Breeding Low-Cadmium Wheat: Progress and Perspectives
Abstract
1. Introduction
2. Cd Effects on Wheat Growth and Development
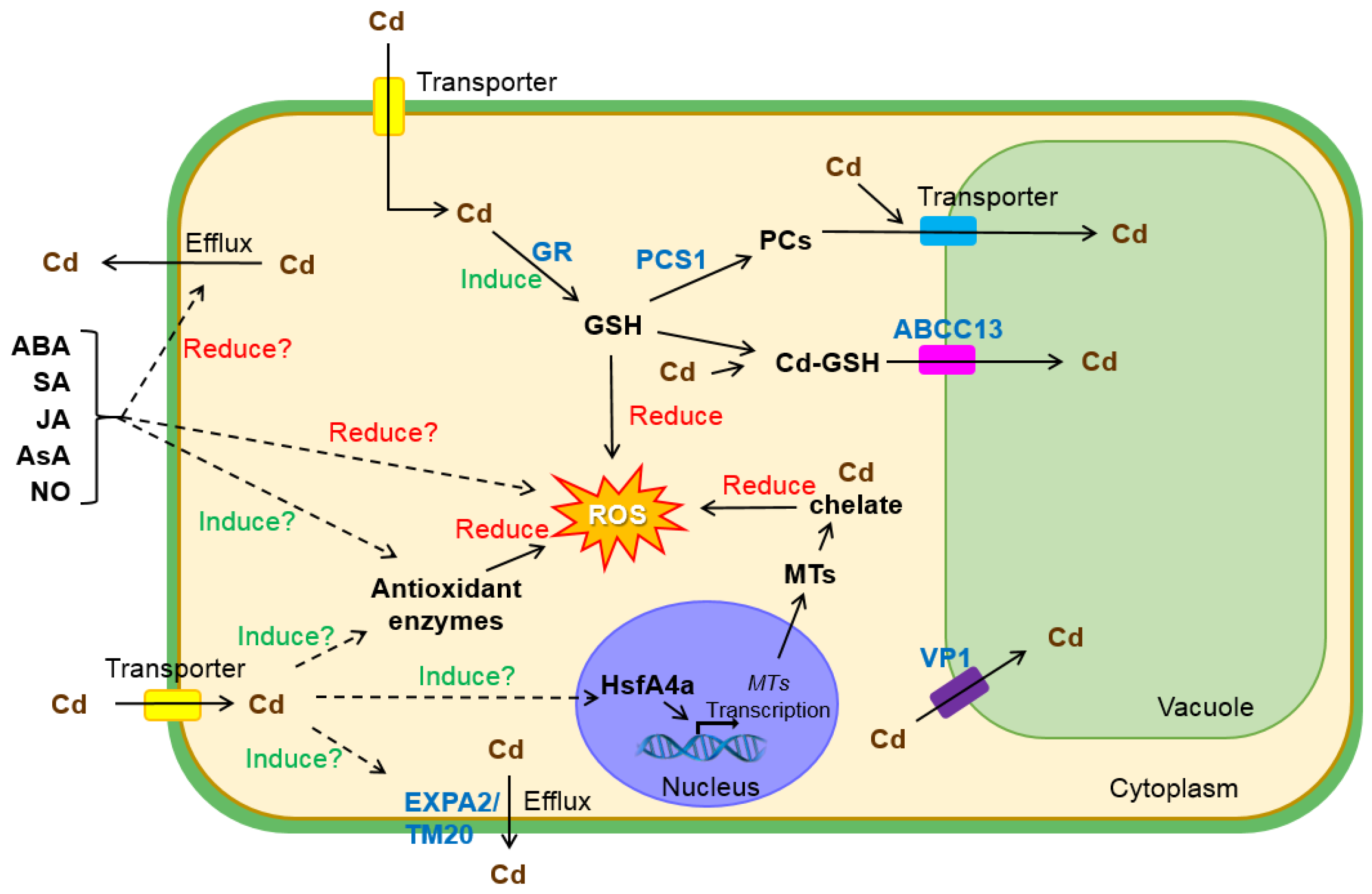
3. Molecular Mechanisms of Cd Resistance in Wheat
3.1. Antioxidation and Sequestration
3.2. Exclusion
3.3. Phytohormone and Signal Molecule Regulation
3.4. Transcriptional Regulation
3.5. Other Mechanisms
4. Breeding Strategies for Low-Cd Wheat Cultivars
4.1. Genetic Variation and Selection of Low-Cd Wheat Cultivars—Conventional Breeding Approaches
4.2. Marker-Quantitative Trait Loci (QTL) Analysis for Cd Toxicity in Wheat
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tolcin, A.C. Mineral resource of the month: Zinc. Earth 2009, 54, 29. [Google Scholar]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 1–33. [Google Scholar] [CrossRef]
- Nziguheba, G.; Smolders, E. Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci. Total Environ. 2008, 390, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Mortvedt, J.J. Cadmium Levels in Soils and Plants from Some Long-term Soil Fertility Experiments in the United States of America. J. Environ. Qual. 1987, 16, 137–142. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of Soil Heavy Metal Pollution on Food Safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.L.; Chang, X.P. Genetic diversity in wheat (T. aestivum) germplasm resources with drought resistance. Acta Bot. Boreal.-Occident. Sin. 2003, 23, 410. [Google Scholar]
- Wu, R. The Pollution Status and Health Risk Assessment of Heavy Metals from the Sewage-Irrigated Wheat and Corn in Shijiazhuang; Hebei Medical University: Shijiazhuang, China, 2015. [Google Scholar]
- Han, J.; Ma, J. Polluting, transferring and accumulating of heavy metals in soil-wheat system in sewage irrigation region: A case study in Huafei River in Kaifeng, Guangdong. Ecol. Environ. 2004, 13, 578–580. [Google Scholar]
- Yang, J.; Cheng, T.; Zheng, Y.; Luo, J.; Liu, H.; Wu, W.; Chen, Y. Dynamic of heavy metals in wheat grains collected from the Liangfeng Irrigated Area, Beijing and a discussion of availability and human health risks. Acta Sci. Circumst. 2005, 25, 1661–1668. [Google Scholar]
- Zhang, X. Risk Assessment of Heavy Metal Toxicity through Wheat and Rice Grow in Tianjin Sewage Irrigated Area; Tianjin Normal Univesity: Tianjin, China, 2014. [Google Scholar]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.P.; Gartrell, J.W.; Tiller, K.G.; Correll, R.; Cozens, G.D.; Youngberg, B.L. Differential responses of Australian wheat cultivars to cadmium concentration in wheat grain. Aust. J. Agric. Res. 1995, 46, 873–886. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Clarke, J.M.; Duguid, S.; Chaney, R.L. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci. Total Environ. 2008, 390, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ashrafzadeh, S.; Leung, D.W.M. Development of Cadmium-Safe Crop Cultivars: A Mini Review. J. Crop Improv. 2016, 30, 107–117. [Google Scholar] [CrossRef]
- Siedlecka, A. Some aspects of interactions between heavy metals and plant mineral nutrients. Acta Soc. Bot. Pol. 1995, 64, 265–272. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Grant, C.; Buckley, W.; Bailey, L.D.; Selles, F. Cadmium accumulation in crops. Can. J. Plant Sci. 1998, 78, 1–17. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Ahmad, A.; Alyemeni, M.N. Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J. Biol. Sci. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maksymiec, W.; Krupa, Z. The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ. Exp. Bot. 2006, 57, 187–194. [Google Scholar] [CrossRef]
