Hoover, R.; Ratnayake, W.S. Current Protocols in Food Analytical Chemistry; John Wiley&Sons: New York, NY, USA, 2001. [Google Scholar]
- Freed, R.D.; Nissen, O. MSTAT-C Version 1.42; Michigan State University: East Lansing, MI, USA, 1992. [Google Scholar]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environmental effects on growth and development of cassava (Manihot esculenta Crantz) II. Crop growth rate and biomass yield. Field Crop. Res. 1982, 5, 283–292. [Google Scholar] [CrossRef]
- Beckles, D.M.; Thitisaksakul, M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch-Stärke 2014, 66, 58–71. [Google Scholar] [CrossRef]
- Madan, P.; Jagadish, S.V.K.; Craufurd, P.Q.; Fitzgerald, M.; Lafarge, T.; Wheeler, T.R. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 2012, 63, 3843–3852. [Google Scholar] [CrossRef]
- Fergason, V.L.; Zuber, M.S. Influence of environment on amylose content of maize endosperm. Crop Sci. 1962, 2, 209–211. [Google Scholar] [CrossRef]
- Lu, T.J.; Jane, J.L.; Keeling, P.L.; Singletary, G.W. Maize starch fine structures affected by ear developmental temperature. Carbohydr. Res. 1996, 282, 157–170. [Google Scholar] [CrossRef]
- Hurkman, W.J.; McCue, K.F.; Altenbach, S.B.; Korn, A.; Tanaka, C.K.; Kothari, K.M.; Johnson, E.L.; Bechtel, D.B.; Wilson, J.D.; Anderson, O.D.; et al. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2009, 49, 184–189. [Google Scholar] [CrossRef]
- Liu, P.; Guo, W.; Jiang, Z.; Pu, H.; Feng, C.; Zhu, X.; Peng, Y.; Kuang, A.; Little, C.R. Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L). J. Agric. Sci. 2011, 149, 159–169. [Google Scholar] [CrossRef]
- Sawatraksa, N.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Hoogenboom, G. Chlorophyll fluorescence and biomass of four cassava genotypes grown under rain-fed upper paddy field condition in tropics. J. Agro. Crop Sci. 2018, 204, 554–565. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Growth rates and yields of cassava at different planting dates in a tropical savanna climate. Sci. Agric. 2018, in press. [Google Scholar]
- El-Sharkawy, M.A.; Cock, J.H.; Held, A.A. Photosynthetic responses of cassava cultivars (Manihot esculenta Crantz) from different habitats to temperature. Photosynth. Res. 1984, 5, 243–250. [Google Scholar] [CrossRef]
- Saithong, T.; Rongsirikul, O.; Kalapanulak, S.; Chiewchankaset, P.; Siriwat, W.; Netrphan, S.; Suksangpanomrung, M.; Meechai, A.; Cheevadhanarak, S. Starch biosynthesis in cassava: A genome-based pathway reconstruction and its exploitation in data integration. BMC Syst. Boil. 2013, 7, 75. [Google Scholar] [CrossRef]
