Stability of the Inherent Target Metallome in Seed Crops and a Mushroom Grown on Soils of Extreme Mineral Spans
Abstract
:1. Introduction
2. Bottlenecks in Plant Mineral Flow
3. The Adopted Seed Metallome in Correspondence with the Geochemical Environment
4. Discrimination of Essential and Non-Essential Elements Masked by Chemical Similarities
5. Conclusion
Author Contributions
Conflicts of Interest
References
- Graham, R.; Senadhira, D.; Beebe, S.; Iglesias, C.; Monasterio, I. Breeding for micronutrient density in edible portions of staple food crops: Conventional approaches. Field Crops Res. 1999, 60, 57–80. [Google Scholar] [CrossRef]
- Hernández Rodríguez, L.; Morales, D.A.; Rodríguez Rodríguez, E.; Díaz Romero, C. Minerals and trace elements in a collection of wheat landraces from the Canary Islands. J. Food Compos. Anal. 2011, 24, 1081–1090. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V. Micronutrient deficiencies in crops and soils in India. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 93–126. [Google Scholar]
- Sillanpää, M. Micronutrient Assessment at Country Level: An International Study; FAO Soils Bulletin No. 63; The Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1990. [Google Scholar]
- Groenenberg, J.E.; Römkens, P.F.A.M.; Comans, R.N.J.; Luster, J.; Pampura, T.; Shotbolt, L.; Tippin, E.; de Vries, W. Transfer functions for solid-solution partitioning of cadmium, copper, nickel, lead and zinc in soils: Derivation of relationships for free metal ion activities and validation with independent data. Eur. J. Soil Sci. 2010, 61, 58–73. [Google Scholar] [CrossRef]
- Schachtschabel, P.; Blume, H.P.; Brümmer, G.; Hartge, K.H.; Schwertmann, U. Lehrbuch der Bodenkunde, 14th ed.; Enke: Stuttgart, Germany, 1998. [Google Scholar]
- Sperotto, R.A.; Boff, T.; Duarte, G.L.; Santos, L.S.; Grusak, M.A.; Fett, J.P. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains. J. Plant Physiol. 2010, 167, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Ismail, A.M.; Graham, R.D. Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil 2008, 306, 37–48. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Yang, X.E.; Chen, W.R.; Feng, Y. Improving human micronutrient nutrition through bio-fortification in the soil-plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, H.; Mattsson, L.; Eriksson, J. Trace element concentration in wheat grain: Results from the Swedish long-term soil fertility experiments and national monitoring program. Environ. Geochem. Health 2009, 31, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic: London, UK, 1995. [Google Scholar]
- Dushenkov, S. Trends in phytoremediation of radionuclides. Plant Soil 2003, 249, 167–175. [Google Scholar] [CrossRef]
- European Communities. Council regulation (EC) No. 1881/2006 of December 2006 on setting maximum levels of certain contaminants in foodstuffs. Off. J. Eur. Communities L Ser. 2006, 364, 5–24. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D. Regulation of the mineral concentrations in pea seeds from uranium mine and reference soils diverging extremely in their heavy metal load. Sci. Hort. 2015, 194, 255–266. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, S.; Sun, B.; Zhao, Q. Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan, China. Sci. Total Environ. 2008, 405, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J.; Ayres, D.C. Chemical Principles of Environmental Pollution; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Gramss, G.; Voigt, K.-D. The legacy of uranium mining: Farming on non-remediable soils? In Uranium: Sources, Exposure and Environmnetal Effects; Nelson, J.R., Ed.; Novapublishers: New York, NY, USA, 2015; pp. 21–52. [Google Scholar]
- Duquène, L.; Vandenhove, H.; Tack, F.; Meers, E.; Baeten, J.; Wannijn, J. Enhanced phytoextraction of uranium and selected heavy metals by Indian mustard and ryegrass using biodegradable soil amendments. Sci. Total Environ. 2009, 407, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Forage and rangeland plants from uranium mine soils: Long-term hazard to herbivores and livestock? Environ. Geochem. Health 2014, 36, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Klang-Westin, E.; Eriksson, J. Potential of Salix as phytoextractor for Cd on moderately contaminated soils. Plant Soil 2003, 249, 127–137. [Google Scholar] [CrossRef]
- Meers, E.; Lamsal, S.; Vervaeke, P.; Hopgood, M.; Lust, N.; Tack, F.M.G. Availability of heavy metals for uptake by Salix viminalis on a moderately contaminated dredged sediment disposal site. Environ. Pollut. 2005, 137, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Regulation of heavy metal concentrations in cereal grains from uranium mine soils. Plant Soil 2013, 364, 105–118. [Google Scholar] [CrossRef]
- Stomph, T.J.; Jiang, W.; Van Der Putten, P.E.L.; Struik, P.C. Zinc allocation and re-allocation in rice. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-F.; Yue, S.-C.; Zhang, Y.-Q.; Cui, Z.-L.; Chen, X.-P.; Yang, F.-C.; Cakmak, I.; McGrath, S.P.; Zhang, F.-S.; Zou, C.-Q. Grain and shoot zinc accumulation in winter wheat affected by nitrogen management. Plant Soil 2012, 361, 153–163. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Clemente, T.E.; Grusak, M.A. Evaluation of constitutive iron reductase (AtFRO2) expression on mineral accumulation and distribution in soybean (Glycine max L.). Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A. Iron transport to developing ovules of Pisum sativum. (I. Seed import characteristics and phloem iron-loading capacity of source regions). Plant Physiol. 1994, 104, 649–655. [Google Scholar] [PubMed]
- Osuji, G.O.; Brown, T.K.; South, S.M.; Duncan, J.C.; Johnson, D.; Hyllam, S. Molecular adaptation of peanut metabolic pathways to wide variations of mineral ion composition and concentration. Am. J. Plant Sci. 2012, 3, 33–50. [Google Scholar] [CrossRef]
- Grusak, M.; Emerick, J. Dynamics of Dry Matter and Mineral Allocation to Pod Walls versus Seeds in Common Bean; Meeting Abstract; Southern Plains Area Home/Children’s Nutrition Research Center: Houston, TX, USA, 2012. [Google Scholar]
- Wu, Y.; Messing, J. Proteome balancing of the maize seed for higher nutritional value. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.P.; Jiang, R.F.; Ueno, D.; Ma, J.F.; Schat, H.; McGrath, S.P.; Zhao, F.J. Variation in root-to-shoot translocation of cadmium and zinc among different accessions of the hyperaccumulators Thlaspi caerulescens and Thlaspi praecox. New Phytol. 2008, 178, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Grillet, L.; Mari, S.; Schmidt, W. Iron in seeds-loading pathways and subcellular localization. Front. Plant Sci. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Castro-Guerrero, N.; Mendoza-Cozatl, D.G. Moving toward a precise nutrition: Preferential loading of seeds with essential nutrients over non-essential toxic elements. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Molecular mechanisms of zinc uptake and translocation in rice. Plant Soil 2012, 361, 189–201. [Google Scholar] [CrossRef]