- Lin, R.; Wang, X.; Luo, Y.; Du, W.; Guo, H.; Yin, D. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 2007, 69, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Quartacci, M.F.; Izzo, R.; Navari-Izzo, F. Relation between lipoic acid and cell redox status in wheat grown in excess copper. Plant Physiol. Biochem. 2002, 40, 591–597. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chugh, L.K.; Sawhney, S.K. Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant Physiol. Biochem. 1999, 37, 297–303. [Google Scholar] [CrossRef]
- Costa, G.; Spitz, E. Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus. Plant Sci. 1997, 128, 131–140. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Davidian, J.-C.; Pokrovsky, O.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Singh, S.; Nazar, R. Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J. Agron. Crop Sci. 2007, 193, 435–444. [Google Scholar] [CrossRef]
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Black, A.; McLaren, R.G.; Speir, T.W.; Clucas, L.; Condron, L.M. Gradient differences in soil metal solubility and uptake by shoots and roots of wheat (T. aestivum). Biol. Fertil. Soils 2014, 50, 685–694. [Google Scholar] [CrossRef]
- Atal, N.; Saradhi, P.P.; Mohanty, P. Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: Analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol. 1991, 32, 943–951. [Google Scholar] [CrossRef]
- Rebekić, A.; Lončarić, Z. Genotypic difference in cadmium effect on agronomic traits and grain zinc and iron concentration in winter wheat. Emir. J. Food Agric. 2016, 28, 772–778. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Stolt, J.; Oscarson, P. Influence of cadmium on net nitrate uptake kinetics in wheat. J. Plant Nutr. 2002, 25, 2763–2774. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; Sanita di Toppi, L. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Smeets, K.; Ruytinx, J.; Opdenakker, K.; Keunen, E.; Remans, T.; Horemans, N.; Vanhoudt, N.; Van Sanden, S.; Van Belleghem, F.; et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011, 168, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yannarelli, G.G.; Fernandez-Alvarez, A.J.; Santa-Cruz, D.M.; Tomaro, M.L. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry 2007, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Li, Z.S.; Lu, Y.P.; Rea, P.A. The GS-X pump in plant, yeast, and animal cells: Structure, function, and gene expression. Biosci. Rep. 1997, 17, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; Muller-Rober, B.; Schulz, B. Multifunctionality of plant ABC transporters—More than just detoxifiers. Planta 2002, 214, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hortensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bhati, K.K.; Alok, A.; Kumar, A.; Kaur, J.; Tiwari, S.; Pandey, A.K. Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation. J. Exp. Bot. 2016, 67, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Bhati, K.K.; Aggarwal, S.; Sharma, S.; Mantri, S.; Singh, S.P.; Bhalla, S.; Kaur, J.; Tiwari, S.; Roy, J.K.; Tuli, R.; et al. Differential expression of structural genes for the late phase of phytic acid biosynthesis in developing seeds of wheat (Triticum aestivum L.). Plant Sci. 2014, 224, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Bhati, K.K.; Sharma, S.; Aggarwal, S.; Kaur, M.; Shukla, V.; Kaur, J.; Mantri, S.; Pandey, A.K. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Front. Plant Sci. 2015, 6, 488. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O’Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, A.; Castagna, A.; Scebba, F.; Careri, M.; Zagnoni, I.; Predieri, G.; Pagliari, M.; di Toppi, L.S. Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol. Biochem. 2005, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Rauser, W.E. MgATP-Dependent Transport of Phytochelatins Across the Tonoplast of Oat Roots. Plant Physiol. 1995, 107, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.M.; Lee, D.A.; Schroeder, J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Moon, J.S.; Ko, T.S.; Petros, D.; Goldsbrough, P.B.; Korban, S.S. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 2003, 131, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wojas, S.; Clemens, S.; Hennig, J.; Sklodowska, A.; Kopera, E.; Schat, H.; Bal, W.; Antosiewicz, D.M. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008, 59, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.I.; Balish, R.S.; Meagher, R.B. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin. 2012, 44, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Brini, F.; Gaxiola, R.A.; Berkowitz, G.A.; Masmoudi, K. Cloning and characterization of a wheat vacuolar cation/proton antiporter and pyrophosphatase proton pump. Plant Physiol. Biochem. 2005, 43, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Khoudi, H.; Maatar, Y.; Gouiaa, S.; Masmoudi, K. Transgenic tobacco plants expressing ectopically wheat H(+)-pyrophosphatase (H(+)-PPase) gene TaVP1 show enhanced accumulation and tolerance to cadmium. J. Plant Physiol. 2012, 169, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Kim, D.Y.; Shim, D.; Song, W.Y.; Lee, J.; Schroeder, J.I.; Kim, S.; Moran, N.; Lee, Y. Expression of the novel wheat gene TM20 confers enhanced cadmium tolerance to bakers’ yeast. J. Biol. Chem. 2008, 283, 15893–15902. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Wu, W.; Guo, J.; Yang, Y. Cadmium stress tolerance in wheat seedlings induced by ascorbic acid was mediated by NO signaling pathways. Ecotoxicol. Environ. Saf. 2017, 135, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, V.; Gondor, O.K.; Szalai, G.; Darko, E.; Majlath, I.; Janda, T.; Pal, M. Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, C.; Du, J.; Liu, H.; Cui, Y.; Zhang, Y.; He, Y.; Wang, Y.; Chu, C.; Feng, Z.; et al. Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012, 158, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; Yu, W.; She, W.; Ma, Y.; Liu, Z.; Zhang, Y. A Ramie bZIP Transcription Factor BnbZIP2 Is Involved in Drought, Salt, and Heavy Metal Stress Response. DNA Cell Biol. 2016, 35, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, W.; Lu, W.; Wang, Y.; Qi, X. Transcription Factors PvERF15 and PvMTF-1 Form a Cadmium Stress Transcriptional Pathway. Plant Physiol. 2017, 173, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.; Hwang, J.-U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Yotsova, E.K.; Borner, A.; Landjeva, S.P.; Apostolova, E.L. The wheat mutant DELLA-encoding gene (Rht-B1c) affects plant photosynthetic responses to cadmium stress. Plant Physiol. Biochem. 