- Fukai, S.; Alcoy, A.B.; Llamelo, A.B.; Patterson, R.D. Effects of solar radiation on growth of cassava (Manihot esculenta Crantz.). I. Canopy development and dry matter growth. Field Crop. Res. 1984, 9, 347–360. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Drought-tolerant cassava for Africa, Asia, and Latin America. Bio. Sci. 1993, 43, 441–451. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H.; Lynam, J.K.; Hernandez, A.D.P.; Cadavid, L.F.L. Relationships between biomass, root–yield and single–leaf photosynthesis in field-grown cassava. Field Crop. Res. 1990, 25, 183–201. [Google Scholar] [CrossRef]
- Boerboom, B.W. A model of dry matter distribution in cassava (Manihot esculenta Crantz). Neth. J. Agri. Sci. 1978, 26, 267–277. [Google Scholar]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environment effects on growth and development of cassava (Manihot esculenta Crantz) III. Assimilate distribution and storage organ yield. Field Crop. Res. 1982, 5, 293–303. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Effect of humidity and wind on leaf conductance of field grown cassava. Rev. Bras. Fisiol. Vegetal. 1990, 2, 17–22. [Google Scholar]
- Aresta, R.B.; Fukai, S. Effects of solar radiation on growth of cassava Manihot esculenta Crantz II. Fibrous root length. Field Crop. Res. 1984, 9, 361–371. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica 2006, 44, 481–512. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Stress tolerant cassava: The role of integrative ecophysiology breeding research in crop improvement. OJSS 2012, 2, 162–186. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Q. Relationships between leaf and stem soluble sugar content and tuberous root starch accumulation in cassava. J. Agric. Sci. 2011, 3, 64–71. [Google Scholar] [CrossRef]
- Dipnaik, K.; Kokare, P. Ratio of amylose and amylopectin as indicators of glycaemic index and in vitro enzymatic hydrolysis of starches of long, medium and short grain rice. J. Res. Med. Sci. 2017, 5, 4502–4505. [Google Scholar] [CrossRef]
- Irikura, V.; Cock, J.H.; Kawano, K. The physiological basis of genotype-temperature interactions in cassava. Field Crop Res. 1979, 2, 227–239. [Google Scholar] [CrossRef]
- Van Dam, J.; Kooman, P.L.; Struik, P.C. Effects of temperature and photoperiod on early growth and final number of tubers in potato (Solanum tuberosum L.). Potato Res. 1996, 39, 51–62. [Google Scholar] [CrossRef]
- Sriroth, K.; Santisopasri, V.; Petchalanuwat, C.; Kurotjanawong, K.; Piyachomkwan, K.; Oates, C.G. Cassava starch granule structure-function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydr. Polym. 1999, 38, 161–170. [Google Scholar] [CrossRef]
- Vasconcelos, L.M.; Brito, A.C.; Carmo, C.D.; Oliveira, P.H.G.A.; Oliveira, E.J. Phenotypic diversity of starch granules in cassava germplasm. Genet. Mol. Res. 2017, 13, 16. [Google Scholar] [CrossRef]
| Soil Physicochemical Properties | April 2016 | June 2016 | October 2016 | December 2016 | ||||
|---|---|---|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | |
| Physical properties | ||||||||
| Sand (%) | 83.9 | 84.4 | 85.4 | 78.5 | 85.5 | 73.0 | 85.8 | 78.3 |