- Paul, S.; Datta, S.K.; Datta, K. miRNA regulation of nutrient homeostasis in plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.C.; Sahu, R.K.; Bhargava, S.K.; Chaterjee, C. Distribution of heavy metals in wheat, mustard, and weed grown in field irrigated with industrial effluents. Bull. Environ. Contam. Toxicol. 2000, 64, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Bhattacharyya, A.K. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere 2008, 70, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-X.; Liu, J.-W.; Wu, M.-Z.; Li, Y.; Zhao, Y.; Li, S.-R. Accumulation and translocation of toxic heavy metals in winter wheat (Triticum aestivum L.) growing in agricultural soil of Zhengzhou, China. Bull. Environ. Contam. Toxicol. 2009, 82, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Sahu, R.K.; Sahu, S.K.; Padhy, R.N. Growth, yield and elements content of wheat (Triticum aestivum) grown in composted municipal solid wastes amended soil. Environ. Dev. Sustain. 2009, 11, 115–126. [Google Scholar] [CrossRef]
- Il’in, V.B. Heavy metals in the soil-crop system. Eurasian Soil Sci. 2007, 40, 993–999. [Google Scholar] [CrossRef]
- Briat, J.F.; Fobisloisy, I.; Grignon, N.; Lobreaux, S.; Pascal, N.; Savino, N.; Thoiron, S.; Vonwiren, N.; Vanwuytswinkel, O. Cellular and molecular aspects of iron metabolism in plants. Biol. Cell 1995, 84, 69–81. [Google Scholar] [CrossRef]
- Deinlein, U.; Weber, M.; Schmidt, H.; Rensch, S.; Trampczynska, A.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Talke, I.N.; Krämer, U.; et al. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 2012, 24, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Stephan, U.W.; Schmidke, I.; Stephan, V.W.; Scholz, G. The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. BioMetals 1996, 9, 84–90. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Díaz-Benito, P.; Abadía, A.; López-Millán, A.-F.; Abadía, J. Metal species involved in long distance metal transport in plants. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.L.; Baker, A.J.M.; Kolev, S.D.; Wedd, A.G. Metal ion ligands in hyperaccumulating plants. J. Biol. Inorg. Chem. 2006, 11, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Bergmann, H. Impact of soil applications of sand, compost and nitrogen on uptake of trace elements by Chinese cabbage. J. Nat. Sci. Sustain. Technol. 2013, 7, 1–28. [Google Scholar]
- Stomph, J.T.; Jiang, W.; Struik, P.C. Zinc biofortification of cereals: Rice differs from wheat and barley. Trends Plant Sci. 2009, 14, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Herren, T.; Feller, U. Transfer of zinc from xylem to phloem in the peduncle of wheat. J. Plant Nutr. 1994, 17, 1587–1598. [Google Scholar] [CrossRef]
- Pearson, J.N.; Rengel, Z.; Jenner, C.F.; Graham, R.D. Transport of zinc and manganese to developing wheat grains. Physiol. Plant. 1995, 95, 449–455. [Google Scholar] [CrossRef]
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Fincher, G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Specht, A.; Horst, W.J. Stable isotope labelling and zinc distribution in grains studied by laser ablation ICP-MS in an ear culture system reveals zinc transport barriers during grain filling in wheat. New Phytol. 2011, 189, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Pearson, J.N.; Marentes, E. The physiology of micronutrient homeostasis in field crops. Field Crops Res. 1999, 60, 41–56. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Ammerlaan, A.M.H.; Wouterlood, M.; Van Aelst, A.C.; Borstlap, A.C. Structure of the developing pea seed coat and the post-phloen transport pathway of nutrients. Ann. Bot. 2003, 91, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Clues for regulatory processes in fungal uptake and transfer of minerals to the basidiospore. Biol. Trace Elem. Res. 2013, 154, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G. Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: A review. J. Nat. Sci. Sustain Technol. 2012, 6, 169–223. [Google Scholar]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 2011, 342, 149–164. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals–Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Drinking Water Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic: London, UK, 1979. [Google Scholar]
- Auermann, E.; Dässler, H.-G.; Jacobi, J.; Cumbrowski, J.; Meckel, U. Untersuchungen zum Schwermetallgehalt von Getreide und Kartoffeln. Die Nahrung 1980, 24, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities Directive EC 466/2001. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Comm. L Ser. 2001, 77, 1–13. [Google Scholar]
- Ghandilyan, A.; Vreugdenhil, D.; Aarts, M.G.M. Progress in the genetic understanding of plant iron and zinc nutrition. Physiol. Plant. 2006, 126, 407–417. [Google Scholar] [CrossRef]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef] [PubMed]
- House, W.A.; Welch, R.M.; Beebe, S.; Cheng, Z. Potential for increasing the amounts of bioavailable zinc in dry beans (Phaseolus vulgaris L) through plant breeding. J. Sci. Food Agric. 2002, 82, 1452–1457. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Ozkan, H.; Braun, H.J.; Welch, R.M.; Romheld, V. Zinc and iron concentrations in seeds of wild, primitive, and modern wheat. Food Nutr. Bull. 2000, 21, 401–403. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Aloupi, M.; Kazakou, E.; Dimitrakopoulos, P.G. Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere 2014, 95, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Kazakou, E.; Adamidis, G.C.; Baker, A.J.M.; Reeves, R.D.; Godino, M.; Dimitrakopoulos, P.G. Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant Soil 2010, 332, 369–385. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.Q.; Rathinasabapathi, B.; Cai, Y.; Liu, Y.G.; Zeng, G.M. Mechanisms of efficient arsenite uptake by arsenic hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2011, 45, 9719–9725. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.; Williams, R.J.P. Order of stability of metal complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Chakraborty, S.; Fretham, S.J.B.; Aschner, M. Cellular transport and homeostasis of essential and nonessential metals. Metallomics 2012, 4, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low-and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Ahmad, S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Ricachenevsky, F.K.; Waldow, V.D.; Fett, J.P. Iron biofortification in rice: It’s a long way to the top. Plant Sci. 2012, 190, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Gill, S.S.; Narendra Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; Teixeira da Silva, J.A.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAP kinase transcript levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Available online: http://www.as-botanicalstudies.com/content/55/1/35 (accessed on 29 May 2015).
