The Elusive Boreal Forest Thaumarchaeota
Abstract
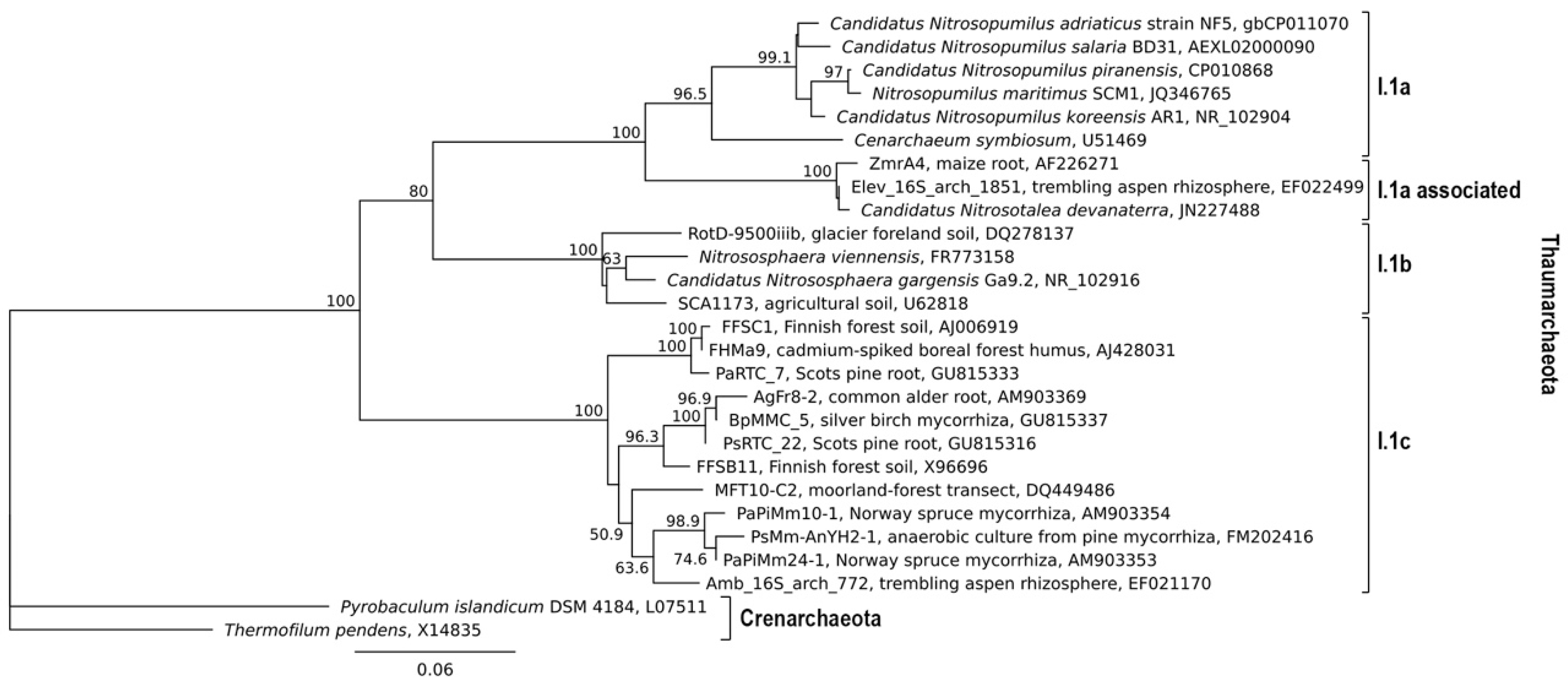
:1. Introduction
2. Factors Affecting the Distribution of the I.1c Thaumarchaeota
2.1. The Influence of the Season on the Abundance of Thaumarchaeota in Soil
2.2. pH
2.3. Association of Thaumarchaeota with Plants
2.4. Growth Requirements by the I.1c ThaumarChaeota?
3. Conclusions
Conflicts of Interest
References
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; McCallum, K.; Davis, A.A. Novel major archaebacterial group from marine plankton. Nature 1992, 356, 148–149. [Google Scholar] [PubMed]
- Hershberger, K.L.; Barns, S.M.; Reysenbach, A.L.; Dawson, S.C.; Pace, N.R. Wide diversity of Crenarchaeota. Nature 1996, 384, 420. [Google Scholar] [CrossRef] [PubMed]
- Schleper, C.; Holben, W.; Klenk, H.P. Recovery of crenarchaeotal ribosomal DNA sequences from freshwater-lake sediments. Appl. Environ. Microbiol. 1997, 63, 321–323. [Google Scholar] [PubMed]
- MacGregor, B.J.; Moser, D.P.; Alm, E.W.; Nealson, K.H.; Stahl, D.A. Crenarchaeota in Lake Michigan sediment. Appl. Environ. Microbiol. 1997, 63, 1178–1181. [Google Scholar] [PubMed]
- Bintrim, S.B.; Donohue, T.J.; Handelsman, J.; Roberts, G.P.; Goodman, R.M. Molecular phylogeny of Archaea from soil. Proc. Natl. Acad. Sci. USA 1997, 94, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Grosskopf, R.; Janssen, P.H.; Liesack, W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 1998, 64, 960–969. [Google Scholar] [PubMed]
- Borneman, J.; Triplett, E.W. Molecular microbial diversity in soils from eastern Amazonia: Evidence for unusual microorganisms and microbial population shifts associated with deforestation. Appl. Environ. Microbiol. 1997, 63, 2647–2653. [Google Scholar] [PubMed]
- Jurgens, G.; Lindstrom, K.; Saano, A. Novel group within the kingdom Crenarchaeota from boreal forest soil. Appl. Environ. Microbiol. 1997, 63, 803–805. [Google Scholar] [PubMed]
- DeLong, E.F. Everything in moderation: Archaea as ‘non-extremophiles’. Curr. Opin. Genet. Dev. 1998, 8, 649–654. [Google Scholar] [CrossRef]
- Schleper, C.; Jurgens, G.; Jonuscheit, M. Genomic studies of uncultivated archaea. Nat. Rev. Microbiol. 2005, 3, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Campbell, C.D.; Chapman, S.J.; Prosser, J.I. Afforestation of moorland leads to changes in crenarchaeal community structure. FEMS Microbiol. Ecol. 2007, 60, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef] [PubMed]
- Quaiser, A.; Ochsenreiter, T.; Klenk, H.P.; Kletzin, A.; Treusch, A.H.; Meurer, G.; Eck, J.; Sensen, C.W.; Schleper, C. First insight into the genome of an uncultivated crenarchaeote from soil. Environ. Microbiol. 2002, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Konstantinidis, K.T.; Putnam, N.; Schleper, C.; Watanabe, Y.; Sugahara, J.; Preston, C.; de la Torre, J.; Richardson, P.M.; DeLong, E.F. Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc. Natl. Acad. Sci. USA 2006, 103, 18296–18301. [Google Scholar] [CrossRef] [PubMed]