2017, 114, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Deng, Q.; Jia, H.; Wei, L.; Wei, J.; Wan, H.; Yang, L.; Cao, W.; Ma, Z. Sequence variations of the partially dominant DELLA gene Rht-B1c in wheat and their functional impacts. J. Exp. Bot. 2013, 64, 3299–3312. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chen, Y.; An, J.; Zhao, Z.; Zhang, G.; Wang, Y.; Wang, W. Wheat expansin gene TaEXPA2 is involved in conferring plant tolerance to Cd toxicity. Plant Sci. 2018, 270, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Y.; Yin, S.; Zhang, M.; Wang, W. Over-expression of TaEXPB23, a wheat expansin gene, improves oxidative stress tolerance in transgenic tobacco plants. J. Plant Physiol. 2015, 173, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mcqueenmason, S.; Cosgrove, D.J. Disruption of Hydrogen-Bonding between Plant-Cell Wall Polymers by Proteins That Induce Wall Extension. Proc. Natl. Acad. Sci. USA 1994, 91, 6574–6578. [Google Scholar] [CrossRef]
- Yan, A.; Wu, M.; Yan, L.; Hu, R.; Ali, I.; Gan, Y. AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. PLoS ONE 2014, 9, e85208. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.R.; Lee, H.J.; Kim, K.H.; Hong, S.W.; Lee, S.J.; Lee, H. Ectopic expression of Expansin3 or Expansinbeta1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnol. Lett. 2008, 30, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, J.; Chai, T.; Zhang, Y.; Feng, S.; Li, Y.; Zhao, H.; Liu, H.; Chai, X. Functional analyses of TaHMA2, a P(1B)-type ATPase in wheat. Plant Biotechnol. J. 2013, 11, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Savadi, S.; Prasad, P.; Kashyap, P.L.; Bhardwaj, S.C. Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathol. 2018, 67, 771–791. [Google Scholar] [CrossRef]
- Forster, B.; Till, B.; Ghanim, A.; Huynh, H.; Burstmayr, H.; Caligari, P. Accelerated plant breeding. CAB Rev. 2014, 9, 1–16. [Google Scholar] [CrossRef]
- Abbasabadi, A.O.; Kumar, A.; Pirseyedi, S.; Salsman, E.; Dobrydina, M.; Poudel, R.S.; AbuHammad, W.A.; Chao, S.; Faris, J.D.; Elias, E.M. Identification and Validation of a New Source of Low Grain Cadmium Accumulation in Durum Wheat. G3 Genes, Genomes Genet. 2018. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Asif, M.; Pozniak, C.; Clarke, J.M.; Graf, R.J.; Fox, S.L.; Humphreys, D.G.; Knox, R.E.; DePauw, R.M.; Singh, A.K. Application of molecular markers to wheat breeding in Canada. Plant Breed. 2013, 132, 458–471. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, L.; Cao, Y.; Liu, Y.; Li, Y.; Wu, W.; Lan, Y.; Jiang, Y.; Gao, S.; Zhang, Z. Genome-wide association analysis and QTL mapping reveal the genetic control of cadmium accumulation in maize leaf. BMC Genom. 2018, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Mujeeb-Kazi, A.; Sawkins, M. Prospects for utilising plant-adaptive mechanisms to improve wheat and other crops in drought-and salinity-prone environments. Ann. Appl. Biol. 2005, 146, 239–259. [Google Scholar] [CrossRef]
- Yue, J.; Wei, X.; Wang, H. Cadmium tolerant and sensitive wheat lines: Their differences in pollutant accumulation, cell damage, and autophagy. Biol. Plant. 2018, 62, 379–387. [Google Scholar] [CrossRef]
- Naeem, A.; Saifullah; Rehman, M.Z.-U.; Akhtar, T.; Ok, Y.S.; Rengel, Z. Genetic Variation in Cadmium Accumulation and Tolerance among Wheat Cultivars at the Seedling Stage. Commun. Soil Sci. Plant Anal. 2016, 47, 554–562. [Google Scholar] [CrossRef]
