Benefits of Precision Farming Technologies for Mechanical Weed Control in Soybean and Sugar Beet—Comparison of Precision Hoeing with Conventional Mechanical Weed Control
Abstract
:1. Introduction
2. Results and Discussion
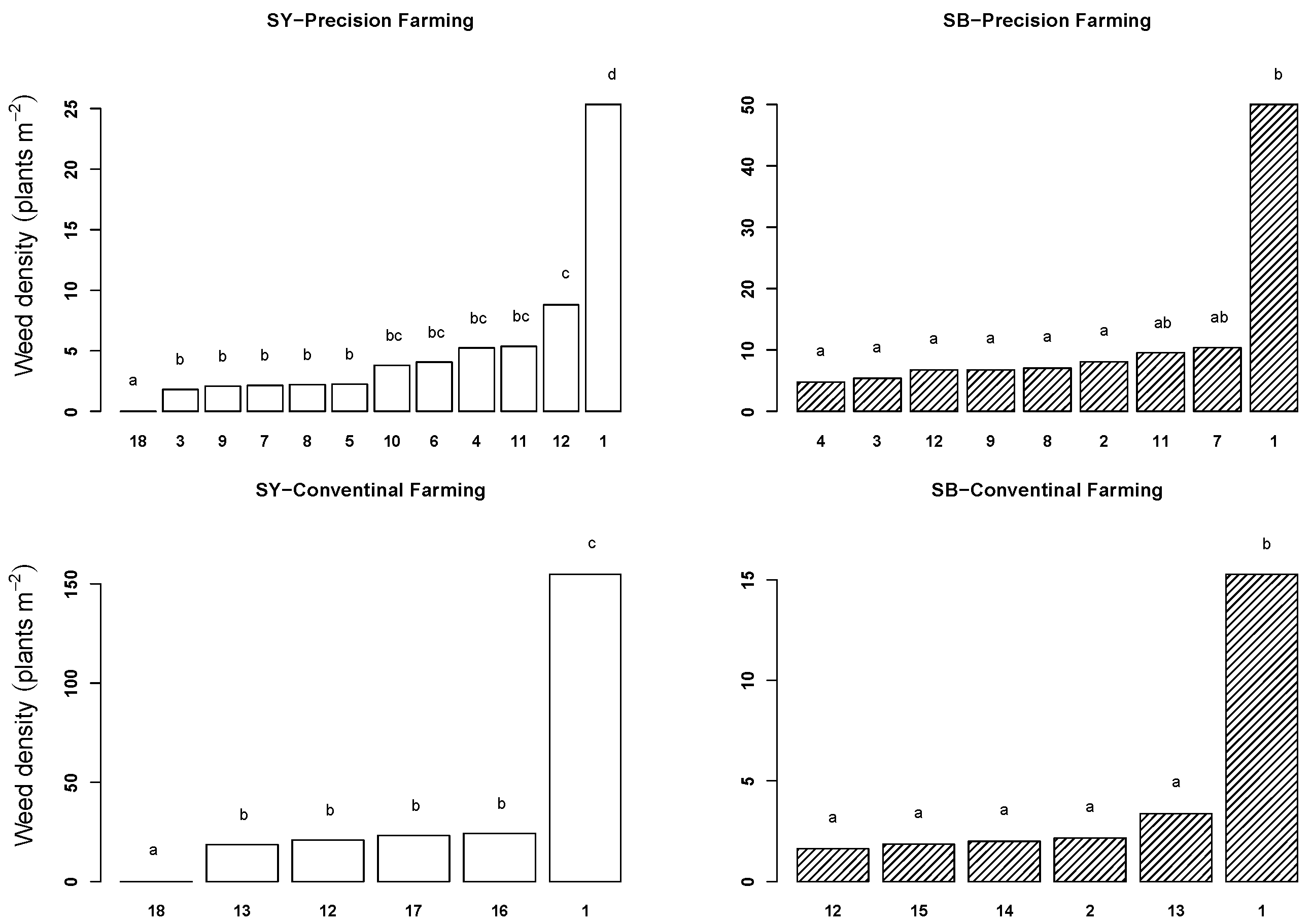
2.1. Weed Density
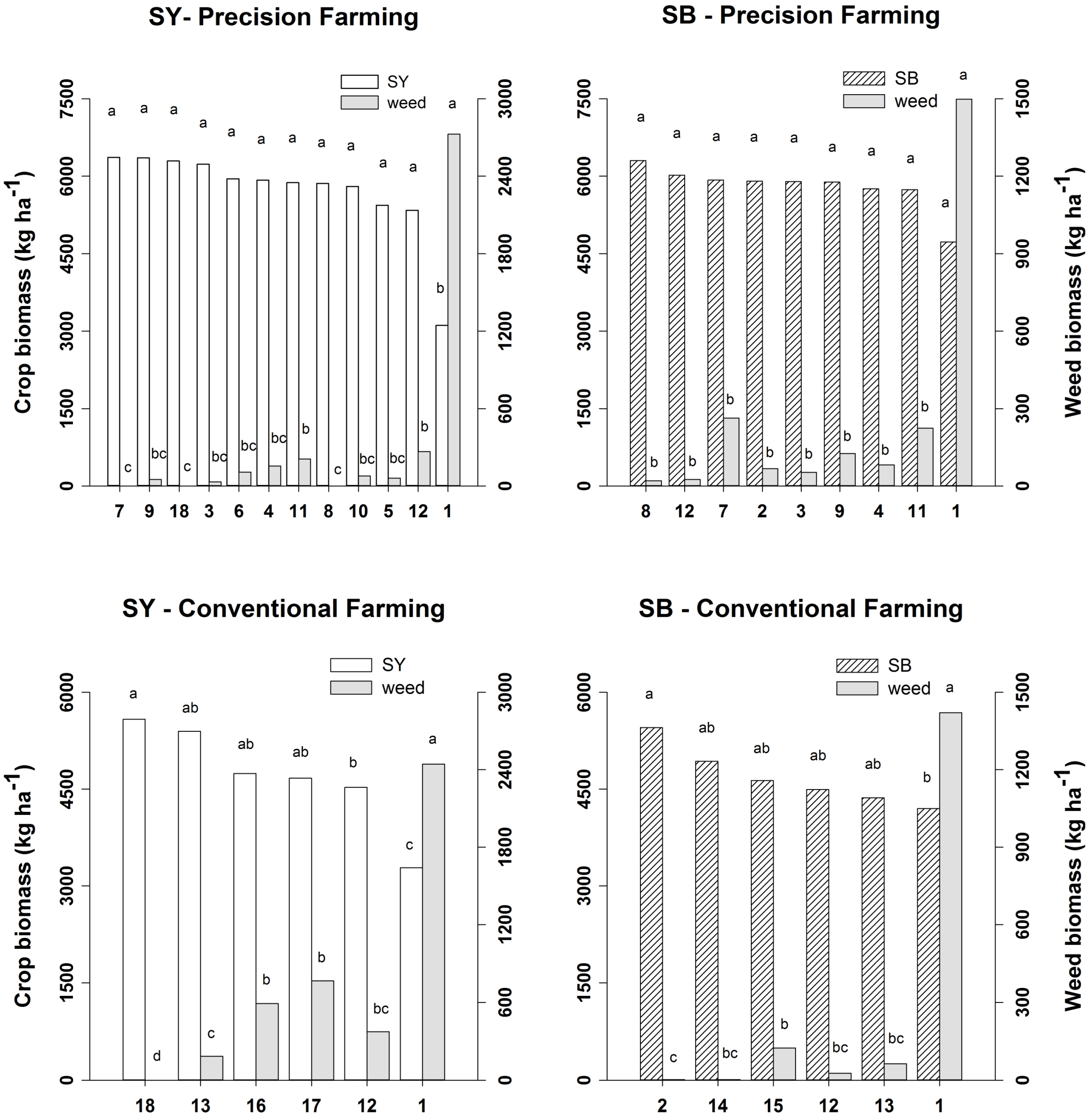
2.2. Crop and Weed Biomass
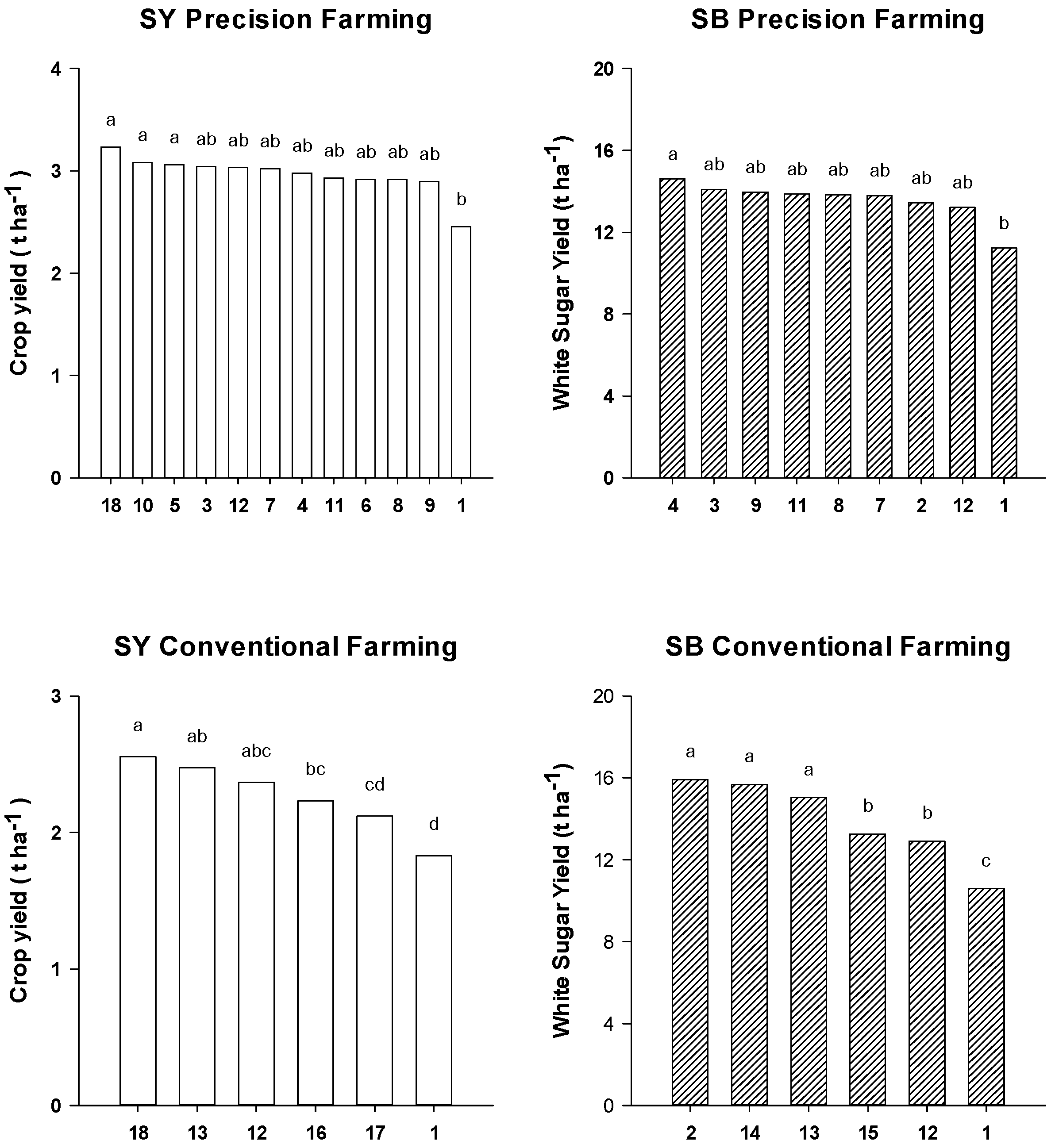
2.3. Crop Yield
3. Experimental Section
3.1. Experimental Sites and Design
3.2. Set up of Experiments
| Crop | Treatment | Description | Speed (km/h) | |
|---|---|---|---|---|
| SB | SY | 1 | No weed control | |
| SB | 2 | Herbicide | 5 | |
| Precision hoeing | ||||
| SB | SY | 3 | RTK hoe, 2 cm deep | 7 |
| SB | SY | 4 | RTK hoe, 2 cm deep | 4 |
| SY | 5 | RTK hoe, 5 cm deep | 7 | |
| SY | 6 | RTK hoe, 5 cm deep | 4 | |
| SB | SY | 7 | RTK hoe 2 cm deep with finger-weeder | 7 |
| SB | SY | 8 | RTK hoe 2 cm deep with finger-weeder | 4 |
| SB | SY | 9 | Camera hoe, 2 cm deep | 10 |
| SB | SY | 10 | Camera hoe, 2 cm deep | 7 |
| SB | SY | 11 | Camera hoe, 2 cm deep | 4 |
| Conventional hoeing | ||||
| SB | SY | 12 | Conventional hoe, 2 cm deep | 4 |
| SB | SY | 13 | Pre-emergence harrow and hoe, 2 cm deep | 7/4 |
| SB | 14 | Pre-emergence harrow and herbicide | 7/5 | |
| SB | 15 | Hoe with cutting blade, 2 cm deep | 4 | |
| SY | 16 | Pre- + post-emergence harrow + hoe 2 cm deep | 7/4 | |
| SY | 17 | Post-emergence harrow, 3 times | 7 | |
| SY | 18 | Repeated hand weeding | ||

| Location | Cultivation Technology | Crop | Year | Treatment | DAS |
|---|---|---|---|---|---|
| MB | CV | SY | 2013 | 12, 13, 16, 17, 18 | 3, 10, 17, 30 |