- Brochier-Armanet, C.; Boussau, B.; Gribaldo, S.; Forterre, P. Mesophilic crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 2008, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B.; de la Torre, J.R.; Klotz, M.G.; Urakawa, H.; Pinel, N.; Arp, D.J.; Brochier-Armanet, C.; Chain, P.S.; Chan, P.P.; Gollabgir, A.; et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Mosier, A.C.; Allen, E.E.; Kim, M.; Ferriera, S.; Francis, C.A. Genome sequence of “Candidatus Nitrosopumilus salaria” BD31, an ammonia-oxidizing archaeon from the San Francisco Bay estuary. J. Bacteriol. 2012, 194, 2121–2122. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, J.G.; Jung, M.Y.; Kim, S.J.; Cha, I.T.; Ghai, R.; Martin-Cuadrado, A.B.; Rodriguez-Valera, F.; Rhee, S.K. Draft genome sequence of an ammonia-oxidizing archaeon, “Candidatus Nitrosopumilus sediminis” AR2, from Svalbard in the Arctic Circle. J. Bacteriol. 2012, 194, 6948–6949. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, J.G.; Jung, M.Y.; Kim, S.J.; Cha, I.T.; Kwon, K.; Lee, J.H.; Rhee, S.K. Draft genome sequence of an ammonia-oxidizing archaeon, “Candidatus Nitrosopumilus koreensis” AR1, from marine sediment. J. Bacteriol. 2012, 194, 6940–6941. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Sayavedra-Soto, L.A.; Gallois, N.; Schouten, S.; Stein, L.Y.; Prosser, J.I.; Nicol, G.W. Identifying potential mechanisms enabling acidophily in the ammonia-oxidising archaeon ‘Candidatus Nitrosotalea devanaterra’. Appl. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Konneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A.; et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Spang, A.; Poehlein, A.; Offre, P.; Zumbragel, S.; Haider, S.; Rychlik, N.; Nowka, B.; Schmeisser, C.; Lebedeva, E.V.; Rattei, T.; et al. The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: Insights into metabolic versatility and environmental adaptations. Environ. Microbiol. 2012, 14, 3122–3145. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.V.; Dias, R.; Leonard, M.T.; Dorr de Quadros, P.; Camargo, F.A.; Drew, J.C.; Farmerie, W.G.; Daroub, S.H.; Triplett, E.W. Genome sequence of Candidatus Nitrososphaera evergladensis from group I.1b enriched from Everglades soil reveals novel genomic features of the ammonia-oxidizing archaea. PLoS ONE 2014, 9, e101648. [Google Scholar] [CrossRef] [PubMed]
- Guy, L.; Ettema, T.J.G. The archaeal ’TACK’ superphylum and the origin of eukaryotes. Trends Microbiol. 2011, 19, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Timonen, S.; Bomberg, M. Archaea in dry soil environments. Phytochem. Rev. 2010, 8, 505–518. [Google Scholar] [CrossRef]