- Clarke, J.M.; Norvell, W.A.; Clarke, F.R.; Buckley, W.T. Concentration of cadmium and other elements in the grain of near-isogenic durum lines. Can. J. Plant Sci. 2002, 82, 27–33. [Google Scholar] [CrossRef]
- Guttieri, M.; Frels, K.; Waters, B.M.; El-Basyoni, I.S.; Akhunov, E.; Baenziger, P.S. Genetic Variation for Grain Cadmium in Hard Winter Wheat. In Proceedings of the International Plant and Animal Genome Conference Xxii, San Diego, CA, USA, 11–15 January 2014. [Google Scholar]
- Jalil, A.; Selles, F.; Clarke, J.M. Effect of cadmium on growth and the uptake of cadmium and other elements by durum wheat. J. Plant Nutr. 1994, 17, 1839–1858. [Google Scholar] [CrossRef]
- Stolt, P.; Asp, H.; Hultin, S. Genetic variation in wheat cadmium accumulation on soils with different cadmium concentrations. J. Agron. Crop Sci. 2010, 192, 201–208. [Google Scholar] [CrossRef]
- Jordaan, J.P. Hybrid wheat: Advances and challenges. Increasing Yield Potential Wheat Break. Barriers 1996, 66, 66–67. [Google Scholar]
- Penner, G.A.; Bezte, L.J.; Leisle, D.; Clarke, J. Identification of RAPD markers linked to a gene governing cadmium uptake in durum wheat. Genome 1995, 38, 543–547. [Google Scholar] [CrossRef] [PubMed]
- AbuHammad, W.A.; Mamidi, S.; Kumar, A.; Pirseyedi, S.; Manthey, F.A.; Kianian, S.F.; Alamri, M.S.; Mergoum, M.; Elias, E.M. Identification and validation of a major cadmium accumulation locus and closely associated SNP markers in North Dakota durum wheat cultivars. Mol. Breed. 2016, 36, 112. [Google Scholar] [CrossRef]
- Knox, R.E.; Pozniak, C.J.; Clarke, F.R.; Clarke, J.M.; Houshmand, S.; Singh, A.K. Chromosomal location of the cadmium uptake gene (Cdu1) in durum wheat. Genome Natl. Res. Counc. Can. 2009, 52, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, K.; Harris, N.S.; Faris, J.D.; Clarke, J.M.; Knox, R.E.; Taylor, G.J.; Pozniak, C.J. Targeted mapping of Cdu1, a major locus regulating grain cadmium concentration in durum wheat (Triticum turgidum L. var durum). Theor. Appl. Genet. 2010, 121, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Bonman, J.M.; Khush, G.S.; Nelson, R.J. Breeding Rice for Resistance to Pests. Ann. Rev. Phytopathol. 1992, 30, 507–528. [Google Scholar] [CrossRef]
- Abe, S. Breeding of a blast resistant multiline variety of rice, Sasanishiki BL. Jpn. Agric. Res. Q. 2014, 38, 149–154. [Google Scholar]
- Perrier, F.; Yan, B.; Candaudap, F.; Pokrovsky, O.S.; Gourdain, E.; Meleard, B.; Bussière, S.; Coriou, C.; Robert, T.; Nguyen, C. Variability in grain cadmium concentration among durum wheat cultivars: Impact of aboveground biomass partitioning. Plant Soil 2016, 404, 307–320. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, J.-M.; Zhao, H.W.; Ma, Y.-B. Accumulation and translocation of cadmium in different wheat cultivars in farmland. J. Agro-Environ. Sci. 2018, 37, 36–44. [Google Scholar]
- Zhang, G.; Fukami, M.; Sekimoto, H. Genotypic differences in effects of cadmium on growth and nutrient compositions in wheat. J. Plant Nutr. 2008, 23, 1337–1350. [Google Scholar] [CrossRef]
- Ji, S.-Q.; Guo, R.; Wang, H.F.; Zhang, D.Q.; Zhao, S.Z.; Xu, L.-C. Estimate of Pollution by Heavy Metals on Wheat in Henan and the Rule of Cadmium Absorption in Wheat. J. Triticeae Crop. 2006, 26, 154–157. [Google Scholar]