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Hayward, A.R.; Coates, K.E.; Galer, A.L.; Hutchinson, T.C.; Emery, R.J.N. Chelator profiling in Deschampsia cespitosa (L.) Beauv. reveals a Ni reaction, which is distinct from the ABA and cytokinin associated response to Cd. Plant Physiol. Biochem. 2013, 64, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.M.; Chien, P.S.; Huang, H.J. Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J. Exp. Bot. 2007, 58, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Titov, V.; Hohn, B.; Kovalchuk, O. Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat. Res. 2005, 570, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Schubert, R.; Bergmann, H. Carbon and nitrogen compounds applied to uranium mine dump soil determine (heavy) metal uptake by Chinese cabbage. Environ. Res. J. 2011, 5, 793–818. [Google Scholar]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Brej, T. Heavy metal tolerance in Agropyron repens (L.) P. Bauv. populations from the Legnica copper smelter area, Lower Silesia. Acta Soc. Bot. Pol. 1998, 67, 325–333. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Ashraf, M.; Ahmad, K.; Akram, N.A. A study on the transfer of cadmium from soil to pasture under semi-arid conditions in Sargodha, Pakistan. Biol. Trace Elem. Res. 2011, 142, 143–147. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Trace elements in human nutrition and health. Available online: http://www.who.int/nutrition/publications/micronutrients/9241561734/en/ (accessed on 14 April 2015).