- Ochsenreiter, T.; Selezi, D.; Quaiser, A.; Bonch-Osmolovskaya, L.; Schleper, C. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 2003, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Putkinen, A.; Juottonen, H.; Juutinen, S.; Tuittila, E.S.; Fritze, H.; Yrjala, K. Archaeal rRNA diversity and methane production in deep boreal peat. FEMS Microbiol. Ecol. 2009, 70, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.N.; Johnson, K.W.; Bräuer, S.L. Southern Appalachian peatlands support high archaeal diversity. Microb. Ecol. 2014, 67, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Liew, K.C.; Yule, C.M. Structural and functional changes with depth in microbial communities in a tropical Malaysian peat swamp forest. Microb. Ecol. 2009, 57, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Schleper, C. Ammonia-oxidising Crenarchaeota: Important players in the nitrogen cycle? Trends Microbiol. 2006, 14, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, M. Archaea in the Mycorrhizosphere of Boreal Forest Trees; University of Helsinki: Helsinki, Finland, 2008; p. 46. [Google Scholar]
- Bomberg, M.; Montonen, L.; Timonen, S. Anaerobic Cren- and Euryarchaeota in boreal forest tree mycorrhiza. EJSB 2010, 46, 356–364. [Google Scholar]
- Fritze, H.; Tikka, P.; Pennanen, T.; Saano, A.; Jurgens, G.; Nilsson, M.; Bergman, I.; Kitunen, V. Detection of Archaeal Diether Lipid by Gas Chromatography from Humus and Peat. Scand. J. For. Res. 1999, 14, 545. [Google Scholar] [CrossRef]
- Bach, L.H.; Frostegard, Å.; Ohlson, M. Variation in soil microbial communities across a boreal spruce forest landscape. Can. J. For. Res. 2008, 38, 1504–1516. [Google Scholar] [CrossRef]
- Long, X.; Chen, C.; Xu, Z.; Linder, S.; He, J. Abundance and community structure of ammonia oxidizing bacteria and archaea in a Sweden boreal forest soil under 19-year fertilization and 12-year warming. J. Soils Sediments 2012, 12, 1124–1133. [Google Scholar] [CrossRef]
- Kemnitz, D.; Kolb, S.; Conrad, R. High abundance of Crenarchaeota in a temperate acidic forest soil. FEMS Microbiol. Ecol. 2007, 60, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.E.; Johansson, T.; Bengtson, P. Archaeal abundance in relation to root and fungal exudation rates. FEMS Microbiol. Ecol. 2012, 80, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Juottonen, H.; Tuittila, E.S.; Juutinen, S.; Fritze, H.; Yrjala, K. Seasonality of rDNA- and rRNA-derived archaeal communities and methanogenic potential in a boreal mire. ISME J. 2008, 2, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, M.; Munster, U.; Pumpanen, J.; Ilvesniemi, H.; Heinonsalo, J. Archaeal communities in boreal forest tree rhizospheres respond to changing soil temperatures. Microb. Ecol. 2011, 62, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta, L.E.; Prosser, J.I.; Nicol, G.W. Soil pH regulates the abundance and diversity of Group 1.1c Crenarchaeota. FEMS Microbiol. Ecol. 2009, 70, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Oton, E.V.; Quince, C.; Nicol, G.W.; Prosser, J.I.; Gubry-Rangin, C. Phylogenetic congruence and ecological coherence in terrestrial Thaumarchaeota. ISME J. 2016, 10, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Glover, L.A.; Prosser, J.I. Spatial analysis of archaeal community structure in grassland soil. Appl. Environ. Microbiol. 2003, 69, 7420–7429. [Google Scholar] [CrossRef] [PubMed]
