Abstract
Greenhouse and field experiments showed that conidia of the fungal pathogen, Phoma commelinicola, exhibited bioherbicidal activity against spreading dayflower (Commelina diffusa) seedlings when applied at concentrations of 106 to 109 conidia·mL−1. Greenhouse tests determined an optimal temperature for conidial germination of 25 °C–30 °C, and that sporulation occurred on several solid growth media. A dew period of ≥ 12 h was required to achieve 60% control of cotyledonary-first leaf growth stage seedlings when applications of 108 conidia·mL−1 were applied. Maximal control (80%) required longer dew periods (21 h) and 90% plant dry weight reduction occurred at this dew period duration. More efficacious control occurred on younger plants (cotyledonary-first leaf growth stage) than older, larger plants. Mortality and dry weight reduction values in field experiments were ~70% and >80%, respectively, when cotyledonary-third leaf growth stage seedlings were sprayed with 108 or 109 conidia·mL−1. These results indicate that this fungus has potential as a biological control agent for controlling this problematic weed that is tolerant to the herbicide glyphosate.
1. Introduction
Spreading dayflower (Commelina diffusa Burm. f.) is a perennial, monocotyledenous weed occurring worldwide in tropical and subtropical areas, and an annual weed in temperate climates. It spreads diffusely, creeping along the ground, branching heavily and rooting at the nodes, obtaining stem lengths up to 1 m [1]. C. diffusa can reproduce vegetatively and by seed, and cut stems root readily in moist ground. This weed prefers moist, fertile soil (e.g., gardens, cultivated fields), but will also grow on roadsides and in non-crop areas. It has a sprawling growth habit, and its long stems can create a tangled web in gardens and flower beds. It is related to several houseplant species e.g., wandering jew (Tradescantia zebrine (Schinz) D.R. Hunt) and perennial spiderwort (Tradescantia virginiana L.). Commelina spp. have been used as a ground cover to reduce soil erosion [2], which may have contributed to their spreading. Its potential as a fodder crop may be useful to provide protein to ruminants on smallholder farms [3].
When growing in rice and other lowland crops, this weed may act as a quasi-aquatic plant that can withstand flooding, and it readily infests cultivated lands, roadsides, pastures and wastelands [1]. C. diffusa is problematic, primarily in young crops (2–5 weeks old), but can also be a problem in mature crops due to its sprawling behavior [4]. It is a troublesome weed of cotton, rice and soybean in warm temperate areas of the U.S. and other countries [5,6,7]. It is also reported as a major weed of bananas in Mexico and Hawaii; beans, oranges, lemons, grapes, apricots, coffee and cotton in Mexico; papaya in Hawaii; sugarcane in Puerto Rico, and sorghum in Thailand [2]. It is also a weed in maize and vegetables in Mexico; bananas, papayas, and pineapples in the Philippines; rice in Colombia; sugarcane in Mexico and Trinidad; taro and pastures in Hawaii and coffee in Costa Rica [2].
C. diffusa is a host of the root-burrowing (Radophilus similis) [8], reniform (Rotylenchulus reniformis), banana lesion (Pratylenchus goodeyi) [9] and root-knot (Meloidogyne exigua) [10] nematodes. Severe outbreaks of cucumber mosaic virus have been correlated with high densities of C. diffusa serving as a reservoir of virus and aphid vectors [11].
Worldwide, there are about 170 species of Commelina and generally, most are difficult to control. Several Commelinia spp. exhibit resistance or tolerance to several chemical herbicides. C. diffusa was one of the first plants reported as being resistant to 2.4-dichlorophenoxy-acetic acid [12]. Herbicidal control of C. diffusa can be variable depending on the herbicide, growth stage, environmental parameters, etc., and various herbicides and combinations of herbicides have exhibited a range of efficacy for control of C. diffusa and other related species as summarized [4,13,14,15]. Recent guidelines for control of C. diffusa in rice in Mississippi (USA) indicate that only ~50% of the 42 single herbicide or herbicide combination treatments provided good to excellent control, while 26% gave fair control and the remainder gave zero to poor control [16]. Some alternative herbicide options can be used to control this weed, alone or in combination with other modes of action in rice, during early post-emergence applications prior to flooding [17].
C. diffusa, Benghal dayflower (C. benghalensis L.), and Asiatic dayflower (C. communis L.) have been reported to be difficult to control with glyphosate in genetically-modified crops [18,19,20,21,22,23,24]. The ecological, biological and physiological factors related to glyphosate-tolerant C. communis in agronomic systems in Iowa have recently been studied [25]. Because of the increasing importance of C. diffusa, and its resistance or tolerance to many herbicides, alterative weed control measures may be required. The use of bioherbicides has been recognized as a potential technological alternative to chemical herbicides in certain situations, and global interest exists in the bioherbicide concept, with active research and development projects established by commercial entities in the U.S., Canada, Europe, Australia, Japan, and other countries [26,27,28,29,30].
A leaf-spot disease (oblong lesions, ca. 1.3–1.8 cm) was observed on C. diffusa in a flooded rice field near Stuttgart, AR, USA (Figure 1). Infected leaf and stem tissues were collected and a fungal pathogen was isolated from this diseased tissue. This fungus was provisionally identified as Phyllosticta commelinicola E. Young, a synonym of Phoma commelinicola (E. Young) Gruyter [31]. The objectives of these studies were to isolate and examine this pathogen with respect to its growth and germination on various growth media, correlate inoculum concentration and bioherbicidal activity (inundative application) with plant growth stage, develop time courses for weed control and disease progression on C. diffusa, and evaluate weed control under field conditions. Knowledge of these basic parameters is essential for evaluating a plant pathogen as a bioherbicide for weed control [32].

Figure 1.
Commelina diffusa photographs. (A): flowering plant in the field; (B): pressed/dried specimen exhibiting leaf spotting incited by P. commelinicola.
2. Materials and Methods
2.1. Seed Sources, Test Plant Propagation
C. diffusa seeds were collected near Stuttgart AR, USA, planted in a 2:1 potting mix of Jiffy mix:sandy soil (Jiffy Mix, Jiffy Products of America, Inc., Batavia, IL, USA) contained in plastic trays (25 × 52 cm) and allowed to germinate. Germinated seedlings were transplanted into 10-cm2 plastic pots (1 plant per pot) containing the soil mixture above, and grown under greenhouse conditions (28 °C to 32 °C, 40 to 60% relative humidity (RH), ~14 h day length, and 1650 μE·m−2·s−1 photosynthetically active radiation (PAR) measured at midday).
2.2. Isolation and Culture of Phoma commelinicola
Several isolates of the fungus were isolated from diseased C. diffusa tissue by surface sterilizing sections of diseased tissue in 0.05% NaOCl for 1 min, rinsing in sterile distilled water and then placing the sections on autoclaved (121 C, 15 min; at 103.42 kPa) potato-dextrose agar (PDA, Difco, Detroit, MI, USA) plates amended with the antibiotics chloramphenicol (0.75 mg·mL−1) and streptomycin sulfate (1.25 mg·mL−1). The plates were incubated for 48 h at 25 °C and then advancing edges of fungal colonies were transferred to PDA plates followed by incubation for 5 days at 25 °C under alternating 12-h light (cool, white fluorescent bulbs)/12-h dark regimens. Tests of these isolates indicated very similar virulence on the host plant. We chose an isolate with the highest virulence (SFN-73) and used it in further studies. When re-inoculated onto healthy seedlings at 1 × 108, the fungus (SFN-73) was highly virulent and killed all inoculated plants within 5 days, while the controls remained healthy, thus fulfilling Koch’s postulates (data not shown). The fungus was then sub-cultured on PDA without antibiotics, and preserved under refrigeration (4 °C to 5 °C) on sterilized sandy loam soil (25% water holding capacity), or on sterile silica gel containing skim milk [33].
Several media were examined for growth and conidial production of the fungus: water agar, 2.0% (WA), potato dextrose agar (PDA), yeast extract agar (YEA) and Czapek-Dox agar (CDA) from Difco (Detroit, MI, USA), V8 agar (V8A) [33] from Campbell Soup Co. (Camden, NJ, USA) and dayflower decoction agar (DFA). DFA was prepared by adding 100 g of finely ground, air-dried dayflower leaf and stem tissue to 2.0% WA to yield a 10% (w:v) product.