- Liu, W.; Liang, L.; Zhang, X.; Zhou, Q. Cultivar variations in cadmium and lead accumulation and distribution among 30 wheat (Triticum aestivum L.) cultivars. Environ. Sci. Pollut. Res. 2015, 22, 8432–8441. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, L.; Eker, S.; Özkutlu, F.; Çakmak, İ. Effect of cadmium on growth and concentrations of cadmium, ascorbic acid and sulphydryl groups in durum wheat cultivars. Turk. J. Agric. For. 2003, 27, 161–168. [Google Scholar]
- Greger, M.; Löfstedt, M. Comparison of Uptake and Distribution of Cadmium in Different Cultivars of Bread and Durum Wheat. Crop Sci. 2004, 44, 501–507. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Duan, Y.P.; Yuan, S.; Tu, S.H.; Feng, W.Q.; Xu, F.; Zhang, Z.W.; Chen, Y.E.; Wang, X.; Shang, J.; Lin, H.H. Effects of cadmium stress on alternative oxidase and photosystem II in three wheat cultivars. Z. Naturforsch. C J. Biosci. 2010, 65, 87–94. [Google Scholar] [CrossRef]
- Idrees, S.; Shabir, S.; Ilyas, N.; Batool, N.; Kanwal, S. Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium. Agrociencia 2015, 49, 917–929. [Google Scholar]
- Khan, N.A.; Ahmad, I.; Singh, S.; Nazar, R. Variation in growth, photosynthesis and yield of five wheat cultivars exposed to cadmium stress. World J. Agric. Sci. 2006, 2, 223–226. [Google Scholar]
- Shafi, M.; Zhang, G.P.; Bakht, J.; Khan, M.A.; Ejazulislam; Khan, M.D.; Raziuddin. Effect of cadmium and salinity stresses on root morphology of wheat. Pak. J. Bot. 2010, 42, 2747–2754. [Google Scholar]
- Zhang, L.; Li, J.M.; Wang, H.X. Physiological and ecological responses of wheat (Triticum aestivm L.) root to cadmium stress. Chin. J. Soil Sci. 2002, 33, 61–65. [Google Scholar]
- Ci, D.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Genetic variance in cadmium tolerance and accumulation in wheat materials differing in ploidy and genome at seedling stage. J. Agron. Crop Sci. 2010, 196, 302–310. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Cadmium stress in wheat seedlings: Growth, cadmium accumulation and photosynthesis. Acta Physiol. Plant. 2010, 32, 365–373. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Li, S.; Wollenweber, B.; Dai, T.; Cao, W. Identification of quantitative trait loci for cadmium tolerance and accumulation in wheat. Acta Physiol. Plant. 2012, 34, 191–202. [Google Scholar] [CrossRef]
- Wiebe, K. Molecular Characterization of Cdu-B1, a Major Locus Controlling Cadmium Accumulation in Durum Wheat (Triticum turgidum L. var durum) Grain; University of Saskatchewan: Saskatoon, SK, Canada, 2012. [Google Scholar]
- Salsman, E.; Kumar, A.; AbuHammad, W.; Abbasabadi, A.O.; Dobrydina, M.; Chao, S.; Li, X.; Manthey, F.A.; Elias, E.M. Development and validation of molecular markers for grain cadmium in durum wheat. Mol. Breed. 2018, 38, 28. [Google Scholar] [CrossRef]
- Rad, B.S.; Shokrpour, M.; Sofalian, O.; Nezhad, S.E.H.; Avanes, A.; Esfandiari, E. Association Analysis of AFLP and RAPD Markers with Cadmium Accumulation in Wheat. J. Crop Breed. 2016, 8, 126–133. [Google Scholar]
- Abuhammad, W.; Elias, E.M.; Mamidi, S.; Mergoum, M. Association Mapping of Cadmium Uptake Locus in Durum Wheat Advanced Breeding Lines. In Proceedings of the American Society of Agronomy International Meeting, Tampa, FL, USA, 3–6 November 2013. [Google Scholar]
- Arduini, I.; Masoni, A.; Mariotti, M.; Pampana, S.; Ercoli, L. Cadmium uptake and translocation in durum wheat varieties differing in grain-Cd accumulation. Plant Soil Environ. 2014, 60, 43–49. [Google Scholar] [CrossRef]