| KH | CV | SY | 2013 | 12, 13, 16, 17, 18 | 3, 10, 17, 30 |
| KH | CV | SY | 2014 | 12, 13, 16, 17, 18 | 3, 10, 17, 30 |
| IHO | PF | SY | 2014 | 3, 4–12 | 27, 37, 50 |
| MB | CV | SY | 2013 | 12, 13, 16, 17, 18 | 3, 10, 17, 30 |
| IHO | PF | SB | 2014 | 2–4, 7–12 | 34 , 49 , 66 |
| GÜ | CV | SB | 2014 | 2, 13, 14 | 4, 35, 47, 60 |
| HD | CV | SB | 2014 | 2, 12, 15 | 11, 22, 43 |
| RE | CV | SB | 2014 | 2, 13 | 3, 27, 45, 59 |
| 12, 14, 15 | 3, 27, 48, 70 |
3.3. Data Collection
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hock, S.M.; Knezevic, S.Z.; Martin, A.R.; Lindquist, J.L. Soybean row spacing and weed emergence time influence weed competitiveness and competitive indices. Weed Sci. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Kropff, M.J.; Spitters, C.J.T. A simple model of crop loss by weed competition from early observations on relative leaf area of the weeds. Weed Res. 1991, 31, 97–105. [Google Scholar] [CrossRef]
- Bastiaans, L.; Kropff, M.J. WEEDS Weed Competition. In Encyclopedia of Applied Plant Sciences; Thomas, B., Ed.; Elsevier: Oxford, UK, 2003; pp. 1494–1500. [Google Scholar]
- Petersen, J. A Review on Weed Control in Sugar beet: From Tolerance Zero to Period Threshold. In Weed Biology and Management, Inderjit; Kluwer Academic Publishers: Dordrecht, Netherlands, 2004; pp. 467–483. [Google Scholar]
- Petersen, J.; Hurle, K. Einführung von herbizidresistenten Sorten: Konsequenzen für die Unkrautbekämpfung. Z. PflKankh. PflSchutz, Sonderh 1998, 16, 365–372. [Google Scholar]
- Schroeder, D. A European weed survey in 10 major crop systems to identify targets for biological control. Weed Res. 1993, 33, 449–458. [Google Scholar] [CrossRef]