| Silt (%) | 10.0 | 9.5 | 7.6 | 7.5 | 8.5 | 10.0 | 10.1 | 12.3 |
| Clay (%) | 6.1 | 6.1 | 7.0 | 14.0 | 6.0 | 17.0 | 4.1 | 9.5 |
| Chemical properties at pre-planting | ||||||||
| Total N (%) | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 |
| Available P (mg kg−1) | 61.2 | 56.5 | 24.5 | 17.2 | 62.9 | 22.7 | 25.7 | 17.4 |
| Exchangeable K (mg kg−1) | 54.6 | 35.6 | 34.2 | 20.2 | 49.0 | 12.8 | 41.4 | 16.6 |
| Exchangeable Ca (mg kg−1) | 339 | 387 | 335 | 379 | 200 | 287 | 245 | 225 |
| Mg (mg kg−1) | 36.3 | 34.9 | 50.4 | 46.3 | 39.7 | 40.1 | 30.8 | 38.4 |
| S (mg kg−1) | 53.5 | 43.2 | 47.5 | 41.1 | 5.4 | 19.3 | 3.0 | 8.3 |
| Exchangeable Na (mg kg−1) | 47.4 | 42.4 | 24.8 | 25.3 | 26.2 | 24.9 | 25.7 | 24.0 |
| pH (1:1 H2O) | 6.6 | 6.7 | 5.8 | 6.1 | 5.6 | 5.3 | 5.6 | 5.5 |
| EC (dS m−1) | 0.05 | 0.04 | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 |
| OM (%) | 0.44 | 0.43 | 0.53 | 0.38 | 0.46 | 0.33 | 0.44 | 0.34 |
| CEC (cmol kg−1) | 3.3 | 5.3 | 1.8 | 2.8 | 2.0 | 5.3 | 3.0 | 4.8 |
| Chemical properties at post-planting | ||||||||
| Total N (%) | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 |
| Available P (mg kg−1) | 57.2 | 53.9 | 15.9 | 10.2 | 28.9 | 14.9 | 16.1 | 12.7 |
| Exchangeable K (mg kg−1) | 33.0 | 27.7 | 26.2 | 15.5 | 29.6 | 20.1 | 42.4 | 31.0 |
| Exchangeable Ca (mg kg−1) | 351 | 364 | 239 | 299 | 216 | 377 | 297 | 312 |
| Mg (mg kg−1) | 44.6 | 33.4 | 52.3 | 47.6 | 35.9 | 62.9 | 58.1 | 57.6 |
| S (mg kg−1) | 24.2 | 29.3 | 8.1 | 35.7 | 9.3 | 36.2 | 12.4 | 20.9 |
| Exchangeable Na (mg kg−1) | 65.4 | 66.2 | 21.0 | 40.7 | 19.2 | 37.4 | 28.5 | 37.4 |
| pH (1:1 H2O) | 7.0 | 6.9 | 6.4 | 5.7 | 5.9 | 5.6 | 6.0 | 6.0 |
| EC (dS m−1) | 0.05 | 0.05 | 0.02 | 0.04 | 0.07 | 0.05 | 0.07 | 0.05 |
| OM (%) | 0.48 | 0.45 | 0.26 | 0.21 | 0.56 | 0.27 | 0.44 | 0.35 |
| CEC (cmol kg−1) | 3.2 | 3.5 | 1.7 | 2.6 | 2.0 | 5.1 | 3.2 | 4.2 |
| Source of Variance | df | Mean Square | |||||
|---|---|---|---|---|---|---|---|
| Biomass (t ha−1) | Starch Content (%) | Starch Yield (kg ha−1) | Granule Size (d (0.05)) | Amylose (%) | Ratio of Amylose and Amylopectin | ||
| Planting date (D) | 3 | 568.2 (71.3) ** | 51.4 (35.3) * | 72700000 (34.9) ** | 1.62 (43.7) * | 3.9 (9.5) ns | 0.00081 (7.2) ns |
| Reps within D | 12 | 12.4 (6.2) | 11.4 (31.2) | 6250739 (12.0) | 0.22 (7.8) | 2.4 (23.0) | 0.00072 (25.3) |
| Genotype (G) | 2 | 55.6 (4.6) ** | 10.5 (4.8) ns | 35470000 (11.4) ** | 1.50 (27.0) ** | 17.0 (27.8) ** | 0.00435 (25.7) ** |
| G × D | 6 | 51.5 (12.9) ** | 6.1 (8.3) ns | 34710000 (33.3) ** | 0.20 (10.9) ns | 4.6 (22.8) ** | 0.00135 (23.8) ** |
| Pooled error | 24 | 4.9 (4.9) | 3.7 (20.4) | 2188152 (8.4) | 0.15 (10.6) | 0.9 (16.8) | 0.00025 (17.9) |
| Planting Date | Starch Content (% of Dry Weight) | Starch Yield (kg ha−1) | ||||
|---|---|---|---|---|---|---|
| CMR 38-125-77 | Kasetsart 50 | Rayong 11 | CMR 38-125-77 | Kasetsart 50 | Rayong 11 | |