- Palmer, L.J.; Palmer, L.T.; Rutzke, M.A.; Graham, R.D.; Stangoulis, J.C.R. Nutrient variability in phloem: Examining changes in K, Mg, Zn and Fe concentration during grain loading in common wheat (Triticum aestivum). Physiol. Plant. 2014, 152, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sandström, J.; Pettersson, J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J. Insect Physiol. 1994, 40, 947–955. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Small RNAs in plants: Recent development and application for crop improvement. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.-D. Seed crops: Alternative for non-remediable uranium mine soils. In Uranium—Past and Future Challenges, Proceedings of the 7th International Conference on Uranium Mining and Hydrogeology, Freiberg, Germany, 21–25 September 2014; Merkel, B.J., Arab, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 777–784. [Google Scholar]
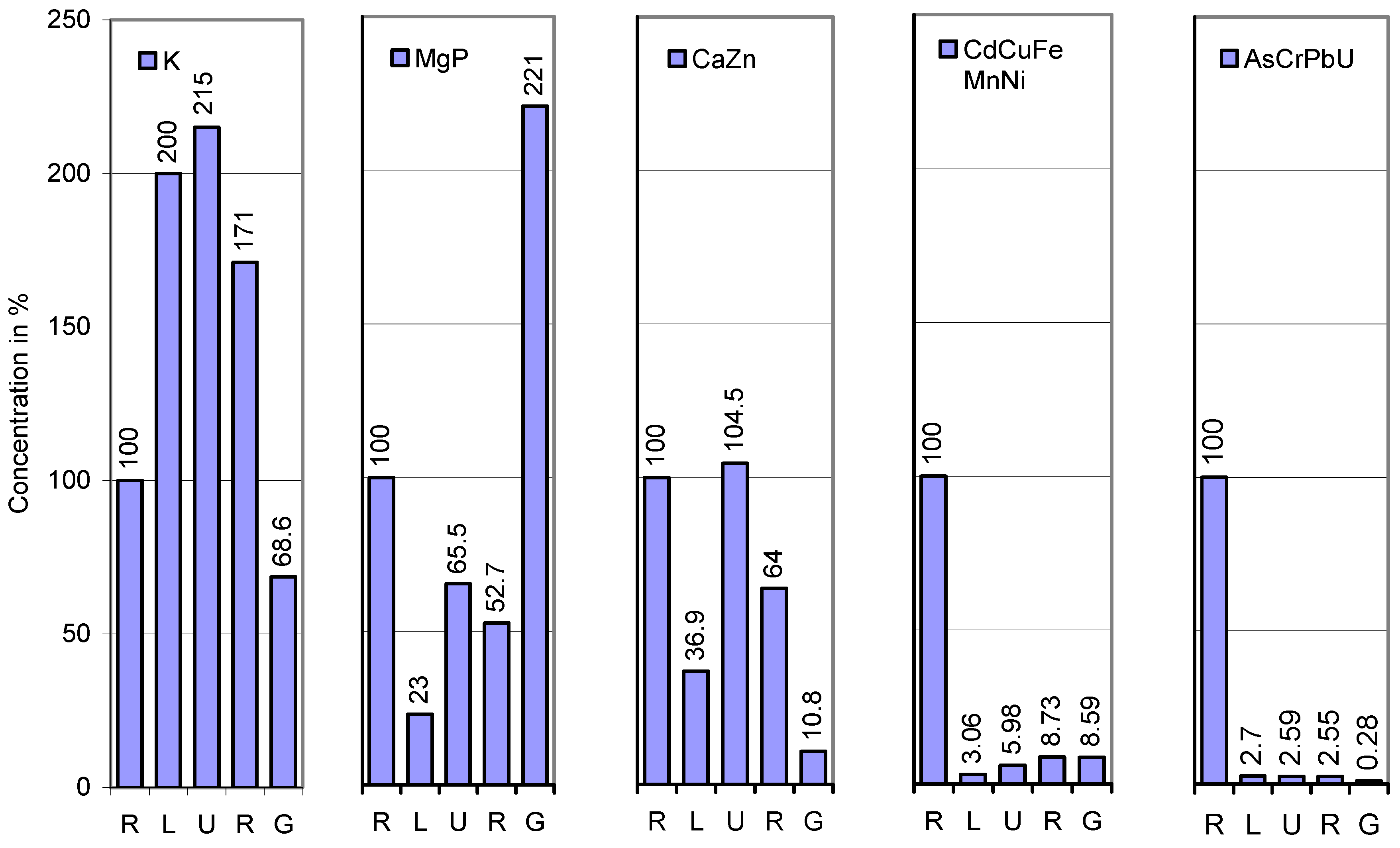
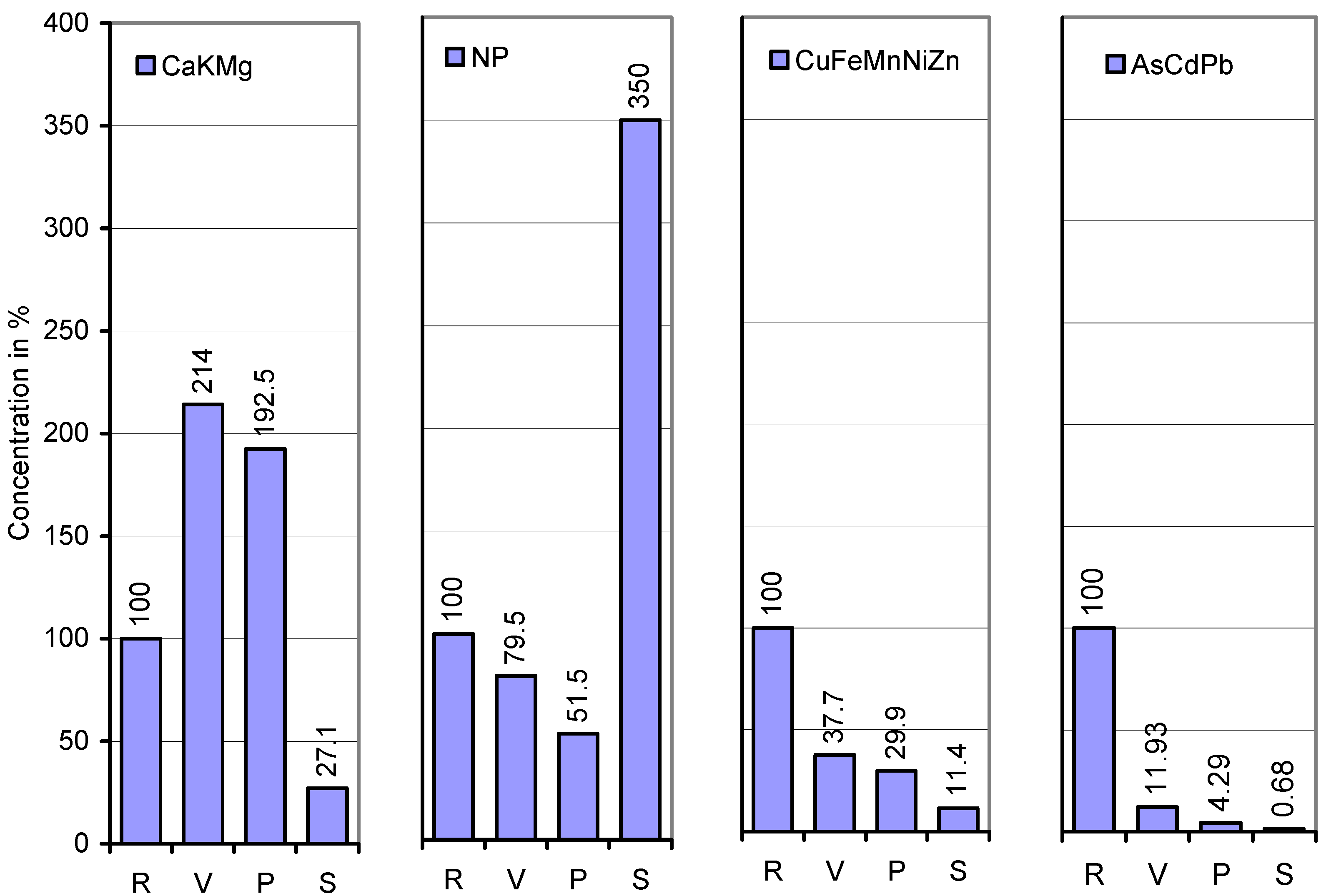
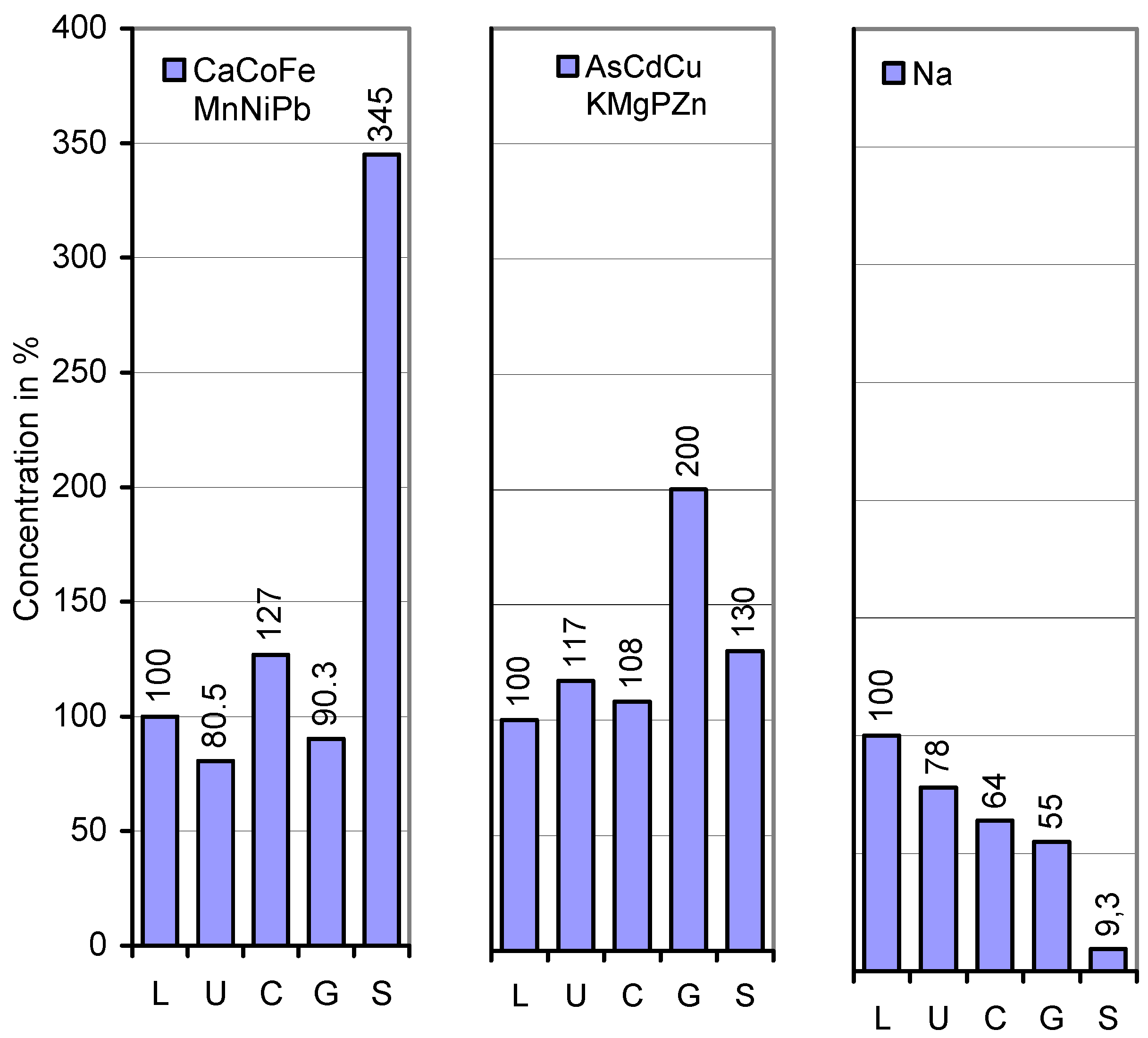
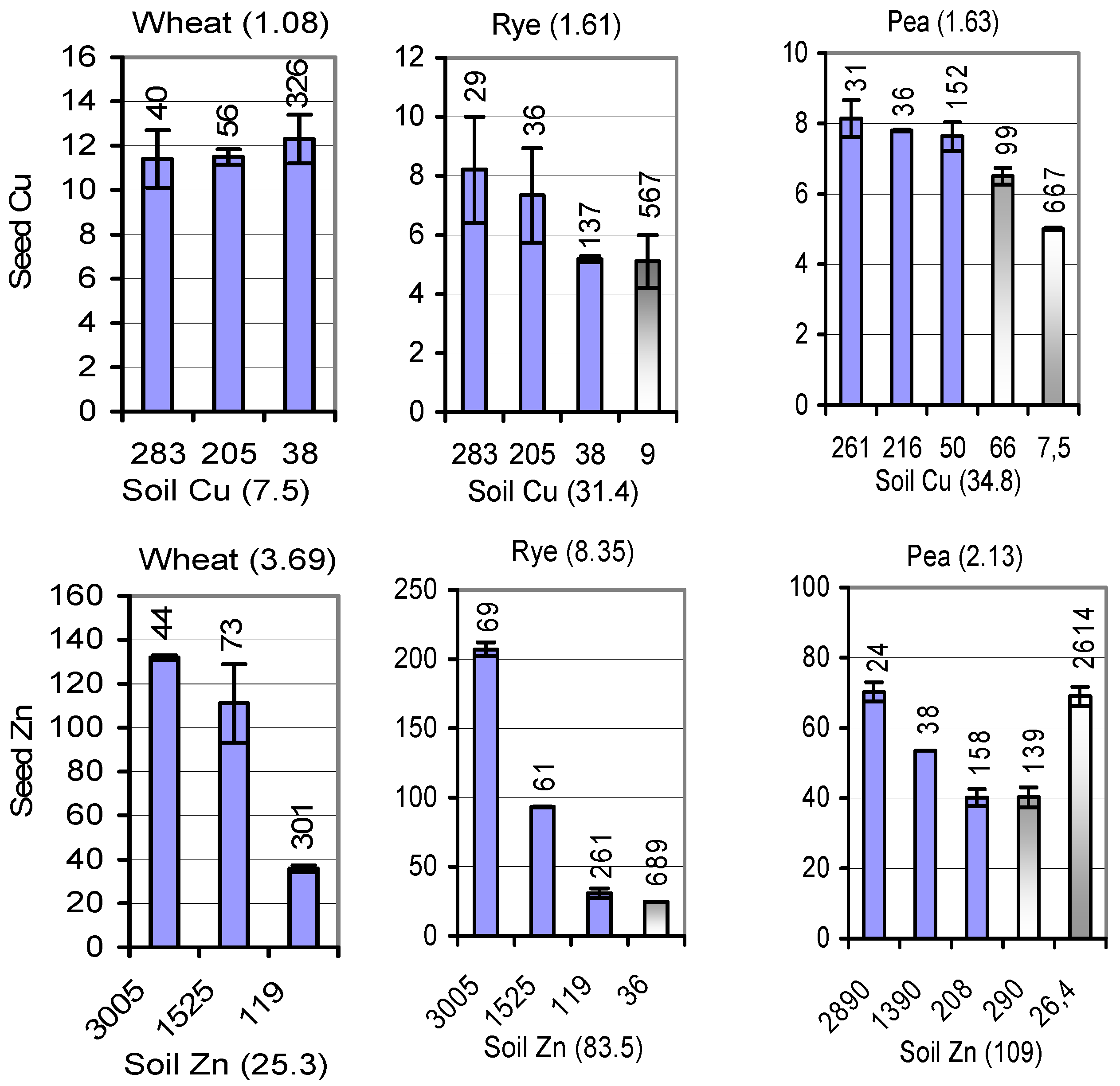
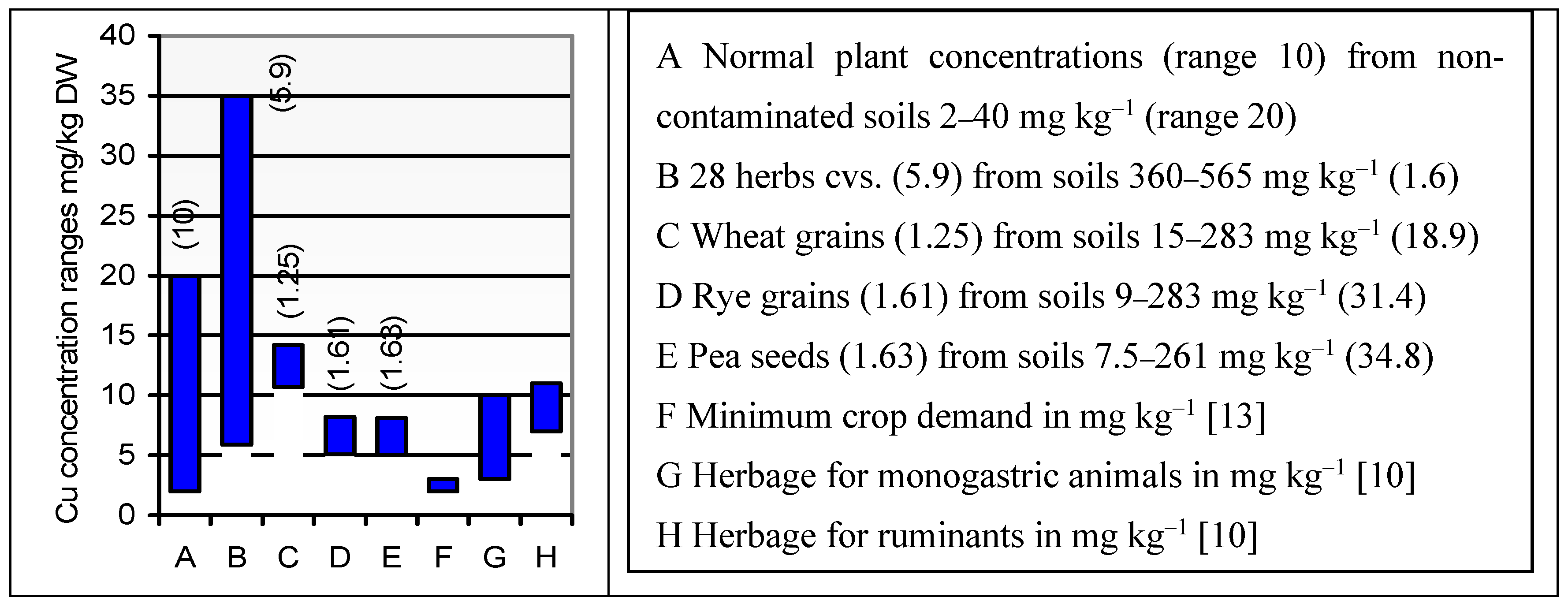
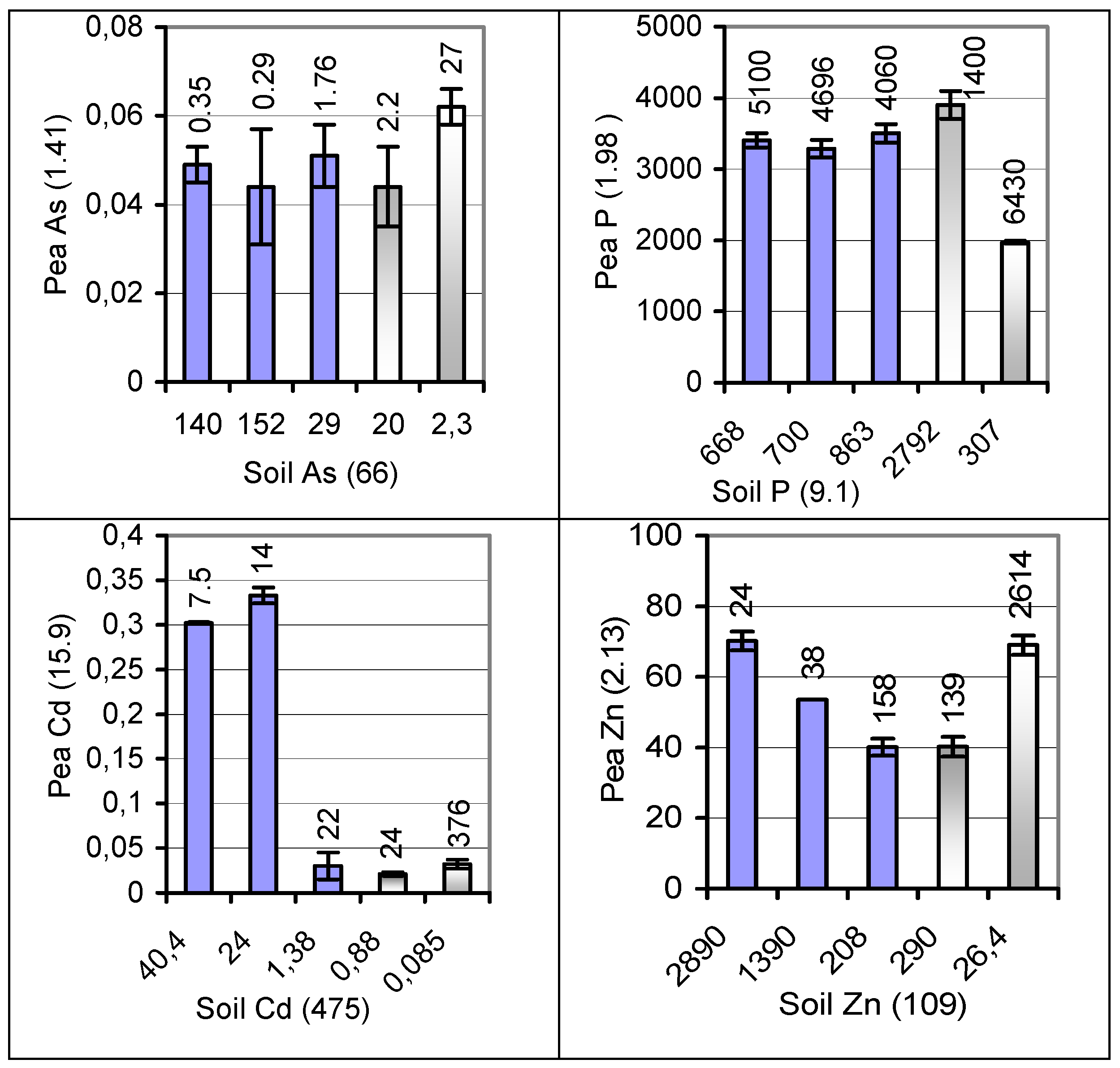
| Element | Root | Lower stem | Upper stem | Rachis | Whole grain |
|---|---|---|---|---|---|
| K | 5910 | 11,820 a (200) | 12,690 (107) | 10,103 (80) | 4052 a (40) |
| Mg | 1060 | 352 a (33) | 965 a (274) | 631 a (65) | 1162 a (184) |
| P | 784 | 102 a (13) | 314 a (308) | 359 (114) | 2612 a (728) |