- Oline, D.K.; Schmidt, S.K.; Grant, M.C. Biogeography and landscape-scale diversity of the dominant Crenarchaeota of soil. Microb. Ecol. 2006, 52, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in Bacterial and Archaeal Community Structure and Functional Diversity along a Geochemically Variable Soil Profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Glover, L.A.; Prosser, J.I. The impact of grassland management on archaeal community structure in upland pasture rhizosphere soil. Environ. Microbiol. 2003, 5, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Tscherko, D.; Embley, T.M.; Prosser, J.I. Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ. Microbiol. 2005, 7, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.M.; Dodsworth, J.A.; Goodman, R.M. Crenarchaeota colonize terrestrial plant roots. Environ. Microbiol. 2000, 2, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.M.; Jahn, C.E.; Bergerud, L.T.; Sliwinski, M.K.; Weimer, P.J.; Willis, D.K.; Goodman, R.M. Cultivation of mesophilic soil crenarchaeotes in enrichment cultures from plant roots. Appl. Environ. Microbiol. 2005, 71, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Chelius, M.K.; Triplett, E.W. The Diversity of Archaea and Bacteria in Association with the Roots of Zea mays L. Microb. Ecol. 2001, 41, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Sliwinski, M.K.; Goodman, R.M. Comparison of crenarchaeal consortia inhabiting the rhizosphere of diverse terrestrial plants with those in bulk soil in native environments. Appl. Environ. Microbiol. 2004, 70, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, M.; Jurgens, G.; Saano, A.; Sen, R.; Timonen, S. Nested PCR detection of archaea in defined compartments of pine mycorrhizospheres developed in boreal forest humus microcosms. FEMS Microbiol. Ecol. 2003, 43, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, M.; Timonen, S. Distribution of Cren- and Euryarchaeota in Scots Pine Mycorrhizospheres and Boreal Forest Humus. Microb. Ecol. 2007, 54, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, M.; Timonen, S. Effect of tree species and mycorrhizal colonization on the archaeal population of boreal forest rhizospheres. Appl. Environ. Microbiol. 2009, 75, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Tscherko, D.; Chang, L.; Hammesfahr, U.; Prosser, J.I. Crenarchaeal community assembly and microdiversity in developing soils at two sites associated with deglaciation. Environ. Microbiol. 2006, 8, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Timonen, S.; Jorgensen, K.S.; Haahtela, K.; Sen, R. Bacterial community structure at defined locations of Pinus sylvestris Suillus bovinus and Pinus sylvestris Paxillus involutus mycorrhizospheres in dry pine forest humus and nursery peat. Can. J. Microbiol. 1998, 44, 499–513. [Google Scholar] [CrossRef]