- Vergine, M.; Aprile, A.; Sabella, E.; Genga, A.; Siciliano, M.; Rampino, P.; Lenucci, M.S.; Luvisi, A.; Bellis, L. Cadmium Concentration in Grains of Durum Wheat (Triticum turgidum L. subsp. durum). J. Agric. Food Chem. 2017, 65, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.W.; Wang, Y.P.; Li, J.; Gao, C.X. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
| Culture Conditions | Number of Cultivars Tested | Treatments | Toxicological Indicators | Cd Concentrations in Traits (Average) (mg kg−1) | Tolerant and Low-Metal Cultivars | High Accumulators | Reference |
|---|---|---|---|---|---|---|---|
| Hydroponic culture | 15 | 0, 15, 30, and 45 μM for 2 weeks | Biomass Cd at seedling stage | 51–67 (60) (shoot Cd at 15 mM treatment) | Lasani-2008 and Iqbal-2000 | Sehar-2006 and Inqlab-91 | [82] |
| Field pot culture | 51 | 20 mg/kg for life cycle | Grain Cd | 1.09–6.15 (3.5) | Ilirija | - | [31] |
| Hydroponic culture | 10 | 2 nM for life cycle | Biomass and Cd among organs | 0.03–0.08 μg/g (grain) | Strongfield | Dakter | [94] |
| Field survey | 59 | 0.107–2.292 mg/kg | Grain Cd | 0.005–0.150 | Jimai518, Heng0628, Heng09, and Guan29 | - | [95] |
| Hydroponic culture | 16 | 1 μmol/L for 7 weeks | Growth parameters and biomass Cd | 32.2-63.0 (48.1) (based on shoot dry weight) | E81513 | - | [96] |
| Field trial | 20 | Around 10. 5 mg/kg | Grain Cd | 0.1–0.17 | Kaimai18 | Zhengmai9405 | [97] |
| Hydroponic culture | 30 | 1 mg/L for 21 days | Seedling biomass Cd | 0.91–6.74 (3.83) (shoot) | LF-13, LF-16, and LF-21 (both root and shoot) | LF-1 | [98] |
| Hydroponic culture | 2 | 0, 6, 30, 75, and 150 µM for 15 days | Root, shoot, and leaf traits under Cd | For BALCALI-85 shoot Cd on average was 135, and root Cd was 3371, for C1252 shoot Cd was 162 and root Cd was 1556 | C-1252 | - | [99] |
| Hydroponic culture | 40 | 0.5 mM | Roots, flag leaf, grain, and grain coats under Cd | Cd in root on average was 29.1, in flag leaf it was 8.4, and in grains it was 2.6 | - | Mjolner, Rental, Tjalve, Hanno, Grandur, and Extradur | [100] |
| Sand was used in thermophore plates | 4 | 0, 5, 20, 50, and 80 mg/L | Seed germination and seedling growth | Seed germination 68.8%, germination index 6.4%, germination energy 60%, and mean germination time 5.1 days | Sehar-06 | - | [101] |
| Hydroponic culture | 3 | 200 μmol/L for 8 days | Root and leaf Cd | 0.125 for 4 days and 0.14 for 8 days | CM42 and CM47 | CY12 | [102] |
| Hydroponic solution | 2 | 150 μM, 200 μM, and 250 μM for 36 days | Seed germination and seedling growth | On average, 20.1% reduction was observed in NARC-11 and 23% in Galaxy | NARC-11 | - | [103] |
| Greenhouse experiment | 5 | 0, 25, 50, and 100 mg Cd/kg | Photosynthesis and yield characteristics | Average Cd for shoot length was 30, shoot dry weight 221, leaf area 29.6, and the net photosynthesis rate was 12.7 (mg Cd/kg) | PBW343 | - | [104] |
| Hydroponic culture | 3 | 0, 2, and 4 μM | Cd root morphology | Cd for shoot dry weight was 0.27 g plant-1, root dry weight was 0.14 g plant-1, root tip was 941, and total root length was 694 cm | Bakhtawar-92 | - | [105] |
| Hydroponic culture | 5 | 1 mg | Biomass production, yield, and yield components of wheat | Average Cd is root was 601.4, in shoot 27.8, and in grains 3.6 mg/kg | Li 667 and Ailuyuang | - | [106] |