| Ca | 3,380 | 989 a (29) | 4,095 a (414) | 1,190 a (29) | 346 a (29) |
| Zn | 1,203 | 536 a (45) | 1,059 a (198) | 1,117 (105) | 138 a (12) |
| Cd | 29.4 | 2.29 a (7.8) | 4.26 a (186) | 2.34 a (55) | 1.55 a (66) |
| Cu | 65.6 | 1.43 a (2.2) | 3.05 a (213) | 2.86 (94) | 6.43 a (225) |
| Fe | 2,410 | 16.6 a (0.7) | 15 (91) | 35.7 a (238) | 24.6 a (69) |
| Mn | 217 | 4.95 a (2.3) | 10.8 a (218) | 18.8 a (174) | 19.9 (106) |
| Ni | 11.8 | 0.28 a (2.4) | 0.615 a (220) | 2.51 a (408) | 2.09 a (83) |
| As | 20.2 | 0.215 a (1.1) | 0.745 a (347) | 0.920 a (123) | 0.100 a (11) |
| Pb | 20.3 | 0.374 a (1.8) | 0.176 (47) | 0.289 (164) | 0.044 a (15) |
| U | 8.49 | 0.571 a (6.7) | 0.360 (63) | 0.185 (51) | 0.003 a (1.6) |
| Element | Root | Whole upper vine | Pod wall | Whole seed |
|---|---|---|---|---|
| Ca | 12,300 | 18,800 (153) | 15,115 (80) | 932 (6.2) |
| K | 8,390 | 11,190 (133) | 18,350 (164) | 10,090 (55) |
| Mg | 2,400 | 6600 (275) | 6290 (95) | 1120 (18) |
| P | 986 | 580 (59) | 414 (71) | 3,406 (823) |
| Cu | 63.9 | 12.2 (19) | 23.4 (192) | 8.14 (35) |
| Fe | 1,145 | 109 (10) | 72 (66) | 49.8 (69) |
| Mn | 160 | 123 (77) | 44 (36) | 11.8 (27) |
| Ni | 3.83 | 1.82 (48) | 2.33 (128) | 0.95 (40) |
| Zn | 684 | 311 (35) | 160 (51) | 70.2 (44) |
| As | 7.45 | 0.833 (11) | 0.106 (13) | 0.049 (46) |
| Cd | 36.7 | 6.18 (17) | 2.81 (45) | 0.302 (11) |
| Pb | 7.24 | 0.548 (8) | 0.273 (50) | 0.040 (15) |
| Element | Lower stipe | Upper stipe | Cap plectenchyma | Gills | Basidiospores |