| 20 April | 82.16 ab | 75.12 b | 82.01 | 12,029 b B | 12,476 ab B | 17,680 a A |
| 30 June | 79.53 b | 76.48 b | 80.32 | 12,940 b A | 8362 b B | 7781 b B |
| 5 October | 82.83 ab | 80.97 ab | 81.32 | 15,252 ab A | 11,830 ab AB | 11,144 b B |
| 15 December | 84.44 a | 82.45 a | 83.49 | 18,609 a A | 16,068 a A | 11,704 b B |
| Planting Date | Genotype | Amylose (%) | Ratio of Amylose and Amylopectin | Granule Size Distribution (µm) | ||
|---|---|---|---|---|---|---|
| d (0.1) | d (0.5) | d (0.9) | ||||
| 20 April | CMR 38-125-77 | 24.3a | 0.32 a | 7.51 | 15.84 a | 29.51 |
| Kasetsart 50 | 21.6b | 0.28 b | 6.75 | 14.41 b | 26.48 | |
| Rayong 11 | 21.6b | 0.28 b | 7.41 | 15.52 ab | 28.68 | |
| Mean | 22.5 | 0.29 | 7.22 | 15.26 B | 28.22 | |
| 30 June | CMR 38-125-77 | 22.6ab | 0.30 ab | 7.89 | 16.39 | 31.52 |
| Kasetsart 50 | 23.2a | 0.30 a | 7.43 | 15.67 | 31.91 | |
| Rayong 11 | 20.5b | 0.26 b | 7.75 | 16.17 | 31.92 | |
| Mean | 22.1 | 0.29 | 7.69 | 16.08 A | 31.78 | |
| 5 October | CMR 38-125-77 | 21.3b | 0.27 b | 8.06 | 16.61 | 31.32 |
| Kasetsart 50 | 23.2a | 0.30 a | 7.30 | 15.67 | 30.57 | |
| Rayong 11 | 20.2c | 0.26 c | 7.54 | 16.00 | 32.17 | |
| Mean | 21.5 | 0.28 | 7.63 | 16.09 A | 31.35 | |
| 15 December | CMR 38-125-77 | 21.0ab | 0.28 ab | 7.71 | 16.39 | 30.86 |
| Kasetsart 50 | 22.3a | 0.29 a | 7.50 | 16.20 | 31.33 | |
| Rayong 11 | 20.4b | 0.26 b | 8.28 | 16.88 | 32.14 | |
| Mean | 21.2 | 0.27 | 7.83 | 16.49 A | 31.44 | |
| Months After Planting | Variable | Coefficient | t | Determination Coefficient (R2) |
|---|---|---|---|---|
| CMR38-125-77 | ||||
| 1–3 | Constant | 115.8 | 8.82 ** | 0.39 |
| Photoperiod (h) | −2.78 | −2.60 * | ||
| 3–6 | Constant | 90.1 | 27.8 ** | 0.37 |
| Relative humidity (%) | −0.13 | −2.61 * | ||
| 6–9 | Constant | 49.8 | 5.01 ** | 0.43 |
| Photoperiod (h) | 2.67 | 3.21 ** | ||
| 9–12 | Constant | 73.47 | 22.9 ** | 0.38 |
| Relative humidity (%) | 0.13 | 2.63 * | ||
| Kasetsart 50 | ||||
| 1–3 | Constant | 82.55 | 69.3 ** | 0.41 |
| Photoperiod (h) | −7.04 | −2.41 * | ||
| 3–6 | Constant | 10.23 | 0.63 ns | 0.60 |
| Photoperiod (h) | 8.11 | 4.02 ** | ||
| Maximum air temperature (°C) | −1.16 | −2.65 * | ||
| 6–9 | Constant | 67.07 | 17.8 ** | 0.48 |
| Relative humidity (%) | 0.21 | 3.5 ** | ||
| 9–12 | Constant | 115.3 | 14.7 ** | 0.61 |
| Total solar radiation | −0.02 | −4.50 ** | ||
| Rayong 11 | ||||
| 1–3 | Constant | 115.5 | 7.48 ** | 0.36 |
| Photoperiod (h) | −2.82 | −2.24 ns | ||
| 3–6 | Constant | 52.8 | 4.5 ** | 0.38 |
| Maximum air temperature (°C) | 0.81 | 2.4 * | ||
| 6–9 | Constant | 79.37 | 80.0 ** | 0.36 |
| Relative humidity (%) | 6.94 | 2.31 * | ||
| 9–12 | Constant | 100.7 | 11.2 ** | 0.36 |
| Maximum air temperature (°C) | −0.49 | −2.21 * | ||
| Months After Planting | Variable | Coefficient | t | Determination Coefficient (R2) |
|---|---|---|---|---|
| CMR38-125-77 | ||||