| Hydroponic culture | 24 | 50 µM for 24 days | Root and shoot parameters under Cd | Average Cd for shoot Cd concentration was 104.0, shoot Cd concentration was 1773, and total Cd accumulation was 0.055 | B and D genomes cultivars showed tolerance | R genome wheat cultivars | [107] |
| Hydroponic culture | 3 | 0, 0.1, 0.5, 1.0, and 2.0 µM | Shoot and root biomass, root length, and leaf area | Concentrations higher than 0.1 (imole/L) significantly decreased the traits’ performances | Kyle and SC84-994 | - | [85] |
| Hydroponic culture | 3 | 0, 10, 20, 30, 40, and 50 μM for 24 days | Cd effect on wheat growth, leaf photon energy conversion, gas exchange, and Cd accumulation | Average Cd shoot dry weight under 6 Cd concentration was 0.27 g/plant, root dry weight 0.08 g/plant, shoot height 20.9 cm, tiller number 3.1 per plant, and secondary root number 15.6 per plant | Jing 411 and Yangmai 10 | - | [108] |
| Wheat Germplasm | Traits Investigated | Marker | Associated Marker/QTL | Breeding Technique | References |
|---|---|---|---|---|---|
| 103 RIL population | 13 traits of germination, growth, and physiology and 6 other traits were investigated for Cd tolerance and accumulation | A linkage map was used, constructed using different markers | 26 QTL | Marker-assisted selection (MAS) | [109] |
| 190 RIL mapping population | Grain Cd content | 90K wheat SNP arrays | A single major QTL | Inclusive composite interval mapping (ICIM) method | [77] |
| 167 RILs | Cd level | 90K wheat SNP arrays | A single putative QTL | Composite interval mapping | [89] |
| 70 F8 lines developed by the single-seed descent method | - | Random amplified polymorphic DNA (RAPD) markers | 2 RAPD markers were found to be associated | MAS | [88] |
| 155 DH lines | Grain Cd concentration | SSR markers | Cdu1 locus | MAS | [90] |
| 155 recombinant substitution lines | Grain Cd concentration | PCR-based markers were developed for ESTs | 2 ESM markers, 1 STS, and 1 minor QTL for grain Cd content were detected | MAS | [91] |
| 155 recombinant substitution lines | Cd concentration | ESTs and STS markers | 2 ESMs and 5 STS markers were identified that co-segregated with Cdu-B1 | MAS | [110] |
| Total of 4178 advance, elite and, uniform regional durum nurseries were used | Grain Cd content | SNP markers | 3 markers on chromosome 5B were found to be linked; 1 marker with Cd was polymorphic while the other 2 were not polymorphic in all of the population | MAB | [111] |
| 14 wheat cultivars | Cd concentration | AFLP and RAPD markers | 113 AFLP and 77 RAPD markers were found to be associated | MAS | [112] |
| 2 durum wheat lines | Cd in grains | SNPs | 1 QTL on chromosome 2B with 3% phenotypic variations and 1 SNP marker on chromosome 5B explaining 34% of the phenotypic variation were detected | Association mapping analysis | [113] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaid, I.U.; Zheng, X.; Li, X. Breeding Low-Cadmium Wheat: Progress and Perspectives. Agronomy 2018, 8, 249. https://doi.org/10.3390/agronomy8110249
Zaid IU, Zheng X, Li X. Breeding Low-Cadmium Wheat: Progress and Perspectives. Agronomy. 2018; 8(11):249. https://doi.org/10.3390/agronomy8110249
Chicago/Turabian StyleZaid, Imdad Ullah, Xin Zheng, and Xiaofang Li. 2018. "Breeding Low-Cadmium Wheat: Progress and Perspectives" Agronomy 8, no. 11: 249. https://doi.org/10.3390/agronomy8110249
APA StyleZaid, I. U., Zheng, X., & Li, X. (2018). Breeding Low-Cadmium Wheat: Progress and Perspectives. Agronomy, 8(11), 249. https://doi.org/10.3390/agronomy8110249