|---|---|---|---|---|---|
| Ca | 176 | 114 a (65) | 382 a (335) | 137 a (36) | 277 a (202) |
| Fe | 87 | 80.5 (93) | 91 (113) | 133 a (146) | 199 a (150) |
| Mn | 47.5 | 42 (88) | 46 (110) | 28.5 a (62) | 73.5 a (260) |
| Ni | 1.40 | 1.45 (104) | 2.30 a (159) | 1.85 a (80) | 13 a (703) |
| Pb | 0.210 | 0.105 a (50) | 0.180 a (171) | 0.090 a (50) | 0.555 a (620) |
| As | 0.069 | 0.061 (88) | 0.088 a (144) | 0.112 a (127) | 0.109 (97) |
| Cd | 0.630 | 1.08 a (171) | 0.300 a (28) | 1.23 a (410) | 0.850 a (69) |
| Cu | 36 | 33.5 (93) | 52 a (155) | 71.5 a (138) | 24 a (34) |
| K | 26,125 | 28,800 (110) | 26,940 (94) | 34,180 a (127) | 8,157 a (24) |
| Mg | 1,130 | 890 a (79) | 1,340 a (151) | 1,580 (118) | 1,140 a (72) |
| P | 2,980 | 3,920 a (132) | 3,280 a (84) | 9,610 a (293) | 5,150 a (54) |
| Zn | 31 | 45.5 a (147) | 33 a (73) | 78.5 a (238) | 75 a (96) |
| Na | 987 | 774 a (78) | 636 a (82) | 539 a (85) | 92 a (17) |
| Element | Concentration spans ( ) in soil and seed, number of test soils (refer to Figure 4) | Balanced dietary pretensions to herb forage | Usual heavy-metal ranges | |||||
|---|---|---|---|---|---|---|---|---|
| Wheat (3 cvs., 3 soils) | Rye (4 soils) | Pea (5 soils) | Monogastric livestock | Ruminants | Arable soils | Herbage | ||
| Ca | Soil | 7235–2296 (3.15) | 7235–2296 (3.15) | 7546–1646 (4.6) | -- | |||
| Seed | 538–281 (1.9) | 411–330 (1.25) | 1256–847 (1.48) | 2400–9000 a | 2000–15,000 a | -- | ||
| K | Soil | 5130–4086 (1.26) | 5130–4086 (1.26) | 2264–1296 (1.75) | -- | |||