2.3. Effect of Temperature on Conidial Germination and Radial Growth Rate
Conidial germination was measured by spreading 100 μL of a suspension (1.0 × 106 conidia·mL−1) prepared in sterile distilled water on PDA plates, and incubating them at 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, or 35 °C on open-mesh wire shelves of an incubator (Precision Scientific Inc., Chicago, IL, USA). A 12-h photoperiod was provided by two 20 W, cool-white fluorescent lamps positioned in the incubator door. The light intensity at the plate level was 200 μE·m−2·s−1 PAR as measured with a light meter (LI-COR Inc., Lincoln, NE, USA). Germinated conidia (500 plate−1) were counted after 16 h using a haemocytomer.
For radial growth studies, 5-mm plugs were taken from the advancing margins of 7-day-old colonies of the fungus and placed in the centers of PDA plates. The plates were incubated at temperatures of 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, or 35 °C as described above for the conidial germination studies. Colony diameters (fungal growth) were measured after 7 days of incubation. In each experiment, five replicate plates for each temperature were utilized. Both experiments were conducted twice, and the results of each experiment were pooled following testing for homogeneity.
2.4. Effect of Dew Period Duration on Weed Control and Dry Weight Reduction of C. diffusa Seedlings
C. diffusa seedlings (cotyledonary to first leaf growth stage) were sprayed (hand held sprayer; Spray-Tool, Aervoe Industries, Gardnerville, NV, USA) until runoff (ca. 100 L·ha−1) occurred with a spray mixture containing 1.0 × 108 conidia·mL−1 in distilled water. Control plants were sprayed with distilled water. The inoculated plants were then placed in darkened dew chambers at 25 °C and 100% RH for periods of 3, 6, 9, 12, 15, 18, 21 or 24 h. Following this dew treatment, the plants were placed on sub-irrigated trays in the greenhouse as described above. Weed control and dry weight reductions were recorded 14 days after treatment (DAT). For dry weight determinations, the above ground biomass was harvested, oven-dried (48 h, 85 °C), weighed, and the percentage biomass reduction (compared with untreated control plants) was determined. The experiment was conducted twice with 3 sets of 10 plants for each experiment.
2.5. Effect of Inoculum Concentration and Plant Growth Stage
C. diffusa plants (cotyledonary, 1 to 2 true-leaf, 3 to 4 true-leaf and 5 to 7 true-leaf growth stages) were sprayed with conidial suspensions of 1.0 × 106 to 1.0 × 109 conidia·mL−1 and held in a dew chamber for 16 h at 25 °C. Control plants were sprayed with distilled water only. Plants were moved to the greenhouse, and mortality and dry weight reductions were recorded 14 DAT. Experiments were conducted twice with 3 sets of 10 plants for each experiment. The experiment was conducted twice with 3 sets of 10 plants for each experiment.
2.6. Effects of Phoma commelinicola on Crop Seedlings
Greenhouse tests were conducted on seedlings of several crops to access the possible detrimental effects of this pathogen. Rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.), cotton (Gossypium hirsutum L.) and corn (Zea mays L.) plants were grown from seeds in the greenhouse under the conditions described above. After 10 to 14 days (when seedlings were ~3–7 cm tall), plants were sprayed with P. commelinicola inoculum concentrations of 1.0 × 106 or 1.0 × 108 conidia·mL−1. Visual disease symptomatology and dry weight analyses of fungal-inoculated plants were monitored 14 DAT. The experiment was conducted twice with 3 sets of 10 plants for each experiment.
2.7. Field Experiments
Field experiments were conducted in 1998 and 1999 at the University of Arkansas, Rice Research and Extension Center, Stuttgart, AR, USA, on Crowley silt loam (fine montmorillonitic, thermic Typic Albaqualfs), pH 6.2 to 6.5, with an organic matter content of ~1.0%. The experiments were established in an irrigated rice field, divided into 1.0 × 1.0 m micro-plots (1.0 × 10−5 ha). The field was naturally infested with dayflower seedlings (avg. 75 seedlings per plot) that were in the cotyledonary to first leaf growth stages. Within each plot, 18–20 test plants were randomly selected and marked using wooden stakes (7.6 cm long). Treatments consisted of P. commelinicola conidia applied at either 0.0 conidia·mL−1 (water control) or conidia in water applied at several concentrations from 1.0 × 106 to 1.0 × 109 conidia·mL−1. The selected plants were monitored for disease development at 3-day intervals for 21 days. All treatments were replicated 4 times and the experiment was repeated.
2.8. Statistical Procedures
The greenhouse and field experiments were arranged as randomized complete block factorial designs with three and four replications, respectively. Data collected over the 2-year field testing period were examined for homogeneity of variance [34], combined, and analyzed using ANOVA. Field data from both years were pooled following subjection to Bartlett’s test for homogeneity, and analyzed using analysis of variance. Because arcsine and square-root transformation of the data did not alter the interpretation, non-transformed data are presented. When significant differences were detected by the F-test, means were separated with Fisher’s protected LSD test at the 0.05 probability level. Disease progression was based on a modified Horsfall and Barratt [35] rating scale of 0 to 5.0, assigning symptom expression as 0 represents unaffected, and 1.0, 2.0, 3.0, 4.0, and 5.0 represents 20, 40, 60, 80 and 100% leaf and stem injury (or dead plants), respectively. Percentage weed control was determined by dividing the number of dead and severely injured plants (symptom expression ratings of 4.0–5.0) by the total number of plants treated × 100. Data were analyzed using standard mean errors and best-fit regression analysis. All data were analyzed using SAS (Version 9.1, SAS Institute, Inc., Cary, NC, USA) statistical software.
3. Results and Discussion
3.1. Isolation of Phoma commelinicola
The fungus was readily isolated from diseased tissue and observed to sporulate abundantly on PDA. Several isolates were collected and found to exhibit similar virulence. We chose an isolate with the highest virulence (SFN-73) and used it in these studies. When re-inoculated onto healthy seedlings at 1.0 × 108, the fungus (SFN-73) was highly virulent and killed all inoculated plants within 5 DAT, while the controls remained healthy, thus fulfilling Koch’s postulates. The organism produced disease symptomatology typical of other diseases incited by other Phoma spp. (i.e., lesions on leaves and stems) (Figure 1). The organism produced typical Phoma lesions on leaves and stems, with pycnidia scattered throughout the lesions. Under moist conditions, slimy masses of conidia accumulated on the upper surface of the leaf, breaking the epidermal layer and cuticle. Conidia were unicellular, hyaline (2.0 to 4.0 × 1.5 to 2.5 μm) extruded through ostioles contained in black pycnidia (60 to 165 × 45 to 140 μm), ostiolate, protruding into plant tissues or agar surfaces.
3.2. Germination and Growth of Phoma commelinicola
Germination of conidia on PDA occurred at 10 to 35 °C with optimal germination at 20 to 30 °C and maximum (85%) at 25 °C (Figure 2). The fungus grew at all temperatures tested (10 to 35 °C), but growth was significantly reduced at 10, 15 and 35°C (Figure 3). The fungus also grew and sporulated prolifically on several different solid substrate media. Dayflower decoction agar (DFA) and PDA produced the most abundant conidia (6.0 × 108 and 5.0 × 108, respectively) (Table 1). The lowest growth rate occurred on Czapek-Dox agar (4.1 mm·day−1) and the highest rate of growth was found on DFA decoction agar (10.5 mm·day−1). Due to the commercial availability of PDA, it was used in all other greenhouse and field experiments. This growth is comparable to conidial yields produced by other Phoma spp. that have been evaluated as bioherbicides [36,37].

Table 1.
Effect of various growth media a on radial growth and conidial production of Phoma commelinicola under various light and dark regimes b.
| Growth | Radial Growth (mm·day−1) | Conidia (108 plate−1) | ||||
|---|---|---|---|---|---|---|
| Light | Dark | Light/Dark | Light | Dark | Light/Dark | |
| WA | - | - | - | - | - | - |
| DFA | 10.2 a,c | 9.0 a | 10.5 a | 4.2 a | 2.8 a | 6.0 a |
| PDA | 9.5 b | 8.0 b | 9.9 b | 3.0 b | 1.3 b | 5.0 b |
| V8A | 8.4 c | 7.0 c | 8.5 c | 2.1 c | 0.9 c | 3.9 c |
| YEA | 7.5 d | 6.9 c | 7.4 d | 1.0 d | 0.3 d | 2.3 d |
| CDA | 4.0 e | 2.8 d | 4.1 e | 1.0 d | 0.2 d | 1.2 e |
a WA, water agar; DFA, dayflower decoction agar; PDA, potato dextrose agar; V8A (V8 vegetable juice); agar; YEA, yeast-extract agar; CDA, Czapek-Dox agar; b Light conditions (24 h continuous light at 28 °C); Dark conditions (24 h continuous light at 28 °C); Light/dark conditions (12 h light/dark at 28 °C); c Means within the same column followed by the same letter do not differ at p = 0.05, according to FLSD.