- Gehring, K.; Festner, T.; Gerhards, R.; Hüsgen, K.; Thyssen, S. Chemische Unkrautregulierung beim Anbau von Sojabohnen (Glycine Max, L.). Julius-Kühn-Archiv 2014, 443, 701–708. [Google Scholar]
- Vasel, E.-H.; Ladewig, E.; Märländer, B. Weed composition and herbicide use strategies in sugar beet cultivation in Germany. J. Kult. 2012, 64, 112–125. [Google Scholar]
- Wilson, R.G. Response of Nine Sugarbeet (Beta vulgaris) Cultivars to Postemergence Herbicide Applications. Weed Technol. 1999, 13, 25–29. [Google Scholar]
- Bender, J. Change: Goals and Obstacles. In Future Harvest-Pesticide-Free Farming; Bender, J., Ed.; University of Nebraska Press: Lincoln, NE, USA, 1995; pp. 1–8. [Google Scholar]
- Heap, I. The International Survey of Herbicide-Resistant Weeds. Available online: http://www.weedscience.org/summary/home.aspx (accessed on 22 September 2014).
- Aper, J.; Mechant, E.; Rubin, B.; Heyerick, A.; Callebaut, G.; Mangelinckx, S. Absorp-tion, translocation and metabolism of metamitron in Chenopodium album. Pest Manag. Sci. 2012, 68, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Kaiser, Y.I.; Andújar-Sánchez, D.; Carmona-Alférez, R.; Mehrtens, J.; Gerhards, R. Development of a geo-referenced database for weed mapping and analysis of agronomic factors affecting herbicide resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy 2013, 3, 13–27. [Google Scholar] [CrossRef]
- Buhler, D.D.; Gunsolus, J.L.; Ralston, D.F. Integrated Weed Management techniques to reduce herbicide inputs in soybean. Agron. J. 1992, 84, 973–978. [Google Scholar] [CrossRef]