| Seed | 6535–4289 (1.52) | 8006–4806 (1.67) | 10,335–9139 (1.13) | 1500–10,000 a | 5000–10,400 a | -- | ||
| Mg | Soil | 3237–2252 (1.44) | 3237–2252 (1.44) | 5595–945 (5.9) | -- | |||
| Seed | 1707–1241 (1.38) | 1267–996 (1,27) | 1236–945 (1.31) | 400–1300 a | 1000–2100 a | -- | ||
| P | Soil | 873–713 (1.22) | 873–713 (1.22) | 2792–307 (9.1) | -- | |||
| Seed | 5290–4206 (1.26) | 4052–3086 (1.31) | 3902–1974 (1.98) | 1700–7000 a | 1600–5900 a | -- | ||
| Cu | Soil | 283–15 (18.9) | 283–9 (31.4) | 261–7.5 (34.8) | 2–40 (30) c | |||
| Seed | 10.7–14.2 (1.33) | 8.21–5.09 (1.61) | 8.14–5 (1.63) | 3–10 (250–800) a | 7–11 (25–100) a | 2–20 (LC 10) d | ||
| Fe | Soil | 23,390–10,170 (2.30) | 23,390–5445 (4.30) | 41,630–4370 (9.53) | -- | |||
| Seed | 39.6–64.3 (1.62) | 28–37 (1.32) | 49.8–72.3 (1.45) | 40–100 (500–3000) a | 15–50 (500–1000) a | -- | ||
| Mn | Soil | 2130–515 (4.14) | 2130–380 (5.61) | 1780–126 (14.1) | 40–1000 (550) | |||
| Seed | 26.4–33.5 (1.27) | 22–16.3 (1.35) | 8.55–20.5 (2.40) | 2–60 (400–2000) a | 14–40 (1000) a | 14–30 | ||
| Ni | Soil | 40.8–11.8 (3.46) | 40.8–4.58 (8.91) | 54.3–3.9 (13.9) | 3–50 (30) | |||
| Seed | 1.41–0.180 (7.83) | 0.811–0.069 (11.8) | 0.626–1.21 (1.93) | 0.05–0.2 (50–300) a | 0.3–0.5 (50) a | 0.1–3 (LC 1) | ||
| Zn | Soil | 3005–48 (62.6) | 3005–36 (83.5) | 2890–26.4 (109) | 10–80 (90) | |||
| Seed | 190–35.8 (5.31) | 207–24.8 (8.35) | 33–70.2 (2.13) | 35–100 (500–1000) a | 20–55 (300–500) a | 10–100 (LC 50) | ||