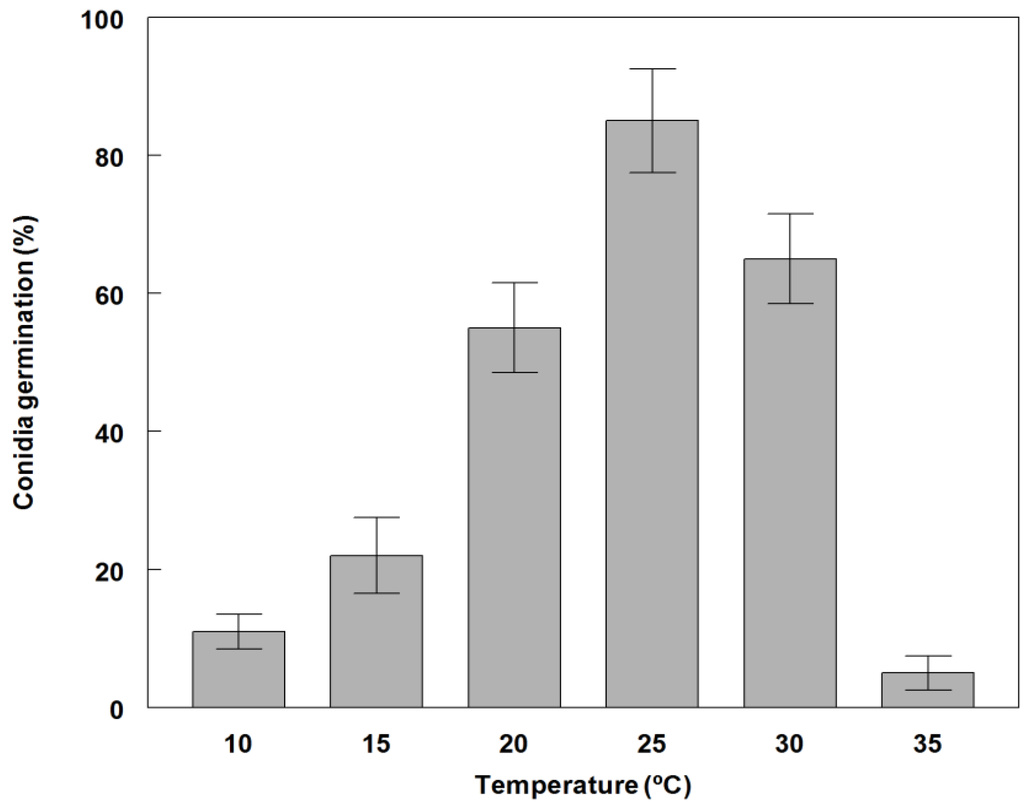
Figure 2.
Effect of temperature on germination of conidia of P. commelinicola on PDA, 7 days after inoculation and growth under alternating light/dark conditions (12 h light/dark at 28 °C). Error bars represent Fisher’s LSD (p = 0.05).
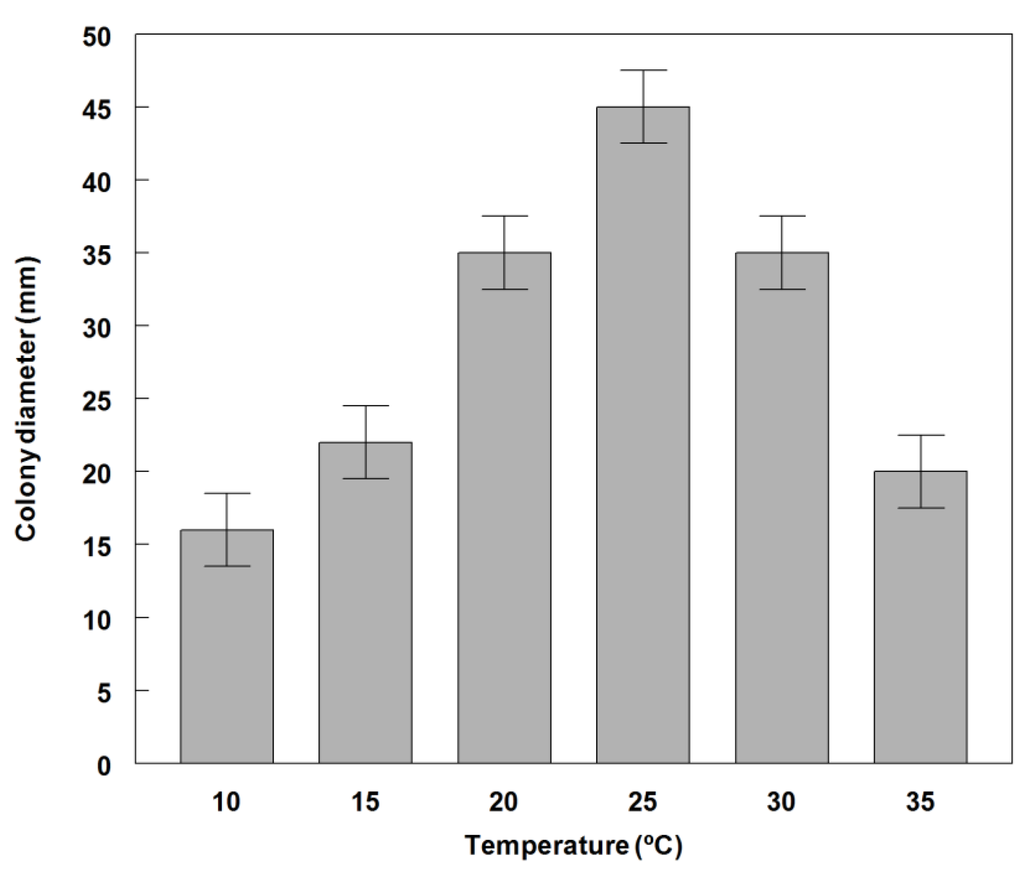
Figure 3.
Effect of temperature on radial growth of P. commelinicola colonies on PDA, 7 days after inoculation and growth under alternating light/dark conditions (12 h light/dark at 28 °C). Error bars represent Fisher’s LSD (p = 0.05).
3.3. Effects of Dew Period Duration on Weed Control and Dry Weight Reduction of C. diffusa Inoculated with P. commelinicola under Greenhouse Conditions
A minimum of 12 h dew at 25 °C was required to achieve ~65% weed control (Figure 4). Optimal weed control occurred at dew period durations of between 15 to 24 h. A similar trend was observed for the dry weight reduction data (~67% at 12 h) (Figure 5). Complete mortality (100%) was not achieved at any dew period, but many plants were severely stunted, which resulted in greatly reduced dry weight. Lengthy dew periods are commonly required for most bioherbicidal plant pathogens [32]. This factor has been a major constraint for commercial development of these biocontrol organisms [38].