- Hyvönen, T. Can conversion to organic farming restore the species composition of arable weed communities? Biol. Conserv. 2007, 137, 382–390. [Google Scholar] [CrossRef]
- Liebman, M.; Liedman, M.; Mohler, C.L.; Staver, C.P. Ecological Management of Agricultural Weeds; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Märländer, B.; Bräutigam, H. Influence of plant population on weed infestation as well as yield and quality of sugar beet-first step of integrated weed management. Gesunde Pflanzen 1994, 46, 117–121. [Google Scholar]
- Gummert, A.; Ladewig, E.; Märländer, B. Guidelines for integrated pest management in sugar beet cultivation-weed control. J. Kult. 2012, 64, 105–111. [Google Scholar]
- Lötjönen, T.; Mikkola, H.J. Three mechanical weed control techniques in spring cereals. Agric. Food Sci. Finl. 2000, 9, 269–278. [Google Scholar]
- Rasmussen, J.; Ascard, J. Weed control in organic farming systems. In Ecology and Integrated Farming Systems: Proceedings of the 13th Long Ashton International Symposium on Arable Ecosystems for the 21st Century; Glen, D.M., Greaves, M.P., Anderson, H.M., Eds.; John Wiley & Sons: Chichester, UK, 1995; pp. 49–67. [Google Scholar]
- Dierauer, H.U.; Stöppler-Zimmer, H. Unkrautregulierung ohne Chemie; Dierauer, H.U., Stöppler-Zimmer, H., Eds.; Eugen Ulmer Verlag: Stuttgart, Germany, 1994. [Google Scholar]
- Bowman, G. Steel in the Field: A Farmer’S Guide to Weed Management Tools; Greg Bowmann: Beltsville, MD, USA, 1997. [Google Scholar]