| 1–3 | Constant | 65,425 | 6.33 ** | 0.68 |
| Photoperiod (h) | −4132 | −4.91 ** | ||
| 3–6 | Constant | −2397 | −0.27 ns | 0.75 |
| Total solar radiation | 16.3 | 3.24 ** | ||
| Relative humidity (%) | −122.8 | 2.7 * | ||
| 6–9 | Constant | −30,760 | −3.68 ** | 0.76 |
| Total solar radiation | −28.3 | −2.73 * | ||
| Photoperiod (h) | 7402 | 4.41 ** | ||
| 9–12 | Constant | 38,113 | 2.93 * | 0.69 |
| Photoperiod (h) | −2860 | −2.64 * | ||
| Relative humidity (%) | 174.0 | 4.32 ** | ||
| Kasetsart 50 | ||||
| 1–3 | Constant | 66,854 | 4.76 ** | 0.61 |
| Total solar radiation | 12.6 | 2.34 * | ||
| Photoperiod (h) | −6074 | −3.93 ** | ||
| 3–6 | Constant | −43,373 | −3.45 ** | 0.70 |
| Photoperiod (h) | 5175 | 4.93 ** | ||
| Relative humidity (%) | −94.94 | −2.19 * | ||
| 6–9 | Constant | −5888 | −1.74 ns | 0.72 |
| Relative humidity (%) | 295.3 | 5.41 ** | ||
| 9–12 | Constant | 7264 | 5.45 ** | 0.73 |
| Photoperiod (h) | −5560 | −5.02 ** | ||
| Relative humidity (%) | 102.8 | 2.49 * | ||
| Rayong 11 | ||||
| 1–3 | Constant | 20,443 | 1.7 ns | 0.76 |
| Total solar radiation | 28.49 | 6.19 ** | ||
| Photoperiod (h) | −4353 | 3.29 ** | ||
| 3–6 | Constant | −50,636 | −5.06 ** | 0.86 |
| Total solar radiation | −39.52 | −6.72 ** | ||
| Photoperiod (h) | 10,200 | 8.61 ** | ||
| 6–9 | Constant | 52,995 | 6.63 ** | 0.82 |
| Total solar radiation | 76.1 | 6.32 ** | ||
| Photoperiod (h) | −15,900 | −7.56 ** | ||
| Relative humidity (%) | 551 | 7.92 ** | ||
| 9–12 | Constant | 40,878 | 3.09 ** | 0.82 |
| Total solar radiation | −92.5 | −5.19 ** | ||
| Photoperiod (h) | 12,742 | 3.62 ** | ||
| Relative humidity (%) | −523 | −6.14 ** | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Banterng, P.; Kesmala, T.; Theerakulpisut, P.; Jogloy, S. Seasonal Variation in Starch Accumulation and Starch Granule Size in Cassava Genotypes in a Tropical Savanna Climate. Agronomy 2018, 8, 297. https://doi.org/10.3390/agronomy8120297
Janket A, Vorasoot N, Toomsan B, Kaewpradit W, Banterng P, Kesmala T, Theerakulpisut P, Jogloy S. Seasonal Variation in Starch Accumulation and Starch Granule Size in Cassava Genotypes in a Tropical Savanna Climate. Agronomy. 2018; 8(12):297. https://doi.org/10.3390/agronomy8120297
Chicago/Turabian StyleJanket, Anon, Nimitr Vorasoot, Banyong Toomsan, Wanwipa Kaewpradit, Poramate Banterng, Thawan Kesmala, Piyada Theerakulpisut, and Sanun Jogloy. 2018. "Seasonal Variation in Starch Accumulation and Starch Granule Size in Cassava Genotypes in a Tropical Savanna Climate" Agronomy 8, no. 12: 297. https://doi.org/10.3390/agronomy8120297
APA StyleJanket, A., Vorasoot, N., Toomsan, B., Kaewpradit, W., Banterng, P., Kesmala, T., Theerakulpisut, P., & Jogloy, S. (2018). Seasonal Variation in Starch Accumulation and Starch Granule Size in Cassava Genotypes in a Tropical Savanna Climate. Agronomy, 8(12), 297. https://doi.org/10.3390/agronomy8120297