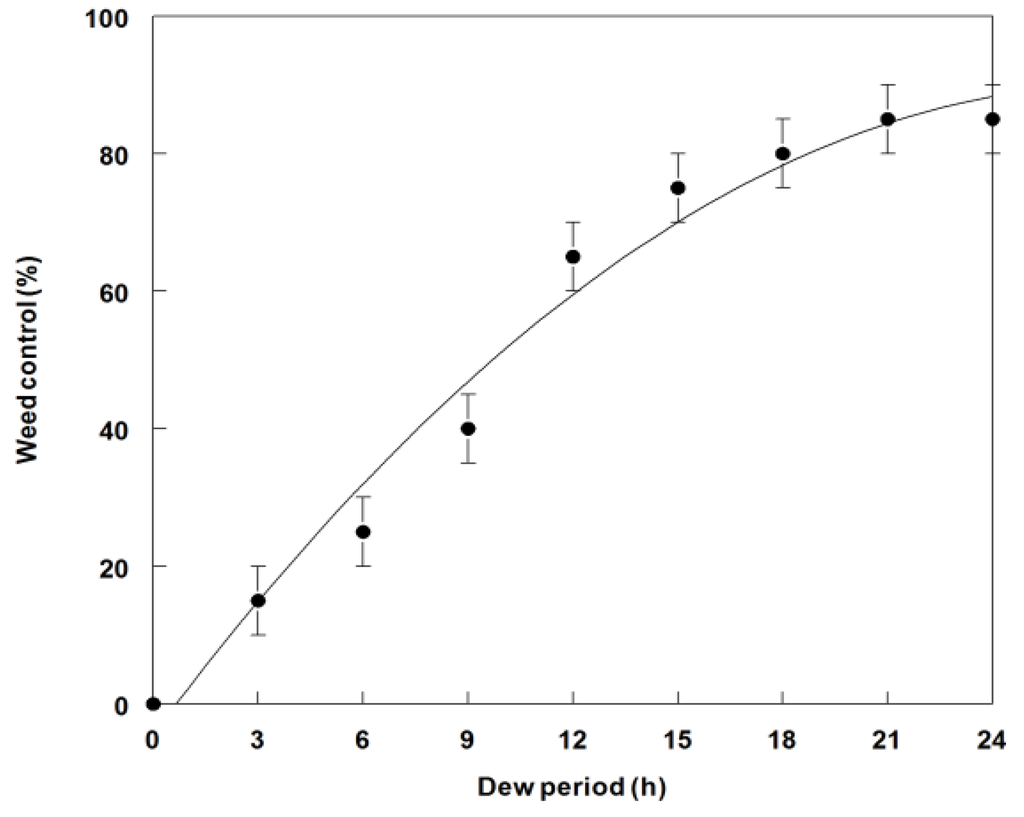
Figure 4.
Effect of dew period duration at 25 °C on weed control of spreading dayflower inoculated with P. commelinicola at 1.0 × 108 under greenhouse conditions. Error bars represent Fisher’s LSD (p = 0.05).
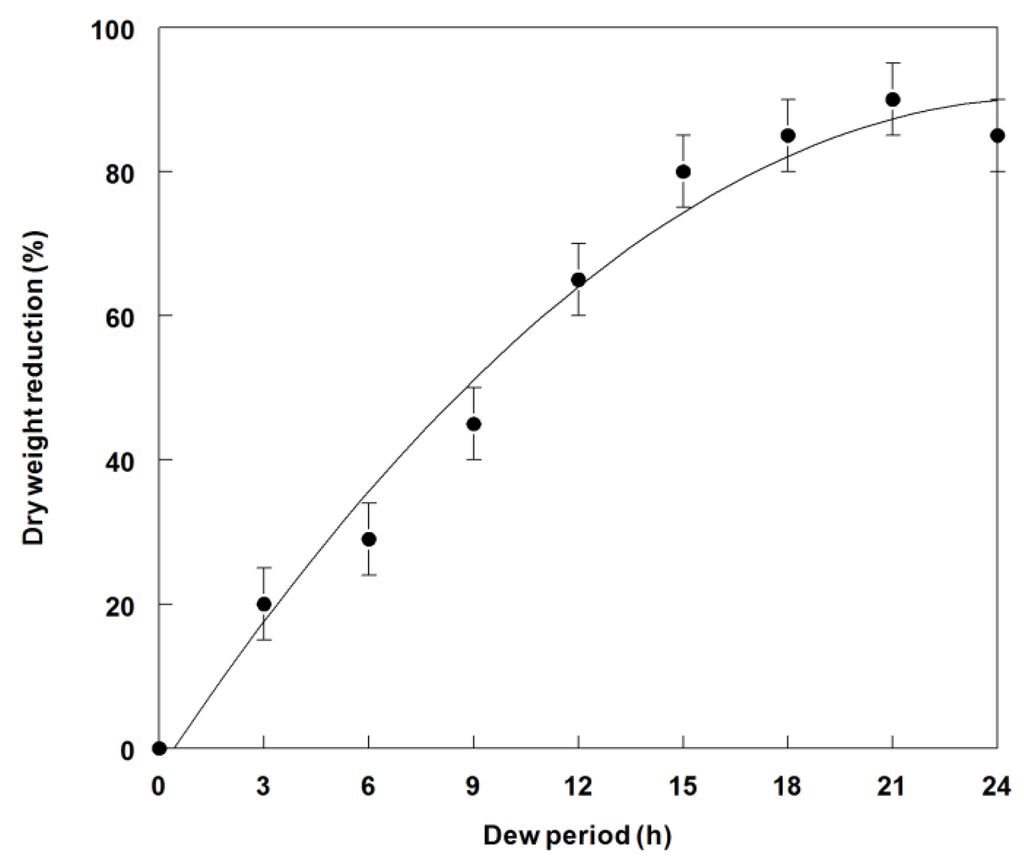
Figure 5.
Effect of dew period duration on dry weight reduction of spreading dayflower inoculated with P. commelinicola at 1.0 × 108 under greenhouse conditions. Error bars represent Fisher’s LSD (p = 0.05).
3.4. Effects of P. commelinicola Inoculum Concentration on Weed Control and Dry Weight Reduction of C. diffusa under Greenhouse Conditions
Generally, weed control on C. diffusa plants under greenhouse conditions was significantly increased at all growth stages as the fungal inoculum concentration increased (Figure 6). For example, at low inoculum concentration (0.001 × 109 conidia·mL−1), the youngest plants were controlled about 5%, while the highest concentration (1.0 × 109 conidia·mL−1) provided ~85% control. Plants in the 6- to 7- and 8- to 9-leaf stages were more resistant to infection than younger plants, i.e., plants in the 8- to 9-leaf growth stages were controlled at the 0 and 15% levels by 0.001 × 109 and 1.0 × 109 conidia·mL−1, respectively. Similar results were obtained for the dry weight reductions of plants at these growth stages and conidia concentrations (Figure 7). Since risk assessment of bioherbicides on non-target plants is necessary and important, we examined the effects of this bioherbicide on several crops under greenhouse conditions. Seedlings of several crops (rice, soybean, cotton and corn), sprayed with P. commelinicola inoculum concentrations at 1.0 × 106 and 1.0 × 108 conidia·mL−1, exhibited no visual disease symptomatology or dry weight reduction when evaluated 14 DAT under greenhouse conditions (data not shown).
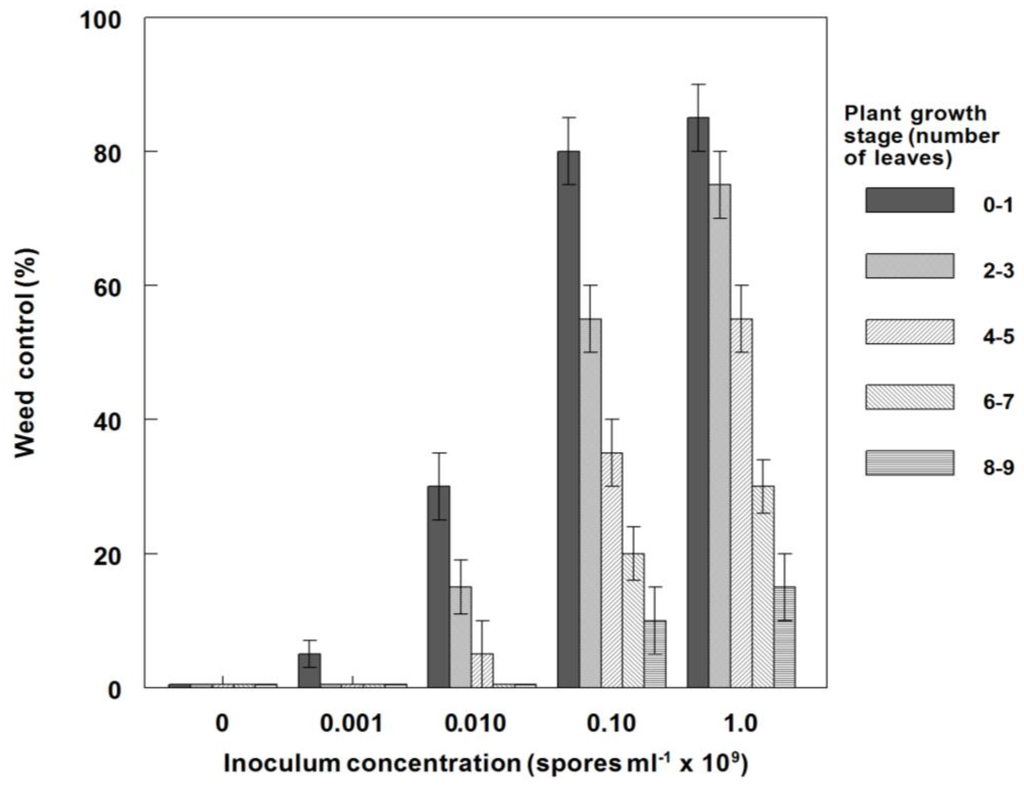
Figure 6.