- Van der Weide, R.Y.; Bleeker, P.O.; Achten, V.T.J.M.; Lotz, L.A.P.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Alliaume, F.; Rossing, W.A.H.; Tittonell, P.; Jorge, G.; Dogliotti, S. Reduced tillage and cover crops improve water capture and reduce erosion of fine textured soils in raised bed tomato systems. Agric. Ecosyst. Environ. 2014, 183, 127–137. [Google Scholar] [CrossRef]
- Nørremark, M.; Griepentrog, H.W.; Nielsen, J.; Søgaard, H.T. Evaluation of an autonomous GPS-based system for intra-row weed control by assessing the tilled area. Precis. Agric. 2012, 13, 149–162. [Google Scholar] [CrossRef]
- Ruckelshausen, A.; Klose, R.; Linz, A.; Marquering, J.; Thiel, M.; Tölke, S. Autonome Roboter zur Unkrautbekämpfung. Z. PflKankh. PflSchutz. Sonderh 2006, 10, 173–180. [Google Scholar]
- Søgaard, H.T.; Olsen, H.J. Determination of crop rows by image analysis without segmentation. Comput. Electron. Agric. 2003, 38, 141–158. [Google Scholar] [CrossRef]
- Tillett, N.D.; Hague, T.; Miles, S.J. Inter-row vision guidance for mechanical weed control in sugar beet. Comput. Electron. Agric. 2002, 33, 163–177. [Google Scholar] [CrossRef]
- Melander, B.; Cirujeda, A.; Jørgensen, M.H. Effects of inter-row hoeing and fertilizer placement on weed growth and yield of winter wheat. Weed Res. 2003, 43, 428–438. [Google Scholar] [CrossRef]
- Kurstjens, D.A.G.; Kropff, M.J. The impact of uprooting and soil-covering on the effectiveness of weed harrowing. Weed Res. 2001, 41, 211–228. [Google Scholar] [CrossRef]
- Glattkowski, H.; Märländer, B. Umrechnung der Ergebnisse der Amino-N-Bestimmung in Zuckerrüben für die Verwendung in der Reinefeld-Formel nach Umstellung auf Aluminiumsulfat-Klärung und Fluorometrische Messung. Zuckerindustrie 1993, 118, 247–249. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Gerhards, R.; Oebel, H. Practical experiences with a system for site-specific weed control in arable crops using real-time image analysis and GPS-controlled patch spraying. Weed Res. 2006, 46, 185–193. [Google Scholar] [CrossRef]
- Wiltshire, J.J.J.; Tillett, N.D.; Hague, T. Agronomic evaluation of precise mechanical hoeing and chemical weed control in sugar beet. Weed Res. 2003, 43, 236–244. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunz, C.; Weber, J.F.; Gerhards, R. Benefits of Precision Farming Technologies for Mechanical Weed Control in Soybean and Sugar Beet—Comparison of Precision Hoeing with Conventional Mechanical Weed Control. Agronomy 2015, 5, 130-142. https://doi.org/10.3390/agronomy5020130
Kunz C, Weber JF, Gerhards R. Benefits of Precision Farming Technologies for Mechanical Weed Control in Soybean and Sugar Beet—Comparison of Precision Hoeing with Conventional Mechanical Weed Control. Agronomy. 2015; 5(2):130-142. https://doi.org/10.3390/agronomy5020130
Chicago/Turabian StyleKunz, Christoph, Jonas Felix Weber, and Roland Gerhards. 2015. "Benefits of Precision Farming Technologies for Mechanical Weed Control in Soybean and Sugar Beet—Comparison of Precision Hoeing with Conventional Mechanical Weed Control" Agronomy 5, no. 2: 130-142. https://doi.org/10.3390/agronomy5020130
APA StyleKunz, C., Weber, J. F., & Gerhards, R. (2015). Benefits of Precision Farming Technologies for Mechanical Weed Control in Soybean and Sugar Beet—Comparison of Precision Hoeing with Conventional Mechanical Weed Control. Agronomy, 5(2), 130-142. https://doi.org/10.3390/agronomy5020130