- Timonen, S.; Hurek, T. Characterization of culturable bacterial populations associating with Pinus sylvestris—Suillus bovinus mycorrhizospheres. Can. J. Microbiol. 2006, 52, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Nemecek-Marshall, M.; MacDonald, R.C.; Franzen, J.J.; Wojciechowski, C.L.; Fall, R. Methanol emission from leaves. Enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiol. 1995, 108, 1359–1368. [Google Scholar] [PubMed]
- Hüve, K.; Christ, M.M.; Kleist, E.; Uerlings, R.; Niinemets, U.; Walter, A.; Wildt, J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. J. Exp. Bot. 2007, 58, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Lesaulnier, C.; Papamichail, D.; McCorkle, S.; Ollivier, B.; Skiena, S.; Taghavi, S.; Zak, D.; van der Lelie, D. Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ. Microbiol. 2008, 10, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Lanzen, A.; Epelde, L.; Garbisu, C.; Anza, M.; Martin-Sanchez, I.; Blanco, F.; Mijangos, I. The Community Structures of Prokaryotes and Fungi in Mountain Pasture Soils are Highly Correlated and Primarily Influenced by pH. Front. Microbiol. 2015, 6, 1321. [Google Scholar] [CrossRef] [PubMed]
- Chronakova, A.; Schloter-Hai, B.; Radl, V.; Endesfelder, D.; Quince, C.; Elhottova, D.; Simek, M.; Schloter, M. Response of Archaeal and Bacterial Soil Communities to Changes Associated with Outdoor Cattle Overwintering. PLoS ONE 2015, 10, e0135627. [Google Scholar] [CrossRef]
- Jurgens, G.; Saano, A. Diversity of soil Archaea in boreal forest before, and after clear-cutting and prescribed burning. FEMS Microbiol. Ecol. 1999, 29, 205–213. [Google Scholar] [CrossRef]
- Stopnisek, N.; Gubry-Rangin, C.; Hofferle, S.; Nicol, G.W.; Mandic-Mulec, I.; Prosser, J.I. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol. 2010, 76, 7626–7634. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.B.; Lehtovirta-Morley, L.E.; Prosser, J.I.; Gubry-Rangin, C. Ammonia oxidation is not required for growth of Group 1.1c soil Thaumarchaeota. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kanto, J.M.; Sinkko, H.; Rajala, T.; Al-Soud, W.A.; Sorensen, S.J.; Tamminen, M.V.; Timonen, S. Natural decay process affects the abundance and community structure of Bacteria and Archaea in Picea abies logs. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bomberg, M. The Elusive Boreal Forest Thaumarchaeota. Agronomy 2016, 6, 36. https://doi.org/10.3390/agronomy6020036
Bomberg M. The Elusive Boreal Forest Thaumarchaeota. Agronomy. 2016; 6(2):36. https://doi.org/10.3390/agronomy6020036
Chicago/Turabian StyleBomberg, Malin. 2016. "The Elusive Boreal Forest Thaumarchaeota" Agronomy 6, no. 2: 36. https://doi.org/10.3390/agronomy6020036
APA StyleBomberg, M. (2016). The Elusive Boreal Forest Thaumarchaeota. Agronomy, 6(2), 36. https://doi.org/10.3390/agronomy6020036