Effect of plant growth stage on weed control (mortality) of spreading dayflower inoculated with P. commelinicola at various inoculum concentrations under greenhouse conditions. Error bars represent Fisher’s LSD (p = 0.05).
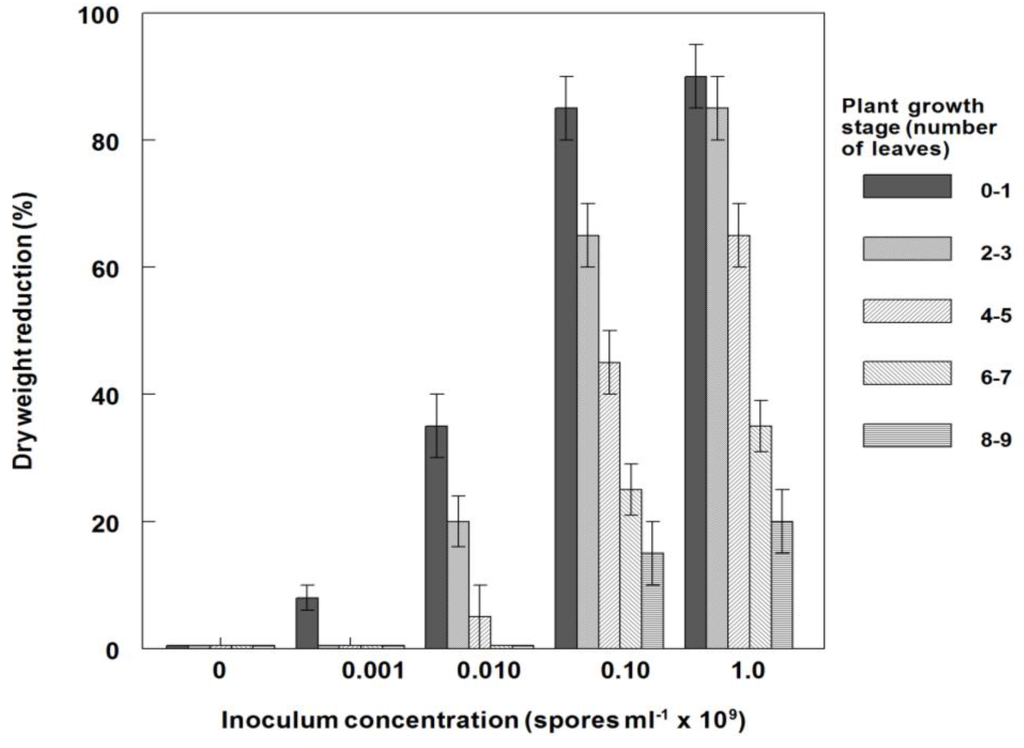
Figure 7.
Effect of plant growth stage on dry weight reduction of spreading dayflower inoculated with P. commelinicola at various inoculum concentrations under greenhouse conditions. Error bars represent Fisher’s LSD (p = 0.05).
3.5. Disease Progression of P. commelinicola on C. diffusa under Greenhouse Conditions
Disease on C. diffusa incited by P. commelinicola progressed in a linear fashion from 3 to 12 DAT under greenhouse conditions, with a disease rating of 3.5 occurring at 12 DAT (Figure 8). Disease progressed to 3.8 at 15 DAT and eventually increased to 4.5 at 21 DAT.
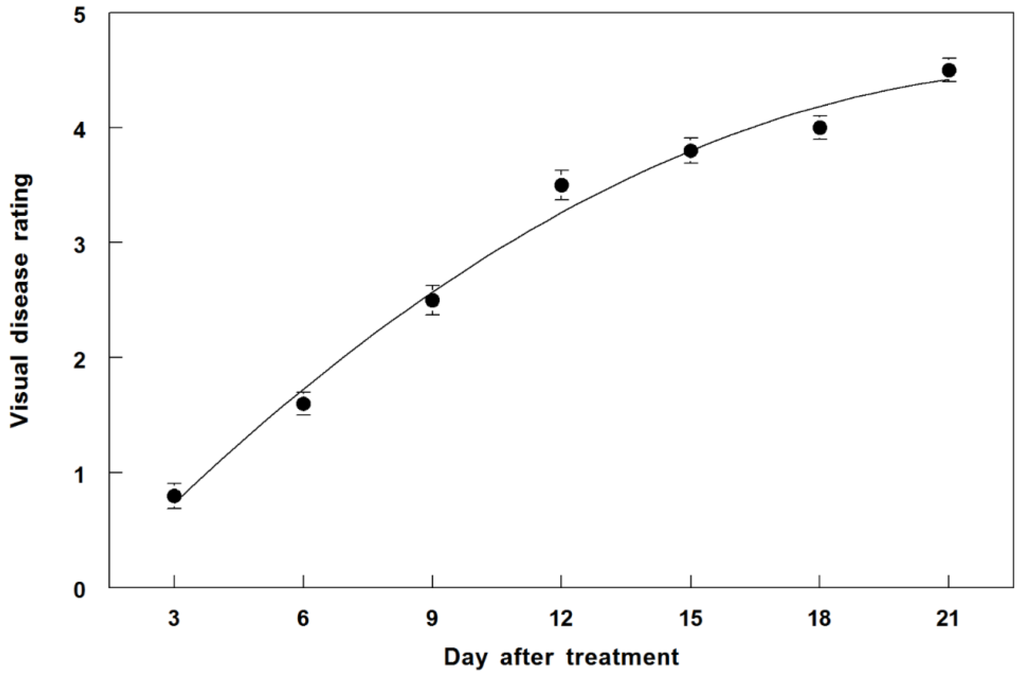
Figure 8.
Disease progression of P. commelinicola on greenhouse-grown C. diffusa over a 21-day period after inoculation. The relationship for P. commelinicola disease progression is best described by the equation: Y = −0.15 + 0.37X − 0.01X2, R2 = 0.98. Error bars represent Fisher’s LSD (p = 0.05).
3.6. Effects of P. commelinicola Inoculum Concentration and C. diffusa Growth Stage on Weed Control and Dry Weight Reduction under Field Conditions
In field experiments, the highest weed control (55 to 70%) occurred on cotyledonary to third-leaf stage plants at 1.0 × 107 to 1.0 × 109 conidia·mL−1, 21 DAT (Figure 9). A similar trend occurred in dry weight reduction with ~80% reduction after 21 DAT at 1.0 × 108 or 109 conidia·mL−1 (Figure 10). The LD50 and GR50 values for weed control (Figure 9) and dry weight reduction (Figure 10) were 2.0 × 107 conidia·mL−1 and 4.0 × 106 conidia·mL−1, respectively.
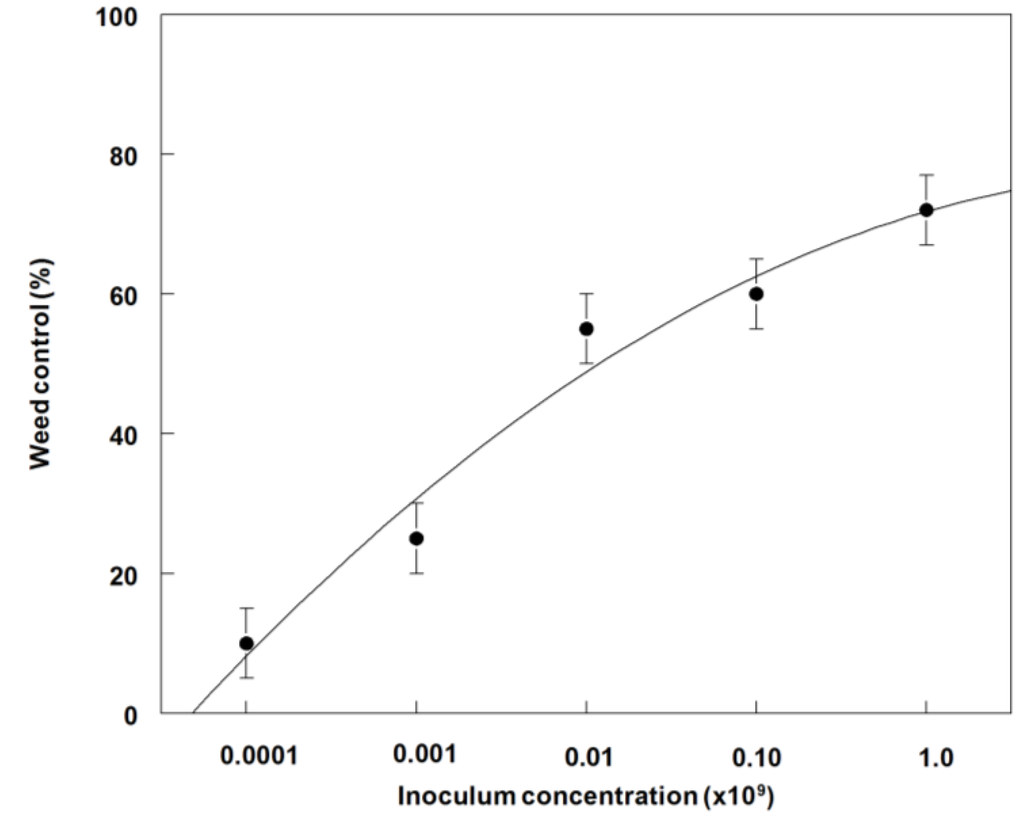
Figure 9.