| As | Soil | 156–6.34 (24.6) | 156–3.53 (44.2) | 152–2.3 (66) | 1–20 (6) | |||
| Seed | 0.433–<0.080 (>5.41) | <0.080 all | 0.044–0.062 (1.41) | 2 (50) b | 2 (50) b | 0.01–1 (L 0.5; LC 0.7) | ||
| Cd | Soil | 41.3–0.231 (179) | 41.3–0.209 (198) | 40.4–0.085 (475) | 0.1–0.6 (0.35) | |||
| Seed | 3.13–0.033 (94.8) | 1.72–0.018 (95.6) | 0.333–0.021 (15.9) | 1 (0.5) b | 1 (0.5) b | 0.05–0.4 (L/LC 0.1) | ||
| Pb | Soil | 150–20.5 (7.32) | 150–16.5 (9.1) | 148–9 (16.4) | 2–80 (35) | |||
| Seed | 0.057–0.140 (2.46) | 0.093–0.033 (2.82) | 0.040–0.019 (2.11) | 30 (30) b | 30 (30) b | 0.1–6 (L 0.2; LC 0.4) | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramss, G.; Voigt, K.-D. Stability of the Inherent Target Metallome in Seed Crops and a Mushroom Grown on Soils of Extreme Mineral Spans. Agronomy 2016, 6, 14. https://doi.org/10.3390/agronomy6010014
Gramss G, Voigt K-D. Stability of the Inherent Target Metallome in Seed Crops and a Mushroom Grown on Soils of Extreme Mineral Spans. Agronomy. 2016; 6(1):14. https://doi.org/10.3390/agronomy6010014
Chicago/Turabian StyleGramss, Gerhard, and Klaus-Dieter Voigt. 2016. "Stability of the Inherent Target Metallome in Seed Crops and a Mushroom Grown on Soils of Extreme Mineral Spans" Agronomy 6, no. 1: 14. https://doi.org/10.3390/agronomy6010014
APA StyleGramss, G., & Voigt, K.-D. (2016). Stability of the Inherent Target Metallome in Seed Crops and a Mushroom Grown on Soils of Extreme Mineral Spans. Agronomy, 6(1), 14. https://doi.org/10.3390/agronomy6010014