Effect of P. commelinicola inoculum concentration on the control of C. diffusa in the cotyledonary to third leaf growth stage under field conditions. Y = −18.8 + 29.2X − 2.2X2, R2 = 0.96.
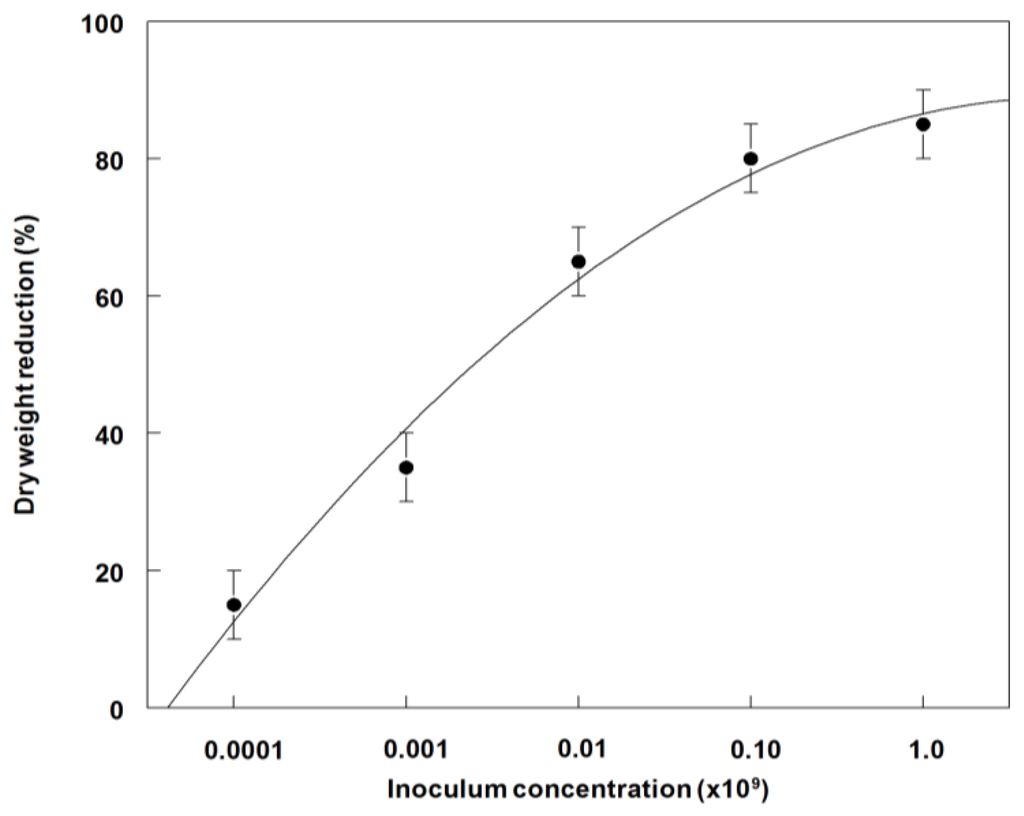
Figure 10.
Effect of P. commelinicola inoculum concentration on the dry weight reduction of C. diffusa in the cotyledonary to third leaf growth stage under field conditions. Y = −22.0 + 37.8X − 3.2X2, R2 = 0.98.
3.7. Disease Progression of P. commelinicola on C. diffusa under Field Conditions
The disease progression of this fungus on C. diffusa under field conditions (Figure 11) was similar to that found under greenhouse conditions (Figure 8). However, 15 days were required to achieve a rating value of 3.5 as compared to 12 days under greenhouse conditions (Figure 8). The maximal disease rating of 3.8 occurred at 21 DAT in the field (Figure 11). No visual infectivity or injury was observed on rice plants using this formulation under field conditions (data not shown).
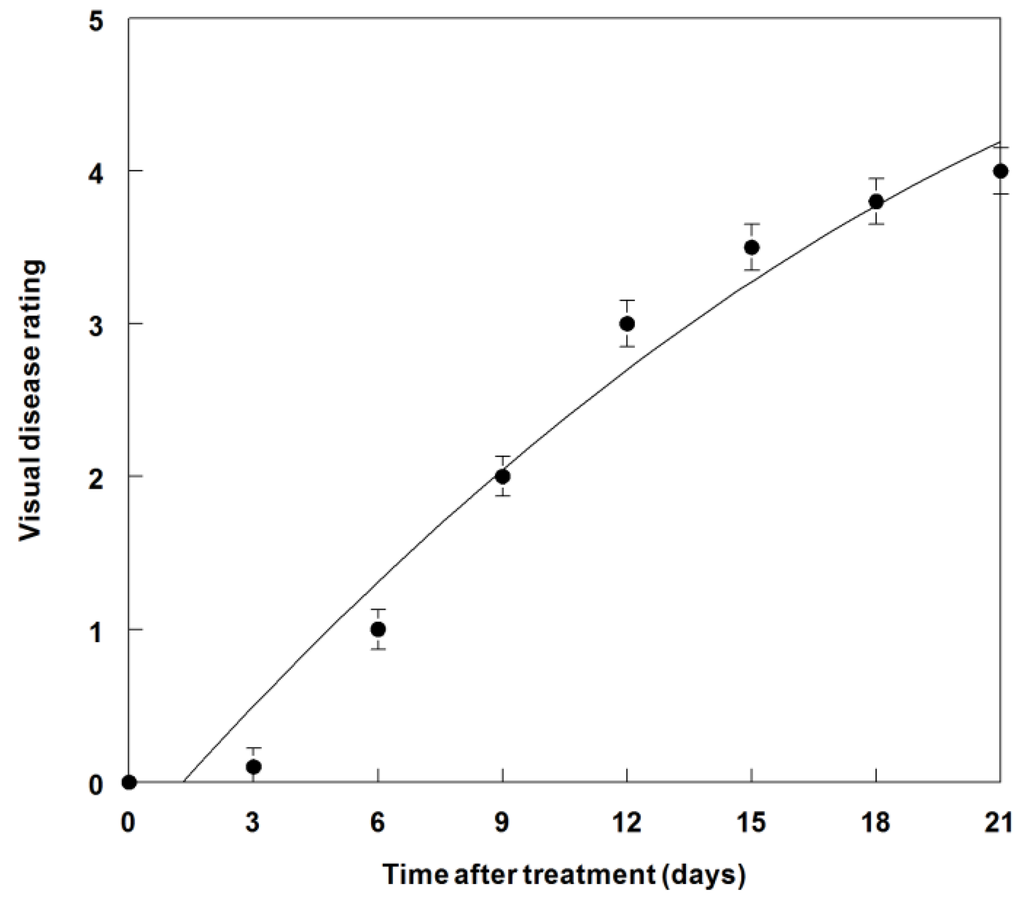
Figure 11.
Disease progression of P. commelinicola on field-grown C. diffusa over a 21-day period after inoculation. The relationship for P. commelinicola disease progression is best described by the equation: Y = −0.39 + 0.31X − 0.04X2, R2 = 0.96. Error bars represent Fisher’s LSD (p = 0.05).
Despite the high phytopathogenic potential shown by various Phoma spp. and pathovars, the bioherbicidal potential of these microbes has been largely ignored. Some attempts to evaluate this diverse genus as bioherbicides have been reported. For example, Heiny [39,40] isolated a highly host specific strain of P. proboscis from diseased field bindweed (Convolvulus arvensis L.). Heiny and Templeton [37] reported significant bioherbicidal effects when conidia of this fungus were applied to weed seedlings, under temperatures from 16–28 °C, and ≥9 h dew period and the compatibility with synthetic herbicides was investigated [40]. Studies of environmental factors on the effectiveness of a Phoma herbarum strain against Commelina communis showed that temperatures of 28–32 °C and a 48-h dew period were required for optimal control [41]. A strain of P. herbarum from diseased leaves of Parthenium hysterophorus L. was found in central India [42]. The fungus caused >90% inhibition of seed germination, seedling mortality and leaf damage, followed by a reduction in the height of this weed [42]. Three strains of Phoma herbarum were isolated from the diseased leaves and stem of Lantana camara L. [43] and all strains incited severe infection of weed seedlings [44]. Other Phoma spp. have also been reported from various regions of India: P. campanulata on Cassia fistula L.; P. exigua on Sesamum indicum L.; P. eupyrena on Achyrenthus aspera L.; P. glomerata on Crotalaria juncea L. and Parthenium hysterophrous L.; P. lantanae on Lantana camara; P. palmarum on Calotropis procera L.; P. tridocis on Tridex procumbens L.; and P. herbarum var. ipomoeae and P. euphorbiae on Euphorbia hirta [45,46]. Recently, Phoma macrotoma has been developed as a commercial bioherbicide (PhomaTM) for controlling various broadleaf weeds in turf [47].
Fungal conidia are the predominant propagules used in bioherbicidal research [26,29,30]. However, mycelial formulations of various fungal bioherbicides, including Phoma spp., have shown weed control potential against some important weeds. For example, mycelial suspensions of P. herbarum incited significantly more disease severity than conidia on dandelion (Taraxacum officinale Weber) [48]. Similarly, mycelial fragments of P. exigua and P. herbarum incited significantly more disease severity of T. officinale than fungal conidia [49]. Zhao and Shamoun [36] found that culture growth media and age of mycelial cultures could affect the disease severity of P. exigua on the perennial evergreen weedy shrub, salal (Gaultheria shallon Pursh).
The Clearfield™ system has become the predominant rice production system in southern rice producing states [50]. The rice cultivars utilized in this system are natural mutants with tolerance to the herbicide imazethapyr (2-(4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl)-5-ethyl-3-pyridine-carboxylic acid) (Newpath™). Although this herbicide controls many grassy and broadleaf weeds, it fails to control some weeds, including C. diffusa, which can result in tremendous weed infestations if other weed control measures are not utilized [51]. Whether this isolate of P. commelinicola could be incorporated in this system remains to be determined.
4. Conclusions
P. commelinicola may be an effective bioherbicide for controlling C. diffusa, a problematic weed in rice production in the southern U.S. Fungal conidia can be readily produced on several solid substrate growth media. The fungus grows and germinates over a wide range of temperatures. Although a rather lengthy dew period is required (15 to 18 h) to achieve levels of control of 75 to 80%, respectively, this free moisture condition is met in flooded rice fields. No visual disease symptomology was observed on rice (cv, Starbonnet). However, further research is in progress to define the host range of the fungus using various rice cultivars and other economically important rice weeds. Special consideration will be given to the effects of this pathogen on other principal Commelina spp., especially C. benghalensis, an exotic, invasive weed that is resistant to herbicides in many areas of the world, including the southern U.S. [52]. We also wish to examine the effects of surfactants and other adjuvants to improve the efficacy and use of mycelial formulations of this organism for control of Commelina spp. Possible interactions (synergistic, additive or antagonistic) of this pathogen with herbicides will be examined since important synergistic interactions of other plant pathogens with herbicides have been discovered [32,53,54]. Furthermore, since another pathogen (C. gloeosporioides f. sp. aeschynomene, LockDownTM) [55] is compatible with the ClearfieldTM rice production system, additional research will also examine the compatibility of P. commelinicola in this rice production protocol.
Acknowledgments
We thank Robin H. Jordan for expert technical assistance during portions of these studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bryson, C.T.; DeFelice, M.S. Weeds of the South; University of Georgia: Athens, GA, USA, 2009; p. 325. [Google Scholar]
- Isaac, W.A.; Gao, Z.; Li, M. Managing Commelina species: Prospects and limitations. Herbic. Curr. Res. Case Stud. Use 2013, 543–561. [Google Scholar] [CrossRef]
- Lanyasunya, T.P.; Wang, R.H.; Abdulrazak, S.A.; Mukisira, E.A. The potential of the weed, Commelina diffusa L., as a fodder crop for ruminants. S. Afr. J. Anim. Sci. 2006, 36, 28–32. [Google Scholar] [CrossRef]
- Invasive Species Compendium: Commelina diffusa (Spreading Dayflower). Available online: http://www.cabi.org/isc/datasheet/14979 (accessed on 2 July 2015).
- Webster, T.M. Weed survey—Southern States 2005: Broadleaf Crops Subsection (Cotton, Peanut, Soybean, Tobacco, and Forestry). Proc. South. Weed Sci. Soc. 2005, 58, 291–306. [Google Scholar]
- Norsworthy, J.K.; Burgos, N.R.; Scott, R.C.; Smith, K.L. Consultant perspectives on weed management needs in Arkansas rice. Weed Technol. 2007, 21, 832–839. [Google Scholar] [CrossRef]
- Singh, G.; Singh, Y.; Singh, V.P.; Johnson, D.E.; Mortimer, M. System level effects in weed management in rice-wheat cropping in India. In Proceedings of the British Crop Protection Conference International Congress on Crop Science and Technology (Glasgow, UK), Alton, Hampshire, UK, 2005; pp. 545–550.
- Queneherve, P.; Chabrier, C.; Auwerkerken, A.; Topart, P.; Martiny, B.; Martie-Luce, S. Status of weeds as reservoirs of plant parasitic nematodes in banana fields in Martinique. Crop Prot. 2006, 25, 860–867. [Google Scholar] [CrossRef]
- Robinson, A.F.; Inserra, R.N.; Caswell-Chen, E.P.; Vovlas, N.; Troccoli, A. Rotylenchulus species: Identification, distribution, host ranges and crop plant resistance. Nematropica 1997, 15, 165–170. [Google Scholar]
- Rich, J.R.; Brito, J.A.; Kaur, R.; Ferrell, J.A. Weed species as hosts of Meloidogyne: A review. Nematropica 2008, 39, 157–185. [Google Scholar]
- Richard, A.; Farreyrol, K.; Rodier, B.; Leoce-Mouk-San, K.; Wong, M.; Pearson, M.; Grisoni, M. Control of virus diseases in intensively cultivated vanilla plots of French Polynesia. Crop Protect. 2009, 28, 870–877. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Hanson, B. Herbicide Resistance and Its Management. Available online: http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=12365 (accessed on 6 July 2015).
- Isaac, W.A.P.; Brathwaite, R.A.I. Commelina species—A review of its weed status and possibilities for alternative weed management in the tropics. Agro. Thesis 2007, 5, 3–18. [Google Scholar]
- Wilson, A.K. Commelinaceae—A review of the distribution, biology and control of the important weeds belonging to this family. Int. J. Pest Manag. 1981, 27, 405–418. [Google Scholar] [CrossRef]
- Anonymous. CABI Crop Protection Compendium, Global Module; CAB International: Wallingford, UK, 2007; Available online: http://www.cabi/compendia/cpc/index.htm (accessed on 21 June 2015).
- Dodds, D.; Calcote, K.; Byrd, J. Weed Control Guidelines for Mississippi; Mississippi State University Extension Service and Mississippi Agricultural and Forestry Experimental Station: Starkville, MS, USA, 2015; p. 64. [Google Scholar]
- Scott, R.C.; Boyd, J.W.; Selden, G.; Norsworthy, J.K.; Burgos, N. Recommended Chemicals for Weed and Brush Control—MP44; University of Arkansas Extension Publication: Fayetteville, AR, USA, 2015; p. 79. [Google Scholar]
- Fawcett, J. Glyphosate tolerant Asiatic dayflower (Commelina communis) control in no-till soybeans. Proc. North Cent. Weed Sci. Soc. 2002, 57, 183. [Google Scholar]
- Santos, L.D.T.; Meira, R.M.S.A.; Santos, I.C.; Ferreira, F.A. Effect of glyphosate on the morpho-anatomy of leaves and stems of C. diffusa and C. benghalensis. Planta Daninha 2004, 22, 101–107. [Google Scholar]
- Webster, T.M.; Burton, M.G.; Culpepper, A.S.; York, A.C.; Prostko, E.P. Tropical spiderwort (Commelina benghalensis): A tropical invader threatens agroecosystems of the Southern United States. Weed Technol. 2005, 19, 501–508. [Google Scholar] [CrossRef]
- Culpepper, A.S. Glyphosate-induced weed shifts. Weed Technol. 2006, 20, 277–281. [Google Scholar] [CrossRef]
- Isaac, W.A.P.; Brathwaite, R.A.I.; Cohen, J.E.; Bekele, I. Effects of alternative weed management strategies on Commelina diffusa Burm. infestations in fair trade banana (Musa spp.) in St. Vincent and the Grenadines. Crop Prot. 2007, 26, 1219–1225. [Google Scholar] [CrossRef]
- Webster, T.M.; Faircloth, W.H.; Flanders, J.T.; Prostko, E.P.; Grey, T.L. The critical period of Bengal dayflower (Commelina benghalensis) control in peanut. Weed Sci. 2007, 55, 359–364. [Google Scholar] [CrossRef]
- Ulloa, S.M.; Owen, M.D.K. Response of Asiatic dayflower (Commelina communis) to glyphosate and alternatives in soybean. Weed Sci. 2009, 57, 74–80. [Google Scholar] [CrossRef]
- Gomez, J.M. Glyphosate-tolerant Asiatic Dayflower (Commelina communis L.): Ecological, Biological and Physiological Factors Contributing to Its Adaptation to Iowa Agronomic Systems. Master Thesis, Iowa State University, Ames, IA, USA, 2012. [Google Scholar]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. Biol. Control 2001, 46, 229–260. [Google Scholar]
- Charudattan, R. Ecological, practical, and political inputs into selection of weed targets: What makes a good biological control target? Biol. Control 2005, 35, 183–196. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelemter, W.; Jackson, T.; Keyhani, N.; Kohl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Weaver, M.A.; Lyn, M.E.; Boyette, C.D.; Hoagland, R.E. Bioherbicides for weed control. In Non-Chemical Weed Management; Updhyaya, M.K., Blackshaw, R.E., Eds.; CABI International: Cambridge, MA, USA, 2007; pp. 93–110. [Google Scholar]
- Duke, S.O.; Scheffler, B.E.; Boyette, C.D.; Dayan, F.E. Biotechnology in weed control. In Encyclopedia of Chemical Technology; Kirk, O., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2015; pp. 1–25. [Google Scholar]
- De Gruyter, J. Contributions towards a monograph of Phoma (Coelomycetes) IX. Section Macrospora. Persoonia 2002, 18, 85–102. [Google Scholar]
- Boyetchko, S.M.; Peng, G. Challenges and strategies for development of mycoherbicides. In Fungal Biotechnology in Agricultural, Food, and Environment Applications; Arora, D.K., Ed.; Marcel and Dekker: New York, NY, USA, 2004; pp. 111–121. [Google Scholar]
- Tuite, J. Plant Pathological Methods: Fungi and Bacteria; Burgess Publication Co.: Minneapolis, MN, USA, 1969. [Google Scholar]
- Steele, R.G.D.; Torrey, J.H.; Dickeys, D.A. Multiple Comparisons. In Principles and Procedures of Statistics—A Biometrical Approach; McGraw Hill: New York, NY, USA, 1997; p. 365. [Google Scholar]
- Horsfall, J.G.; Barratt, R.W. An improved grading system for measuring diseases. Phytopathology 1945, 35, 655. [Google Scholar]
- Zhao, S.; Shamoun, S.F. Effects of culture media, temperature, pH, and light on growth, sporulation, germination, and bioherbicidal efficacy of Phoma exigua, a potential biological control agent for salal (Gaultheria shallon). Biocontrol Sci. Technol. 2006, 16, 1043–1055. [Google Scholar] [CrossRef]
- Heiny, D.K.; Templeton, G.E. Effects of spore concentration, temperature, and dew period on disease of field bindweed caused by Phoma proboscis. Phytopathology 1991, 81, 905–909. [Google Scholar] [CrossRef]
- Auld, B.A.; Morin, L. Constraints in the development of bioherbicides. Weed Technol. 1995, 9, 638–652. [Google Scholar]
- Heiny, D.K. Phoma probocis sp. nov. pathogenic on Convolvoulus arvensis. Mycotaxon 1990, 36, 457–471. [Google Scholar]
- Heiny, D.K. Field survival of Phoma proboscis and synergism with herbicides for control of field bindweed. Plant Dis. 1994, 78, 1156–1164. [Google Scholar] [CrossRef]
- Gu, Z.M.; Ji, M.S.; Li, X.H.; Qi, Z.Q. Effects of environmental factors on effectiveness of Phoma herbarum strain SYAU-06 against Commelina communis. Chin. J. Biol. Control 2009, 25, 355–358. [Google Scholar]
- Rajak, R.C.; Farkya, S.; Hasija, S.K.; Pandey, A.K. Fungi associated with congress weed (Parthenium hysterophorus L.). Proc. Nat. Acad. Sci. India 1990, 60, 165–168. [Google Scholar]
- Pandey, A.K.; Luka, R.S.; Hasija, S.K.; Rajak, R.C. Pathogenicity of some fungi to Parthenium and obnoxious weed in Madhya Pardesh. J. Biol. Control 1991, 5, 113–115. [Google Scholar]
- Pandey, S.; Pandey, A.K. Mycoherbicidal potential of some fungi against Lantana camara L.: A preliminary observation. J. Trop. For. 2000, 16, 28–32. [Google Scholar]
- Pandey, A.K. Microorganism associated with weeds: Opportunities and challenges for their exploitation as herbicides. Int. J. Mendel 2000, 17, 59–62. [Google Scholar]
- Deshmukh, P.; Rai, M.K.; Kövics, G.; Irinyi, L.M.; Karaffa, E.M. Phomas—Can these fungi be used as biocontrol agents and sources of secondary metabolites? In Proceedings of the 4th International Plant Protection Symposium at Debrecen University and 11th Trans-Tisza Plant Protection Forum, Debrecen, Hungary, 18–19 October 2006; pp. 224–232.
- Quarles, W. New Biopesticides for IPM and Organic Production. IPM Pract. 2011, 33, 1–20. [Google Scholar]
- Neumann, S.; Boland, G.J. Influence of host and pathogen variables on the efficacy of Phoma herbarum, a potential biological control agent of Taraxacum officinale. Can. J. Bot. 2002, 80, 425–429. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M.; Boland, G.J. Selected cultural and environmental parameters influence disease severity of dandelion caused by the potential bioherbicidal fungi, Phoma herbarum and Phoma exigua. Biocontrol Sci. Technol. 2004, 14, 561–569. [Google Scholar] [CrossRef]
- Burgos, N.R.; Singh, V.; Tseng, T.-M.; Black, H.; Young, N.D.; Huang, Z.; Hyma, K.E.; Gealy, D.R.; Caicedo, A.L. The impact of herbicide-resistant rice technology on phenotypic diversity and population structure of United States weedy rice. Plant Physiol. 2014, 166, 1208–1220. [Google Scholar] [CrossRef]
- Scott, R.C.; Meins, K.B.; Smith, K.L. Tank-mix partners with Newpath herbicide for hemp sesbania control in a Clearfield rice-production system. Ark. Agric. Res. Ser. 2005, 54, 225–229. [Google Scholar]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Hoagland, R.E.; Boyette, C.D.; Vaughn, K.C. Interactions of quinclorac with a bioherbicidal strain of Myrothecium verrucaria. Pest Technol. 2011, 5, 88–96. [Google Scholar]
- Boyette, C.D.; Hoagland, R.E.; Weaver, M.A.; Stetina, K.C. Interaction of the bioherbicide Myrothecium verrucaria and glyphosate for kudzu control. Am. J. Plant Sci. 2014, 5, 3943–3956. [Google Scholar] [CrossRef]
- Cartwright, K.; Boyette, C.D.; Roberts, M. Lockdown: Collego bioherbicide gets a second act. Phytopathology 2010, 100. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).